Abstract
We tested the hypothesis that increased dietary protein augments distal nephron acidification through an endothelin-dependent mechanism. Munich-Wistar rats ate minimum electrolyte diets of 50% (HiPro) and 20% (CON) casein-provided protein, the latter comparable to standard chow. HiPro vs. CON had higher distal nephron H+ secretion (41.3 ± 4.0 vs. 23.0 ± 2.1 pmol/mm.min, p < 0.002) mediated by augmented Na+/H+ exchange and H+-ATPase activity. Renal cortex of HiPro vs. CON had higher ET-1 addition to microdialysate and higher ET-1 mRNA, consistent with increased renal ET-1 production. Bosentan, an endothelin A/B receptor antagonist, decreased HiPro distal nephron H+ secretion (28.4 ± 2.4 vs. 41.3 ± 4.0 pmol/mm.min, p < 0.016) through decreased Na+/H+ exchange and decreased H+-ATPase activity. Increased dietary protein augments distal nephron acidification through an endothelin-sensitive increase in Na+/H+ exchange and H+-ATPase activity, supporting an endothelin role in the distal nephron response to this common challenge to acid-base status.
Key words: acidosis, ammonium, bafilomycin, bicarbonate, bosentan, EIPA, light cycler
Introduction
The routine acid challenges to systemic acid-base status faced by humans are modest compared to the large acid loads administered to animals in most experimental protocols. Augmented distal rather than proximal nephron acidification is the predominant renal regulatory response in experimental animals to modest dietary acid loads induced by acid-producing mineral salts (1,2). Augmented distal nephron acidification induced by dietary acid is mediated by multiple mechanisms including 1) increased net HCO3 reabsorption (3), consistent with increased H+ secretion; 2) reduced HCO3 delivery to the terminal distal nephron (4) that facilitates NH4+ secretion (5) and permits secreted H+ to effect acid excretion rather than HCO3 reclamation; and 3) decreased distal nephron HCO3 secretion (1) mediated by endogenous endothelins (2).
In contrast with the acid-producing mineral salts that are most commonly used to induce an acid challenge in experimental protocols, increased intake of dietary protein containing acid-producing amino acids constitutes the acid challenge that humans more routinely face. Intake of acid-producing amino acids increases systemic acid production and urine net acid excretion (6) and distal nephron acidification (7) but the hormonal and/or transport mediators of this response are not known. Because endothelins mediate increased distal nephron acidification induced by modest dietary acid loads due to intake of acid-producing mineral salts (1,2), the present studies tested the hypothesis that increased intake of acid-producing amino-acids as dietary protein increases distal nephron acidification and that this increased acidification is mediated by enhanced endothelin activity.
Materials and Methods
Animals and diet protocol.
Male and female Munich-Wistar rats (Harlan Sprague-Dawley, Houston, TX, 200 to 220 g) ate standard rat chow (Prolab RMH 2500 with 23% protein) for 1 week (week 0) then ate a custom minimum electrolyte diet with protein as purified high nitrogen casein (ICN Nutritional Biochemicals, Cleveland, OH) for 3 weeks (weeks 1, 2, and 3). High protein rats (HiPro) ate custom diet with 50% protein and controls (CON) ate 20%. In preliminary studies similar weight rats ate 24.6 ± 0.9 and 27.1 ± 1.2 g/day, respectively, (n = 4, p = 0.15) and so all rats received 24 g/day diet to assure similar diet intake. Some animals received bosentan (Hoffman-LaRoche, Basel, Switzerland), a nonpeptide endothelin A/B receptor antagonist (8), mixed with study diet at 100 mg/kg body wt/day and so was completely ingested. This oral dose blocks action of pressor doses of i.v. big ET-1 for more than 24 hr (8). All drank distilled H2O except a separate CON group given 4.0% dextrose drinking solution to approximate the increased urine output associated with the HiPro diet. Animals drank ad libitum.
Urine net acid and ET-1 excretion.
We measured daily excretion of urine net acid (NAE) (9) and ET-1 (10) in a 24-hr sample collected on days 7 (week 0), 14 (week 1), 21 (week 2), and 28 (week 3) of the protocol from eight each of HiPro and CON in metabolic cages. We examined the effect of endothelin receptor blockade with bosentan on urine NAE in paired and separate groups of eight each (four with and four without drug) of HiPro and CON. NAE was the mean for each animal group.
Arterial blood parameters.
We measured pH, PCO2, calculated [HCO3] (IRMA Blood Analysis System, Diametrics Medical, Inc., St. Paul, MN) and total CO2 (TCO2) by ultrafluormetry (see below) in 1.0 ml of blood from a chronic carotid arterial catheter in eight each of awake, gently restrained, and calm HiPro and CON at weeks 1 and 3 to assess the effects of HiPro on plasma acid-base parameters. We also measured arterial blood pressure through this chronic arterial catheter as done previously (11).
Microdialysis technique for measurement of renal cortical fluid ET-1.
Renal cortical fluid ET-1 addition was measured using microdialysis of the renal cortex as done in our laboratory (10) at weeks 0, 1, 2, and 3. Three consecutive 20 min collection periods were done in four each of HiPro, CON, and CON + 4.0% dextrose animals for microdialysate ET-1 measurements.
Micropuncture protocol.
Animals were prepared for micropuncture of accessible distal tubules (11) at weeks 1 and 3. In situ early distal flow rate for HiPro and CON was 9.4 ± 0.7 (n = 6) and 6.4 ± 0.4 nl/min (n = 8), respectively. Separate superficial distal nephrons of HiPro and CON were each perfused 9 and 6 nl/min with a Hampel pump to approximate respective in situ flow rates. We measured distal tubule transepithelial potential difference to calculate blood-to-lumen HCO3 permeability (11). After weighing the micropunctured (left) kidney, perfused nephron length was determined by measuring the length of a latex cast injected after micropuncture, recovered after acid digestion of the kidney (11). We measured [HCO3] in stellate vessel plasma to determine peritubular blood-to-lumen HCO3 gradient for calculating transepithelial H+/HCO3 passive permeability (11). Diet, but not H2O, was withheld the evening before micropuncture to yield higher baseline HCO3 reabsorption (12), as done previously (11).
The perfusion solutions used are in table 1. Solution (#1) contained 5 mM HCO3 and 40 mM Cl− to approximate their concentrations at the early distal nephron in situ (13). Solution #2 contained Cl− but no HCO3 to measure Cl−-dependent luminal HCO3 accumulation and to calculate an “apparent” blood-to-lumen H+/HCO3 permeability (11). Solution #3 was HCO3−-and Cl−-free and contained 0.5 mM acetazolamide to inhibit transtubule H+/HCO3 transport and was used to determine “passive” blood-to-lumen H+/HCO3 permeability (11,14). We used this “passive” permeability determined using solution #3 to calculate passive blood-to-lumen HCO3 secretion when perfusing with the HCO3-containing solution, #1 (11). We used the “apparent” blood-to-lumen H+/HCO3 permeability determined from perfusing with solution #2 to calculate “total” HCO3 secretion when perfusing with HCO3-containing solution #1 (11,14). We subtracted calculated “passive” HCO3 secretion from calculated “total” HCO3 secretion to obtain “net” HCO3 secretion when perfusing with solution #1 (11,14). The HCO3 secretion reported herein is the “net” HCO3 secretion that excludes the passive HCO3 secretion calculated as described above. Distal nephron H+ secretion was calculated by subtracting the calculated “net” HCO3 secretion (a negative value) from the measured net HCO3 reabsorption (HCO3 perfused into the distal nephron minus HCO3 collected) (14). All perfusing solutions contained raffinose to minimize fluid transport and gluconate substituted for Cl− when necessary (11). Each surface distal nephron was perfused with each perfusing solution in the following order: #1, #2, #3. Previous studies doing random perfusions of these solutions show that the order of perfusing solutions did not affect calculations of the components of distal nephron HCO3 reabsorption (11).
TABLE 1.
Perfusate composition (mM)
| #1 | #2 | #3 | |
|---|---|---|---|
| Na+ | 61 | 61 | 61 |
| K+ | 4 | 4 | 4 |
| Cl− | 40 | 40 | 0 |
| HCO3− | 5 | 0 | 0 |
| Gluconate | 20 | 25 | 65 |
| Acetazolamide | 0 | 0 | 0.5 |
| Raffinose | 200 | 200 | 200 |
Identification of the H+ transport mediators of HiPro-induced changes in distal nephron acidification.
We compared the net decrease in distal nephron H+ secretion in response to specific H+ transport inhibitors in HiPro vs. CON to determine the contribution of Na+/H+ exchange (EIPA, 10−5 M), H+-ATPase (bafilomycin, 10−7 M), and H+, K+-ATPase (Sch 28080, 10−5 M) as done previously in our laboratory (15). Greater inhibitor-induced decrease in H+ secretion in HiPro vs. CON determined increased activity of the H+-transported inhibited by that compound (15).
Qualitative comparison of ET-1 mRNA expression
Total RNA extraction and RT-PCR.
After treatment, kidneys were removed from anesthetized rats. Pieces of tissues were immediately frozen in liquid nitrogen and stored at −80°C until use.
Total RNA isolation.
Total RNA was isolated using 1 ml of TRI-Reagent™ (Molecular Research Center, Inc., Ohio), for 50 mg of tissue a commercial variant of the guanidium thiocyanate-phenol-chloroform reagent, using the manufacturer's suggested protocol (16). The resulting RNA was dissolved in DNase/RNase free water and stored at −20°C until use. Only RNA preparations whose A260/A280 ratio exceeded 1.6 were further analyzed. The RNA quality was assessed by running the samples in a 1% formaldehyde agarose gel following standard protocol.
Reverse transcription.
We then performed reverse-transcription with 2 μg of RNA, preheated 5 min at 65°C, in a final volume of 20 μl containing 50 mM Tris-HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2, 0.2 mmoles of dNTPs (Roche, Indianapolis, IN), 10 mM dithiothreitol, 1 mg of oligo(dT)12–18 primers (Roche), 40 units of RNasin (Promega, Madison, WI) and 200 units of Moloney murine leukemia virus reverse transcriptase (Promega). After 1 hour at 37°C, the enzyme was inactivated by boiling (10 min at 95°C).
PCR.
Specific oligonucleotide primers (5′-CTCTGCTGTTTGTGGCTTTC-3′ and, 5′-GTCTGTGGTCTTTGTGGGA-3′ for sense and antisense primers respectively) were designed to hybridize the rat endothelin-1 (rET-1) mRNAs using Vector NTI 7 (InforMax Inc., Frederick, MD). Rat ET-1 cDNA amplification was carried out as follows: one μl of the reverse transcribed mixture was added to the PCR mixture containing 100 mM Tris-HCl (pH 8.3), 500 mM KCl, 15 mM MgCl2, 200 μM of dNTPs, 400 nmoles of each primers, 2.5 units of Taq DNA polymerase (Roche) up to a final volume of 50 μl. After 2 min. at 94°C, samples were submitted to 30 cycles under the following conditions: 45 s at 94°C, 45 s at a 48°C (specific annealing temperature for the rET-1 primers) and 45 s at 72°C. After the final cycle, an additional elongation period of 7 min was performed at 72°C.
Real-Time PCR.
Because rat kidneys express very little endothelin-1 mRNAs, we performed a Real-Time PCR using the LightCycler apparatus (Roche) after the conventional PCR. The SYBR Green, which has a high affinity for double-stranded DNA (dsDNA) and exhibits enhancement of fluorescence upon binding to the dsDNA, was chosen as the fluorescent dye. Each reaction contained 2 μl of cDNA from the conventional PCR, 3 mM MgCl2, 0.5 mM of each primers and, 1X of FastStart DNA Master SYBR Green I mix (Roche) in a 20 μl final volume. Samples were then placed in the LightCycler instrument in duplicate and underwent the following thermal cycling profile: cDNA was denatured by a preincubation of 30s at 95°C and the template was amplified for 35 cycles of: 1. denaturation for 0s at 95°C; 2 annealing at 48°C for 10s; 3. extension at 72°C for 25s. The increase in fluorescence, dependent on the initial template concentration, was acquired after each extension phase at 83°C, a temperature above the Tm of the primer dimers and below the Tm of the specific PCR product, thus minimizing acquisition of nonspecific fluorescence intensities. After amplification, a melting curve was generated by cooling the samples to 55°C for 30s and slowly heating the samples at 0.1°C/s to 95°C while the fluorescence was measured continuously. The LightCycler run was concluded with a 40°C incubation for 30 s. Groups were qualitatively compared, with earlier amplification indicating greater number of ET-1 mRNA copies.
Product identity was confirmed by sequence analysis and electrophoresis on a 1% agarose gel stained with ethidium bromide (Expected PCR product size: 290 bp).
Analytical methods.
Immediately after experiment termination, initial and collected perfusate, as well as stellate vessel plasma samples, were analyzed for inulin (11) and for total CO2 (TCO2) using flow-through ultrafluorometry (17) as described previously (18). All tubule fluid and plasma TCO2 were measured on the experimental day by comparing fluorescence of a 7 to 8 nl sample aliquot (corrected for a distilled H2O blank run with each sample group) to a standard curve as previously described (18). This technique actually measures TCO2 but we will refer to this measured value as HCO3 for simplicity.
Microdialysate and urine [ET-1] was measured using a RIA kit (Peninsula Laboratories, Inc., Belmont, CA) after disposable column extraction (Sep-Pak C18, Milford MA) preconditioned with methanol, H2O, and acetic acid as done previously (10) at weeks 0, 1, 2, and 3.
Statistical analysis.
Data were expressed as means ± SE. Paired perfusions of the same tubule were compared using paired t-test; otherwise, ANOVA was used for multiple group comparisons. We used the Bonferroni method for multiple comparisons (p < 0.05) of the same parameter among groups.
Results
Effect of high protein diet (HiPro) on animal/kidney/tubule growth and urine volume.
HiPro and CON had similar body weight at week 0 (214 ± 4 vs. 216 ± 5 g, respectively) but HiPro gained more weight (159 ± 4 vs. 114 ± 3 g, respectively, p < 0.001) by week 3. Daily food intake was identical between HiPro and CON (see Methods) but HiPro daily urine volume was higher at week 3 (44.0 ± 5 vs. 15 ± 2 ml, respectively, p < 0.001). Left kidney weights in HiPro and CON were similar at week 1 (1.011 ± 0.019 vs. 0.988 ± 0.022 g, respectively, p = 0.44) but were higher in HiPro at week 3 (1.251 ± 0.028 vs. 1.048 ± 0.025 g, respectively, p < 0.001). In addition, length of accessible distal tubule was similar in HiPro and CON at week 1 (1.041 ± 0.032 vs. 0.978 ± 0.030 mm, respectively, p = 0.17) but was greater in HiPro at week 3 (1.289 ± 0.041 vs. 1.023 ± 0.033 mm, respectively, p < 0.001). Bosentan did not affect animal or kidney weight, tubule length, food intake, or urine output in either group.
Effect of HiPro on arterial acid-base parameters of conscious animals.
Table 2 shows that Weeks 1 and 3 arterial pH and PCO2 by blood gases with calculated [HCO3] were not different but plasma TCO2 by ultrafluorometry was lower in HiPro than CON. Mean blood pressure was not different in HiPro and CON (111.5 ± 2.4 vs. 110.1 ± 2.2 mm Hg, p = 0.67) at week 3.
TABLE 2A.
Plasma acid-base data in conscious animals after three weeks of dietary protein (HiPro)
| pH | PCO2 (mm Hg) | Calculated [HCO3] (mM) | Measured [TCO2] (mM) | |
|---|---|---|---|---|
| CON (20% Protein) Week 1 (n = 8) | 7.42 ± 0.02 | 39.9 ± 1.0 | 24.0 ± 0.8 | 25.0 ± 0.5 |
| HiPro (50% Protein) Week 1 (n = 8) | 7.37 ± 0.02 | 39.1 ± 1.0 | 22.0 ± 0.7 | 23.1* ± 0.6 |
Values are means ± SE. * p < 0.05 vs. 20% protein; n = number of animals.
TABLE 2B.
Plasma acid-base data in conscious animals after three weeks of dietary protein (HiPro)
| pH | PCO2 (mm Hg) | Calculated [HCO3] (mM) | Measured [TCO2] (mM) | |
|---|---|---|---|---|
| CON (20% Protein) Week 3 (n = 8) | 7.41 ± 0.02 | 39.2 ± 1.0 | 24.1 ± 0.9 | 25.2 ± 0.6 |
| HiPro (50% Protein) Week 3 (n = 8) | 7.38 ± 0.02 | 38.5 ± 1.0 | 22.1 ± 0.8 | 23.3* ± 0.5 |
Values are means ± SE. * p < 0.05 vs. 20% protein; n = number of animals.
Effect of HiPro on renal acidification.
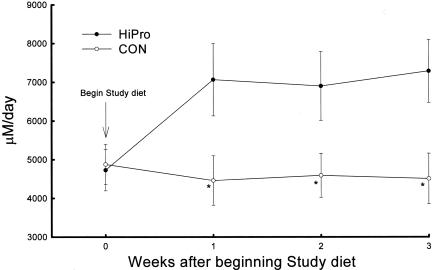
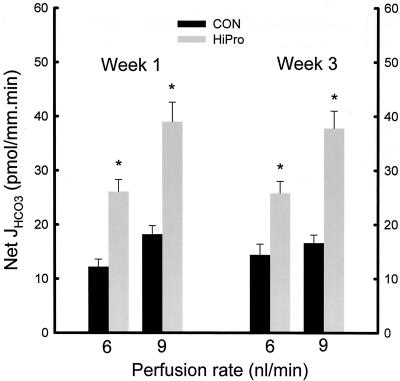
Figure 1 shows higher urine NAE in HiPro than CON at weeks 1, 2, and 3 (7067 ± 937 vs. 4460 ± 639 μM/day, p < 0.04 at week 3). Higher NAE in HiPro was due to higher ammonium (NH4+) excretion (5117 ± 613 vs. 2455 ± 353 μM/day, p < 0.003) and lower HCO3 excretion (56 ± 21 vs. 257 ± 68 μM/day, p < 0.002) but titratable acid excretion was not different between HiPro and CON (2006 ± 338 vs. 2261 ± 345 μM/day, respectively, p = NS). Distal nephron net HCO3 reabsorption was higher at week 1 in HiPro than CON whether perfused at 6 nl/min (26.1 ± 2.2 vs. 12.2 ± 1.4 pmol/mm.min, p < 0.001) or 9 nl/min (39.0 ± 3.6 vs. 18.2 ± 1.6 pmol/mm.min, p < 0.001) as shown in Figure 2. Higher distal nephron net HCO3 reabsorption in HiPro than CON at week 1 was due to higher H+ secretion (29.1 ± 2.7 vs. 21.6 ± 2.0 pmol/mm.min, p < 0.02 for 6 nl/min, and 43.5 ± 3.9 vs. 26.2 ± 2.5 pmol/mm.min, p < 0.002 for 9 nl/min) and less so to lower HCO3 secretion (−3.0 ± 0.5 vs. −6.1 ± 0.8 pmol/mm.min, p < 0.001 for 6 nl/min and −4.5 ± 0.6 vs. −8.0 ± 1.0 pmol/mm.min, p < 0.001 for 9 nl/min). Similarly, Figure 2 shows that distal nephron net HCO3 reabsorption was higher at week 3 in HiPro than CON whether perfused at 6 nl/min (25.8 ± 2.2 vs. 14.4 ± 2.0 pmol/mm.min, p < 0.004) or 9 nl/min (37.8 ± 3.2 vs. 16.6 ± 1.5 pmol/mm.min, p < 0.001) as shown in figure 2. Higher distal nephron net HCO3 reabsorption in HiPro than CON at week 3 was due to higher H+ secretion (28.7 ± 2.8 vs. 19.9 ± 1.9 pmol/mm.min, p < 0.03 for 6 nl/min, and 41.3 ± 4.0 vs. 23.0 ± 2.1 pmol/mm.min, p < 0.002 for 9 nl/min) and less so to lower HCO3 secretion (−2.8 ± 0.3 vs. −5.5 ± 0.5 pmol/mm.min, p < 0.001 for 6 nl/min and −3.5 ± 0.4 vs. −6.4 ± 0.8 pmol/mm.min, p < 0.001 for 9 nl/min).
Fig. 1.
Daily urine net acid excretion measured in weekly intervals in conscious rats initially eating standard chow with 23% protein before being changed to the study diets with 50% (HiPro) and 20% (CON) protein provided as purified casein. *p < 0.05 vs. CON.
Fig. 2.
Distal nephron net HCO3 reabsorption (Net JHCO3) in tubules microperfused in vivo at 6 or 9 nl/min in HiPro and CON at weeks 1 and 3 of the protocol. *p < 0.05 vs. CON.
Transport mediators of HiPro-induced enhanced distal nephron acidification.
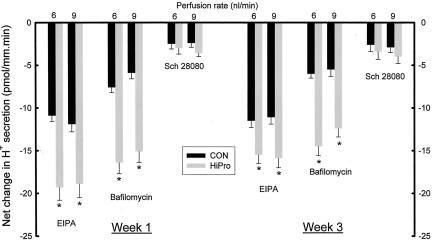
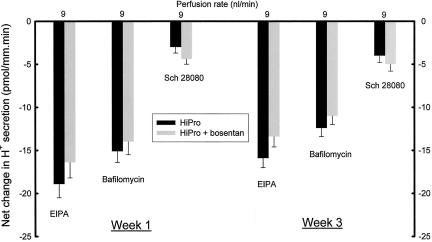
Figure 3 shows that at week 1, the net decrease in distal nephron H+ secretion was greater in HiPro than CON with EIPA (−19.3 ± 1.5 vs. −10.9 ± 0.7 pmol/mm.min, p < 0.006 at 6 nl/min and −18.9 ± 1.6 vs. −11.9 ± 0.9 pmol/mm.min, p < 0.006 at 9 nl/min) and bafilomycin (−16.4 ± 1.3 vs. −7.6 ± 0.6 pmol/mm.min, p < 0.001 at 6 nl/min and −15.1 ± 1.3 vs. −5.9 ± 0.7 pmol/mm.min, p < 0.006 at 9 nl/min), consistent with enhanced Na+/H+ exchange and H+-ATPase activity, respectively. Net decrease in H+ secretion induced by Sch 28080 was not different in HiPro and CON (−3.0 ± 0.7 vs. −2.5 ± 0.6 pmol/mm.min, p = 0.60 at 6 nl/min and −3.6 ± 0.7 vs. −2.4 ± 0.5 pmol/mm.min, p = 0.18 for 9 nl/min), consistent with no increased H+, K+-ATPase activity in HiPro. Similarly, net decrease in distal nephron H+ secretion was greater in HiPro than CON at week 3 with EIPA (−15.5 ± 1.0 vs. −11.3 ± 0.8 pmol/mm.min, p < 0.006 at 6 nl/min and −15.9 ± 1.1 vs. −11.1 ± 0.8 pmol/mm.min, p < 0.004 at 9 nl/min) and bafilomycin (−14.5 ± 1.1 vs. −6.0 ± 0.5 pmol/mm.min, p < 0.001 at 6 nl/min and −12.4 ± 1.0 vs. −5.5 ± 0.8 pmol/mm.min, p < 0.001 at 9 nl/min), consistent with enhanced Na+/H+ exchange and H+-ATPase activity, respectively, as shown in Figure 3. Net decrease in H+ secretion induced by Sch 28080 was not different in HiPro and CON (−3.4 ± 0.9 vs. −2.6 ± 0.8 pmol/mm.min, p = 0.52 at 6 nl/min and −4.0 ± 0.8 vs. −2.9 ± 0.6 pmol/mm.min, p = 0.29 for 9 nl/min), consistent with no increased activity of H+, K+-ATPase activity in HiPro.
Fig. 3.
Net change in distal nephron proton (H+) secretion at weeks 1 and 3 in response to in vivo microperfusion at 6 or 9 nl/min with inhibitors of Na+/H+ exchange (EIPA), H+-ATPase (Bafilomycin), and H+, K+-ATPase (Sch 28080). *p < 0.05 vs. CON.
Effect of HiPro on renal ET-1 production.
Figure 4 shows that HiPro compared to CON had similar urine ET-1 excretion at week 0 (42.9 ± 5.8 vs. 33.2 ± 3.7 fmole/kg bw/day, p = 0.18) but HiPro was higher at week 1 (122.4 ± 26.8 vs. 39.5 ± 3.9 fmole/kg bw/day, p < 0.009), week 2 (89.6 ± 16.1 vs. 30.8 ± 3.4 fmole/kg bw/day, p < 0.004), and week 3 (80.0 ± 15.7 vs. 29.0 ± 3.9 fmole/kg bw/day, p < 0.008). In addition, Figure 4 shows that HiPro and CON had similar ET-1 addition to microdialysate at week 0 (275.2 ± 81.0 vs. 249.3 ± 30.5 fmole/g kidney wt/min, p = 0.77) but HiPro had greater renal cortical microdialysate addition at week 1 (612.4 ± 81.0 vs. 255.2 ± 32.5 fmole/g kidney wt/min, p < 0.002), week 2 (456.8 ± 62.5 vs. 216.3 ± 34.1 fmole/g kidney wt/min, p < 0.005) and week 3 (386.1 ± 49.4 vs. 230.8 ± 29.2 fmole/g kidney wt/min, p < 0.02). In addition, figure 5 shows a qualitative increase in renal cortical mRNA in HiPro than CON at week 3. HiPro had higher urine flow than CON and high urine flow might itself increase urine ET-1 excretion (19). Consequently, we studied CON ingesting 20% protein diet and distilled H2O compared with those ingesting 4% dextrose-containing drinking water (CON-D4W) to increase daily urine volume to a level comparable to that of HiPro without providing additional dietary protein. At week 3, CON-D4W compared to CON had higher daily urine volume (40.2 ± 3 vs. 14 ± 1 ml/day, respectively, n = 8, p < 0.001) and numerically higher urine ET-1 excretion (43.6 ± 9.5 fmole/kg bw/day vs. respective week 3 CON, n = 8, p = 0.18). Nevertheless, ET-1 addition to renal cortical microdialysate in animals with compared to those without the dextrose-containing drinking solution was not different (238 ± 39 fmole/g kidney wt/min vs. respective week 3 CON, n = 8, p = 0.88). Although qualitative comparison of CON-D4W and CON ET-1 mRNA expression suggested a small difference (Figure 5), absolute quantitative analysis failed to show any differences (data not shown).
Fig. 4.
Daily urine endothelin-1 excretion (UET-1 V) (Panel A) and ET-1 microdialysate addition (Panel B) at weekly intervals in HiPro and CON conscious rats. *p < 0.05 vs. CON.
Fig. 5.
Qualitative comparison of ET-1 mRNA using real-time PCP and light cycler technology. CON indicates animals ingesting the 20% experimental diet and CON-D4W indicates CON drinking 4% dextrose solution to increase urine flow to that comparable to HiPro. Earlier amplification indicates greater number of ET-1 mRNA copies.
Effect of ET-1 receptor blockade on arterial blood and urine parameters.
Table 3a shows that bosentan did not affect CON arterial plasma acid-base parameters at weeks 1 or 3. By contrast, Table 3b shows that HiPro receiving bosentan had lower plasma TCO2 at week 1 but not week 3. In addition, mean blood pressure was not different in bosentan-ingesting compared to non-ingesting HiPro (109.7 ± 2.3 vs. 111.5 ± 2.4 mm Hg, p = 0.60) or CON (110.4 ± 2.3 vs. 110.1 ± 2.2 mm Hg, p = 0.93). Figure 6 shows that HiPro receiving bosentan had lower urine NAE (5704 ± 594 vs. 7067 ± 937 μM/day, p < 0.05, paired t) at week 1 but NAE was comparable without and with bosentan at week 3. Lower NAE at week 1 in HiPro with bosentan was due to lower NH4+ excretion (3715 ± 416 vs. 5117 ± 613 μM/day, p < 0.03, paired t) and higher HCO3 excretion (231 ± 42 vs. 56 ± 21 μM/day, p < 0.001, paired t) but titratable acid excretion was not different (2006 ± 338 vs. 2221 ± 304 μM/day, respectively, p = 0.64).
TABLE 3A.
Plasma acid-base parameters of Control animals in response to bosentan
| pH | PCO2 (mm Hg) | Calculated [HCO3] (mM) | Measured [TCO2] (mM) | |
|---|---|---|---|---|
| 20% Protein (Control) Week 1 (n = 8) | 7.41 ± 0.02 | 39.2 ± 1.0 | 24.1 ± 0.9 | 25.2 ± 0.6 |
| 20% Protein (+Bosentan) Week 1 (n = 8) | 7.40 ± 0.02 | 38.9 ± 0.9 | 23.7 ± 0.6 | 24.8 ± 0.6 |
| 20% Protein (+Bosentan) Week 3 (n = 8) | 7.39 ± 0.02 | 39.8 ± 1.0 | 23.6 ± 0.7 | 24.5 ± 0.5 |
| 20% Protein (+Bosentan) Week 3 (n = 8) | 7.40 ± 0.02 | 38.2 ± 0.9 | 23.1 ± 0.6 | 24.1 ± 0.5 |
Values are means ± SE. * p < 0.05 vs. 20% protein; n = number of animals.
TABLE 3B.
Plasma acid-base parameters of HiPro animals in response to bosentan
| pH | PCO2 (mm Hg) | Calculated [HCO3] (mM) | Measured [TCO2] (mM) | |
|---|---|---|---|---|
| 50% Protein (HiPro) Week 1 (n = 8) | 7.37 ± 0.02 | 39.1 ± 1.0 | 22.0 ± 0.7 | 23.1 ± 0.6 |
| 50% Protein (+Bosentan) Week 1 (n = 8) | 7.34 ± 0.02 | 36.1 ± 1.1 | 19.0 ± 0.9 | 19.9* ± 0.5 |
| 50% Protein (+Bosentan) Week 3 (n = 8) | 7.38 ± 0.02 | 38.3 ± 1.0 | 22.2 ± 0.6 | 23.0 ± 0.5 |
| 50% Protein (+Bosentan) Week 3 (n = 8) | 7.37 ± 0.02 | 37.6 ± 1.0 | 21.3 ± 0.6 | 22.2 ± 0.5 |
Values are means ± SE. * p < 0.05 vs. HiPro protein; n = number of animals.
Fig. 6.
Daily urine net acid excretion (NAE) in HiPro not-ingesting and ingesting the endothelin A/B receptor antagonist bosentan. *p < 0.05 vs. CON.
Effect of ET-1 receptor blockade on HiPro-induced changes in distal nephron acidification.
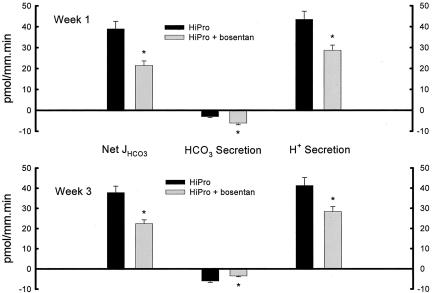
Distal nephron acidification was not different between CON receiving and not receiving bosentan (data not shown). At week 1, Figure 7 shows that HiPro receiving bosentan had lower distal tubule net HCO3 reabsorption when perfused at 9 nl/min (21.5 ± 2.1 vs. 39.0 ± 3.6 pmol/mm.min, p < 0.001). Lower net HCO3 reabsorption at week 1 was due to lower H+ secretion (28.7 ± 2.5 vs. 43.5 ± 3.9 pmol/mm.min, p < 0.007) and higher HCO3 secretion (−6.1 ± 0.8 vs. −3.0 ± 0.5 pmol/mm.min, p < 0.006). Figure 7 also shows that week 3 HiPro animals receiving bosentan and perfused at 9 nl/min had lower distal tubule net HCO3 reabsorption (22.4 ± 1.9 vs. 37.8 ± 3.2 pmol/mm.min, p < 0.001). This lower distal tubule net HCO3 reabsorption was due to lower H+ secretion (28.4 ± 2.4 vs. 41.3 ± 4.0 pmol/mm.min, p < 0.016) and higher HCO3 secretion (−6.0 ± 0.7 vs. −3.5 ± 0.4 pmol/mm.min, p < 0.006).
Fig. 7.
Distal tubule net HCO3 reabsorption (Net JHCO3) and its components, HCO3 and H+ secretion, at weeks 1 and 3 by in vivo microperfusion in HiPro not-ingesting and ingesting the endothelin A/B receptor antagonist bosentan. *p < 0.05 vs. CON.
Effect of ET-1 receptor blockade on enhanced H+ transporter activity induced by HiPro.
At week 1, net decrease in distal nephron H+ secretion was not different in bosentan-ingesting compared to non-ingesting HiPro perfused at 9 nl/min with EIPA (−16.4 ± 1.8 vs. −18.9 ± 1.6 pmol/mm.min, p = 0.32) and bafilomycin (−14.0 ± 1.5 vs. −15.1 ± 1.3 pmol/mm.min, p = 0.59) consistent with no additional effect of these H+ inhibitors on Na+/H+ exchange and H+-ATPase activity, respectively, in HiPro with endothelin A/B receptor blockade. There was no difference in net H+ secretion decrease in HiPro perfused with Sch 28080 (−4.4 ± 0.6 vs. −3.0 ± 0.7 pmol/mm.min, p = 0.15), consistent with no additional effect of endothelin A/B receptor blockade on H+, K+-ATPase activity in HiPro. Similarly, net decrease in distal nephron H+ secretion at week 3 was not different in the bosentan-ingesting compared to the non-ingesting HiPro animals perfused at 9 nl/min with EIPA (−13.4 ± 1.2 vs. −15.9 ± 1.1 pmol/mm.min, p = 0.15) and bafilomycin (−11.0 ± 1.0 vs. −12.4 ± 1.0 pmol/mm.min, p = 0.34) consistent with no additional effect of these H+ inhibitors on Na+/H+ exchange and H+-ATPase activity, respectively, in HiPro with endothelin A/B receptor blockade. The was also no difference in net in H+ secretion decrease in HiPro perfused with Sch 28080 (−5.0 ± 0.8 vs. −3.4 ± 0.9 pmol/mm.min, p = 0.21), consistent with no additional effect of endothelin A/B receptor blockade on H+, K+-ATPase activity in HiPro.
Discussion
The present studies show that increased dietary protein as purified casein augments distal nephron acidification and does so by increasing H+ secretion through increased Na+/H+ exchange and increased H+-ATPase activity and to a lesser extent by decreasing HCO3 secretion. Increased dietary protein increased renal ET-1 production and the data support that each component of increased distal nephron acidification was mediated by increase endothelin activity. That the studies used in vivo microperfusion of the distal nephron supports that the observed endothelin effects were mediated through effects on transport rather than hemodynamics. These studies show that endothelin is a mediator of increased renal acidification in response to dietary protein, the common acid challenge faced by humans.
Endothelin increases Na+/H+ exchange in renal epithelia in vitro (20,21) and endothelin A/B receptor antagonism inhibits Na+/H+ exchange in the distal nephron in vivo (15) but we are not aware of studies showing that endothelin increases H+-ATPase activity. This suggests that the increased H+-ATPase activity that is reduced by endothelin A/B receptor blockade is an indirect effect of endothelin, possibly acting through another agent. A possible scenario is that increased renal endothelin production induced by dietary protein increases adrenal secretion of aldosterone (22) that in turn increases distal nephron H+-ATPase activity (23). Further studies will be necessary to explore this hypothesis.
Table 2 shows lower plasma TCO2 in HiPro compared to CON, consistent with a relative metabolic acidosis in HiPro compared to CON. Because plasma TCO2 remained at this slightly reduced level at weeks 1 and 3, it appears that increased dietary protein as casein leads to a steady-state but not progressive acidosis. The fact that the metabolic acidosis was not progressive is likely due to the marked increase in urine NAE (Figure 1). Dietary mineral acid causes mild net acid retention that mediates the sustained associated increase in urine NAE (24). Also, increased [H+] in vitro increases ET-1 release from renal microvascular endothelium (25) and renal epithelium (21) and so increased endogenous endothelins might contribute to the untoward effects postulated for chronic metabolic acidosis (26).
Figure 1 shows that bosentan decreased urine NAE in HiPro at week 1 but not at week 3, suggesting greater endothelin dependence of HiPro-induced acidification at week 1. Although the net reduction of distal nephron acidification measured per mm tubule length was not different at weeks 1 and 3, the perfused distal nephron segment was longer at week 3 consistent with tubule hypertrophy induced by HiPro. Because there was residual acidification in animals ingesting bosentan the longer tubule at week 3 might have allowed for more overall endothelin-independent acidification at this time point.
Endothelin is more commonly recognized as a vasoconstrictor and a promoter of collagen synthesis and smooth muscle cell proliferation (27). More recent studies show that it mediates the enhanced distal nephron acidification in response to dietary NH4+ salts (2). The present studies show that increased dietary protein increases renal endothelin production and that this increased endothelin activity mediates augmented distal nephron acidification induced by this dietary maneuver. Increased dietary protein provides a physiologic acid challenge to acid-base status that animals, including humans, routinely face. The present studies show that increased renal endothelin activity has the physiologic benefit of enhancing distal nephron acidification that appears necessary to help excrete the increment in metabolic acid production caused by the increment in dietary protein. On the other hand, possible untoward effects of endothelin including its ability to enhance collagen synthesis and smooth muscle cell proliferation might contribute to nephropathy progression as described in some experimental models of renal failure (28). Further studies will be necessary to determine whether increased renal endothelin activity induced by dietary protein is associated with a “trade off” of enhanced renal acid excretion but increased risk for renal injury.
In summary, increased dietary protein augments distal nephron acidification through an endothelin-dependent mechanism. The data support that endothelins contribute to the overall acidification response to this common dietary challenge to systemic acid-base homeostasis faced by humans.
Fig. 8.
Net change in distal nephron proton (H+) secretion in HiPro at weeks 1 and 3 in response to in vivo microperfusion at 6 or 9 nl/min with inhibitors of Na+/H+ exchange (EIPA), H+-ATPase (Bafilomycin), and H+, K+-ATPase (Sch 28080). *p < 0.05 vs. CON.
ACKNOWLEDGMENTS
We are grateful to Ms. Jeri Tasby and Ms. Cathy Hudson for expert technical assistance. We are also grateful to Martine Clozel M.D. for generously providing bosentan for use in these studies.
This work was supported by funds from the Larry and Jane Woirhaye Memorial Endowment in Renal Research the Texas Tech University Health Sciences Center.
DISCUSSION
Wolf, Boston: High protein diets damage kidneys in rats, and actually decrease their lifespan. Are the changes you see reversible? In other words if you feed an animal with high-pro for three or four weeks, and then put them on a normal protein diet and study them, what happens?
Wesson, Lubbock, TX: That's a very good question. If you keep them on the diet the high-protein diet they continue to get worse. But if you stop the high-protein diet the changes remain as they are but do not reverse.
Kaul, Charlottesville: What is the effect of high-protein diet on renal blood flow?
Wesson: Other investigators have shown that high protein diet increases renal blood flow.
Dunn, Milwaukee: Don, I'm curious why you chose endothelin as a possible mediator. The actions of endothelium are broad. Stimulating endothelin secretion, in an adaptive way, with increased protein load is unanticipated. Why did you pick it?
Wesson: That's where our studies have lead us. We were originally interested in chronic metabolic alkalosis and the model that we used additionally has mild hypertension. A number of years ago, one of our fellows starting to look at some of the hormonal profiles in this alkalotic model to try to see why the blood pressure was elevated. He found increased urine endothelin excretion in these alkalotic animals with increased distal nephron acidification.
Boucher, Chapel Hill: Is it possible there was a fourth mechanism that perhaps that EIPA blocked the epithelial channel and changed the driving force for proton secretion through a proton conductance.
Wesson: That's entirely possible. As you know that EIPA also has effects on tubule voltage, although we would like to believe the other studies that show that EIPA is a little bit more specific for sodium hydrogen exchange at the doses that we used, but certainly we have to acknowledge that possibility.
Luke, Cincinnati: I am so pleased to see Don talking about acid base balance. The Society needs to hear more about that subject! I have several questions. Did you look at histology in terms of the A and B cells - although some of the acute changes tend to be not so great. Secondly, was there any change in systemic plasma acid base, and if there was not, (and I realize that these may not be detectable), what's the stimulus? And thirdly, was there a change in sodium delivery to the distal nephron that might affect sodium proton exchange?
Wesson: We are beginning at the histology and it looks like the A type cells are stimulated. These animals have a slight metabolic acidosis compared to control, in that their serum total CO2's are slightly lower than controlled. And Robin I forgot the third one.
Luke: Sodium delivery.
Wesson: Sodium delivery is increased because these animals have increased tubular flows.
Billings, Baton Rouge: I was curious as I heard your talk, about the Atkins diet. There must be an enormous clinical population that you can study to prove the point, in humans, that you are proposing.
Wesson: You are leading to where we think that there is human counterpart to these studies. Yes, in fact we would argue against not just Atkins, but most dietitians would tell you that we in western societies already eat more animal protein or acid producing protein than we really need. So certainly the studies have connotations for the dramatically increased protein intake that the Atkins and South Beach Diet provide, but also the so-called “normal” protein intake of western societies may well be injurious.
Billings: When Dr. Atkins slipped on the ice and died, his wife refused the autopsy. It was suggested that he died of something other than just a slip on the ice. Would you propose that the atherosclerotic possibilities were higher in him since he lived and maybe died, by his own advice.
Wesson: It's a big leap (pun intended), and we are trying to work toward that, but our hypothesis is that high protein diet possibly mediated through endothelin and/or increased aldosterone production in the kidney may well contribute to a cascade leading to cardiovascular disease.
Cohen, Washington: I wanted to join Dr. Luke in welcoming an acid base paper to this meeting. But I want to ask you what I am sure is a very naïve question. Is it possible to show a direct effect of endothelin on acidification. Can you administer endothelian either in the tubular system or to the whole animal and see what happens.
Wesson: We've done that (Am. J. Physiol. 273: F586, 1997), and have shown that endothelin increases proton secretion and decreases bicarbonate secretion in the rat distal nephron in vivo. It does so directly by stimulating sodium hydrogen exchange and indirectly stimulates proton ATPase indirectly through endothelin-stimulated aldosterone. Those are studies we are going to talk about at the upcoming ASN annual meeting.
REFERENCES
- 1.Wesson DE. Reduced HCO3 secretion mediates increased distal nephron acidification induced by dietary acid. Am J Physiol. 1996;271:F670–F678. doi: 10.1152/ajprenal.1996.271.3.F670. (Renal Fluid and Electrolyte Physiol 40) [DOI] [PubMed] [Google Scholar]
- 2.Wesson DE. Endogenous endothelins mediate increased distal tubule acidification induced by dietary acid in rats. J Clin Invest. 1996;99:2203–2211. doi: 10.1172/JCI119393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vandorpe D, Levine DZ. Distal tubule bicarbonate reabsorption in NH4Cl acidotic rats. Clin Invest Med. 1989;12:224–229. [PubMed] [Google Scholar]
- 4.Buerkert J, Martin D, Trigg D. Segmental analysis of the renal tubule in buffer production and net acid formation. Am J Physiol. 1983;244:F442–F4454. doi: 10.1152/ajprenal.1983.244.4.F442. (Renal Fluid Electrolyte Physiol 13) [DOI] [PubMed] [Google Scholar]
- 5.Knepper MA, Packer R, Good DW. Ammonium transport in the kidney. Physiol Rev. 1989;69:179–248. doi: 10.1152/physrev.1989.69.1.179. [DOI] [PubMed] [Google Scholar]
- 6.Remer T. Influence of nutrition on acid-base balance-metabolic aspects. Eur J Nutr. 2001;40:214–220. doi: 10.1007/s394-001-8348-1. [DOI] [PubMed] [Google Scholar]
- 7.Kunau RT, Walker KA. Total CO2 absorption in the distal tubule of the rat. Am J Physiol. 1987;252:F468–F473. doi: 10.1152/ajprenal.1987.252.3.F468. Renal Fluid Electrolyte Physiol 21. [DOI] [PubMed] [Google Scholar]
- 8.Clozel M, Breu V, Gray G, Kalina B, Loffler B-M, Burri K, Cassal J-M, Hirth G, Muller M, Neidhart W, Ramuz H. Pharmacological characterization of Bosentan, a new potent orally active nonpeptide endothelin receptor antagonist. J Pharmacol Exp Ther. 1994;270:228–235. [PubMed] [Google Scholar]
- 9.Wesson DE. Dietary bicarbonate reduces rat distal nephron acidification evaluated in situ. Am J Physiol. 1990;258:F870–F876. doi: 10.1152/ajprenal.1990.258.4.F870. (Renal Fluid Electrolyte Physiol 27) [DOI] [PubMed] [Google Scholar]
- 10.Wesson DE. Endogenous endothelins mediate increased distal tubule acidification induced by dietary acid in rats. J Clin Invest. 1997;99:2203–2211. doi: 10.1172/JCI119393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wesson DE, Dolson GM. Augmented bidirectional HCO3 transport by rat distal tubules in chronic alkalosis. Am J Physiol. 1991;261:F308–F317. doi: 10.1152/ajprenal.1991.261.2.F308. (Renal Fluid Electrolyte Physiol 30) [DOI] [PubMed] [Google Scholar]
- 12.Levine DZ, Iacovitti M, Nash L, Vandorpe D. Secretion of bicarbonate by rat distal 1988; tubules in vivo. Modulation by overnight fasting. J Clin Invest. 1988;81:1873–1878. doi: 10.1172/JCI113533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wesson DE. Depressed distal tubule acidification corrects chloride-deplete alkalosis in rats. Am J Physiol. 1990;259:F636–F644. doi: 10.1152/ajprenal.1990.259.4.F636. (Renal Fluid Electrolyte Physiol 28) [DOI] [PubMed] [Google Scholar]
- 14.Wesson DE. Endogenous endothelins mediate augmented acidification in remnant kidneys. J Am Soc Nephrol. 2001;12:1826–1835. doi: 10.1681/ASN.V1291826. [DOI] [PubMed] [Google Scholar]
- 15.Wesson DE. Na+/H+ exchange mediates increased distal tubule acidification in chronic alkalosis. Kid Int. 1998;53:945–951. doi: 10.1111/j.1523-1755.1998.00838.x. [DOI] [PubMed] [Google Scholar]
- 16.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Analytical Biochemistry. 1987;162(1):156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 17.Star RA. Quantitation of total carbon dioxide in nanoliter samples by flow-through fluorometry. Am J Physiol. 1990;258:F429–F432. doi: 10.1152/ajprenal.1990.258.2.F429. (Renal Fluid Electrolyte Physiol 27) [DOI] [PubMed] [Google Scholar]
- 18.Wesson DE. Dietary HCO3 reduces distal tubule acidification by increasing cellular HCO3 secretion. Am J Physiol. 1996;271:F132–F142. doi: 10.1152/ajprenal.1996.271.1.F132. (Renal Fluid Electrolyte Physiol 40) [DOI] [PubMed] [Google Scholar]
- 19.Zeiler M, Loffler BM, Bock HA, Thiel G. ET-1 excretion is urine flow-dependent in kidney donors and transplant recipients. J Cardiovasc Pharmacol. 1995;26(Suppl 3):S513–S515. [PubMed] [Google Scholar]
- 20.Eiam-Ong S, Hilden SA, King AJ, Johns CA, Madias NE. Endothelin-1 stimulates the Na+/H+ and Na+/HCO3-transporters in rabbit renal cortex. Kid Int. 1992;42:18–24. doi: 10.1038/ki.1992.255. [DOI] [PubMed] [Google Scholar]
- 21.Chu T-S, Peng Y, Cano A, Yanagisawa M, Alpern RJ. EndothelinB receptor activates NHE-3 by a Ca2+-dependent pathway in OKP cells. J Clin Invest. 1996;97:1454–1462. doi: 10.1172/JCI118567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazzocchi G, Rebuffat P, Gottardo G, Meneghelli V, Nussdorfer GG. Evidence that both ETA and ETB receptor subtypes are involved in the in vivo aldosterone secretagogue effect of endothelin-1 in rats. Research Experimental Med. 1996;196:145–152. doi: 10.1007/BF02576836. [DOI] [PubMed] [Google Scholar]
- 23.Eiam-Ong S, Kurtzman NA, Sabatini S. Regulation of collecting tubule adenosine triphosphatases by aldosterone and potassium. J Clin Invest. 1993;91:2385–2392. doi: 10.1172/JCI116471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wesson DE. Dietary acid increases blood and renal cortical acid content in rats. Am J Physiol. 1997;274:F97–F102. doi: 10.1152/ajprenal.1998.274.1.F97. (Renal Fluid Electrolyte Physiol 43) [DOI] [PubMed] [Google Scholar]
- 25.Wesson DE, Simoni J, Green DF. Reduced extracellular pH increases endothelin-1 secretion by human renal microvascular cells. J Clin Invest. 1998;101:578–583. doi: 10.1172/JCI854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alpern RJ, Sakhaee K. The clinical spectrum of chronic metabolic acidosis: homeostatic mechanisms produce significant morbidity. Am J Kid Dis. 1997;29:291–302. doi: 10.1016/s0272-6386(97)90045-7. [DOI] [PubMed] [Google Scholar]
- 27.Kohan DE. Endothelins in the normal and diseased kidney. Am J Kid Dis. 1996;29:2–26. doi: 10.1016/s0272-6386(97)90004-4. [DOI] [PubMed] [Google Scholar]
- 28.Benigni A, Zola C, Corna D, Orisio S, Facchinetti D, Benati L, Remuzzi G. Blocking both A and B endothelin receptors in the kidney attenuates renal injury and prolongs survival in rats with remnant kidney. Am J Kid Dis. 1996;27:416–423. doi: 10.1016/s0272-6386(96)90366-2. [DOI] [PubMed] [Google Scholar]