Abstract
Recent studies demonstrate that Eph receptors exert their action mainly through the regulation of actin reorganization. Here, we show a novel mode of action for EphB receptors. We identified synaptojanin 1, a phosphatidylinositol 5′-phosphatase involved in clathrin-mediated endocytosis, as a physiological substrate for EphB2. EphB2 causes tyrosine phosphorylation in the proline-rich domain of synaptojanin 1, and inhibits the interaction with endophilin and the 5′-phosphatase activity of synaptojanin 1. Treatment with the EphB ligand, ephrin-B2, elevates the cellular level of phosphatidylinositol 4,5-bisphosphate and promotes transferrin uptake. A kinase inactive mutant of EphB2 and a phosphorylation site mutant of synaptojanin 1 both neutralize the increase of transferrin uptake following ephrin-B2 treatment. These mutants also inhibit AMPA glutamate receptor endocytosis in hippocampal neurons. Interestingly, incorporated transferrin does not reach endosomes, suggesting dual effects of EphB signaling on the early and late phases of clathrin-mediated endocytosis. Our results indicate that ephrin-B-EphB signaling regulates clathrin-mediated endocytosis in various cellular contexts by influencing protein interactions and phosphoinositide turnover through tyrosine phosphorylation of synaptojanin 1.
The Eph receptors are a large family of receptor tyrosine kinases. Upon stimulation with their ephrin ligands, these receptors are clustered and initiate intracellular signaling cascades through tyrosine phosphorylation of target molecules and interactions with intracellular ligands1. Proteins binding the cytoplasmic domains of Eph receptors include SH2 domain-containing proteins2, PDZ domain-containing proteins3, and guanine nucleotide exchange factors (GEF)4,5. Proteins that are phosphorylated by Eph receptors include non-receptor tyrosine kinases6,7, adaptor proteins7,8, the small GTPase R-Ras9, the Rho-GEF kalirin10, and the transmembrane proteoglycan syndecan-211. The majority of these molecules are involved in the modulation of the actin cytoskeleton, which is consistent with the fact that the predominant cellular response to ephrin-Eph signaling is a rapid change in cell shape, such as during growth cone collapse. However, the downstream effects of Eph receptor activation may not be restricted to actin cytoskeleton rearrangements.
To identify new pathways downstream of ligand-activated EphB receptors, we analyzed proteins that are specifically tyrosine phosphorylated in response to ephrin-B2 stimulation. For this purpose, we generated a neuroblastoma-like cell line that stably expresses EphB2 (B35-EphB2 cells). B35-EphB2 cells were treated for 15 min with an ephrin-B2-Fc fusion protein multimerized with an anti-Fc antibody. Tyrosine-phosphorylated proteins were captured by anti-phosphotyrosine-agarose and separated by SDS-PAGE. Protein bands that were present in ephrin-B2-treated cells but not in control Fc-treated cells were subjected to MALDI-TOF mass spectrometry analysis. One of the major bands identified in this experiment was the 145 kDa form of synaptojanin 1 (Supplementary Table. 1), a phosphatidylinositol 5′-phosphatase required for clathrin-mediated endocytosis12,13. To confirm the biological significance of this observation, we investigated whether ephrin-B-dependent phosphorylation of synaptojanin 1 occurs in cells in which both synaptojanin 1 and EphB2 are endogenously expressed. In cultured rat hippocampal neurons, ephrin-B2 treatment caused phosphorylation of endogenous synaptojanin 1 as well as autophosphorylation of endogenous EphB2 (Fig. 1a). Moreover, cotransfection assays using 293T cells showed that synaptojanin 1 is phosphorylated downstream of EphB2, but not kinase-inactive EphB2 or EphA4 (Fig. 1b). Together, these results demonstrate that synaptojanin 1 is a physiological downstream target of EphB2 (and possibly other B-type Eph receptors), and suggest a role for ephrin-B-EphB signaling in endocytosis.
Figure 1.
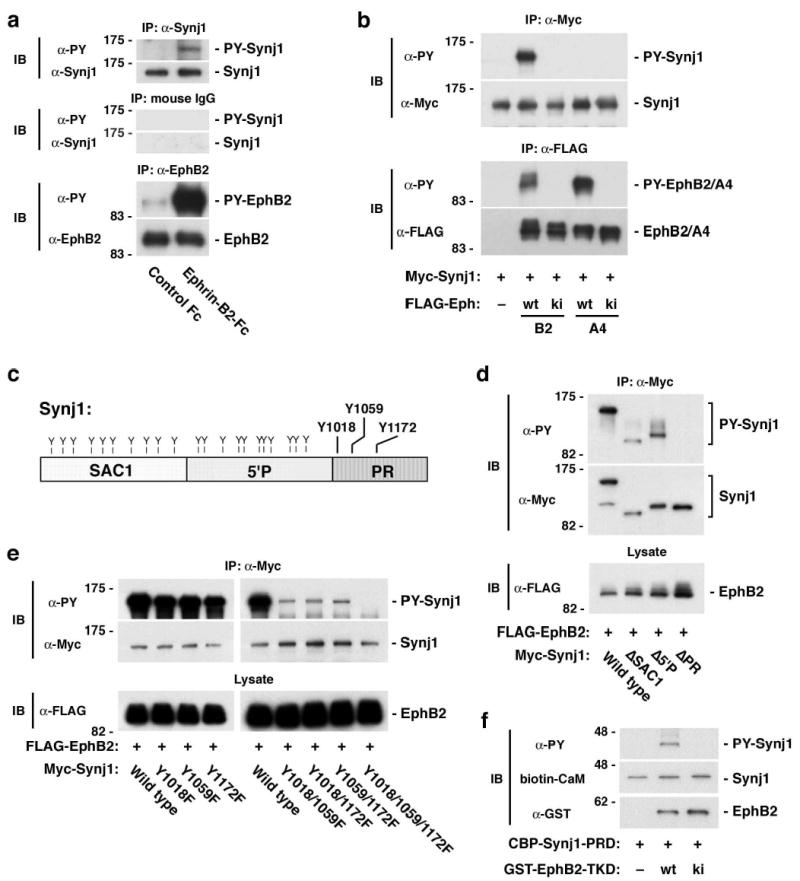
Tyrosine phosphorylation of synaptojanin 1 by EphB receptor. (a) Ephrin-B2-Fc treatment induces tyrosine phosphorylation of endogenous synaptojanin 1 in hippocampal neurons (top panels). Also shown are controls for immunoprecipitation (with normal mouse IgG; middle panels) and for the expression and activation of endogenous EphB2 (bottom panels). (b) EphB2, but not EphA4, phosphorylates synaptojanin 1 in 293T cells (upper panels). Lower panels show controls for the kinase activity of transfected wild-type and kinase-inactive EphB2 and EphA4. (c) Domain structures of synaptojanin 1. Y, tyrosine residues. (d) Phosphorylation of truncated synaptojanin 1 mutants lacking the SAC1 ( SAC1), 5′ phosphatase ( 5′P), and proline-rich ( PR) domains in 293T cells. (e) Phosphorylation of synaptojanin 1 point mutants at the three tyrosine residues in the proline-rich domain (Y1018, Y1159, Y1172; see c) in 293T cells. (f) In vitro phosphorylation assay. Calmodulin-binding peptide-tagged proline-rich domain of synaptojanin 1 (CBP-Synj1-PRD) was phosphorylated by glutathione S-transferase-fused tyrosine kinase domain of wild type EphB2 (GST-EphB2-TKD wt), but not by kinase inactive (ki) EphB2. CBP-Synj1-PRD was detected by blotting with biotinylated calmodulin (CaM). IP; immunoprecipitation. IB; immunoblotting.
Next we identified the EphB2-dependent tyrosine phosphorylation sites in synaptojanin 1. Synaptojanin 1 is a multidomain protein consisting of a Sac1 homology domain, an inositol 5′-phosphatase domain, and a proline-rich domain (Fig. 1c). In 293T cells transfected with EphB2, both a synaptojanin 1 deletion mutant lacking the Sac1 homology domain and another mutant lacking the 5′-phosphatase domain were efficiently phosphorylated, whereas a deletion mutant lacking the proline-rich domain was not phosphorylated (Fig. 1d). This suggests that EphB2 causes phosphorylation in the proline-rich domain. The proline-rich domain contains three tyrosine residues: Tyr1018, Tyr1059 and Tyr1172 (Fig. 1c). Point mutants at single tyrosine residues (designated as Y1018F, Y1059F, and Y1172F) were still phosphorylated downstream of EphB2 (left panel in Fig. 1e). Mutation of two of the three tyrosine residues (Y1018/1059F, Y1018/1172F, and Y1059/1172F) resulted in a significant reduction in phosphorylation, and mutation of all three tyrosine residues (Y1018/1059/1172F) completely eliminated phosphorylation (right panel in Fig. 1e). These results indicate that EphB2-dependent phosphorylation of synaptojanin 1 occurs predominantly at these tyrosine residues in the proline-rich domain. Furthermore, the EphB2 kinase domain can directly phosphorylate the proline-rich domain of synaptojanin 1 in an in vitro phosphorylation assay using recombinant proteins (Fig. 1f).
The proline-rich domain of synaptojanin 1 is known to be involved in protein interactions with SH3 domain-containing endocytic proteins, such as amphiphysin and endophilin14,15. These interactions between endocytic accessory proteins play a pivotal role in clathrin-mediated endocytosis16,17. Since EphB2-dependent phosphorylation occurs in the proline-rich domain, we examined how phosphorylation affects these interactions. In control 293T cells not transfected with EphB2, endophilin was efficiently coimmunoprecipitated with synaptojanin 1 (fourth lane in Fig. 2a). Cotransfection with EphB2, however, greatly reduced coimmunoprecipitation of endophilin (fifth lane in Fig. 2a, and Fig. 2c). In contrast, coimmunoprecipitation of amphiphysin with synaptojanin 1 was not affected by phosphorylation (second and third lanes in Fig. 2a, and Fig. 2c). The selective effect of phosphorylation on the interaction between synaptojanin 1 and endophilin but not amphiphysin was confirmed in an in vitro binding assay (Fig. 2b and 2d) as well as in hippocampal neurons treated with ephrin-B2, where the components of the pathway are endogenously expressed (Fig. 2e and 2f). The differential effect of EphB2-mediated synaptojanin 1 phosphorylation may be due to the fact that amphiphysin and endophilin bind to different sites in the proline-rich domain of synaptojanin 118,19. Consistent with this notion, binding of the Y1059F and Y1172F mutants to endophilin was much less sensitive to phosphorylation than binding of the Y1018F mutant (Fig. 2g and 2h), while the mutations did not affect the interaction of synaptojanin with endophilin in the absence of EphB2 (data not shown). These observations suggest that Tyr1059 and Tyr1172, but not Tyr1018, are the sites critical for the inhibition of the synaptojanin-endophilin interaction by phosphorylation. Taken together, these results indicate that EphB2-dependent phosphorylation at Tyr1059 and Tyr1172 differentially regulates the interactions of synaptojanin 1 with its binding partners.
Figure 2.
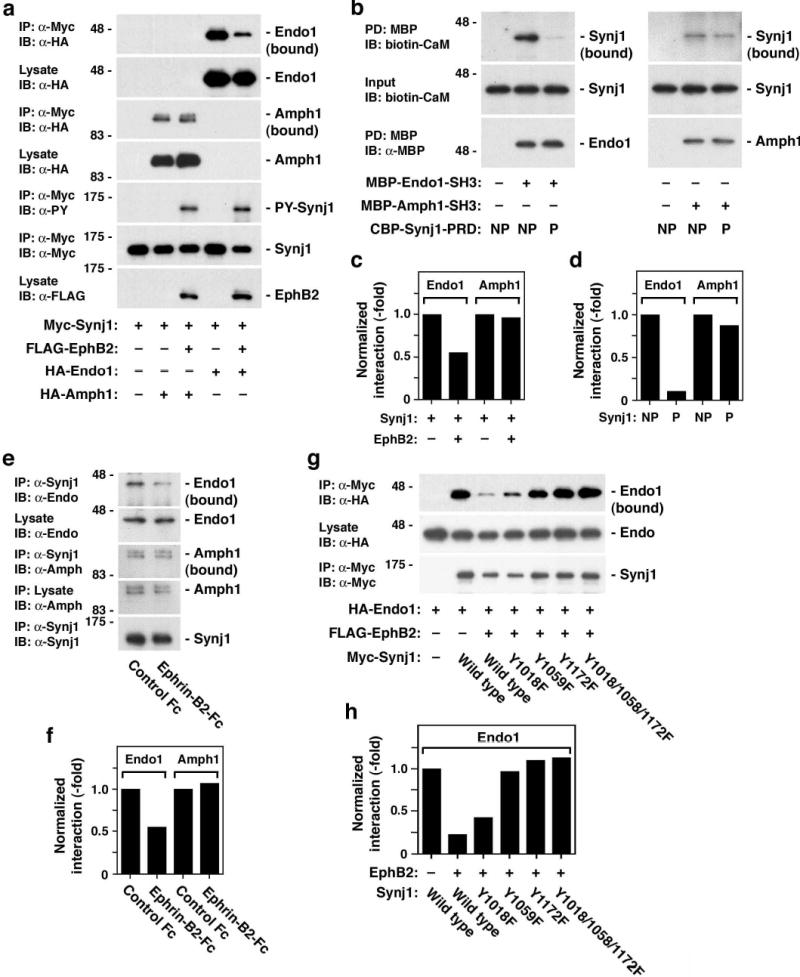
Inhibition of synaptojanin 1-endophilin interaction by EphB2-mediated tyrosine phosphorylation. (a) Effects of EphB2-mediated phosphorylation of synaptojanin 1 on the interactions of synaptojanin 1 with endophilin (Endo1) and amphiphysin (Amph1). 293T cells were transfected with indicated constructs and cell lysates were subjected to immunoprecipitation/immunoblotting assays. Note that the interaction with endophilin 1 is inhibited by EphB2-mediated phosphorylation (uppermost panel marked Endo1 (bound)), whereas the interaction with amphiphysin 1 is not affected (third panel from top marked Amph1 (bound)). (b) In vitro binding assay. Phosphorylated (P; by GST-EphB2-TKD) or control non-phosphorylated (NP) CBP-Synj1-PRDs were incubated with maltose-binding protein-tagged SH3 proteins of endophilin 1 (MBP-Endo1-SH3) and amphiphysin 1 (MBP-Amph1-SH3). The binding of synaptojanin 1 to SH3 proteins was examined by pull down (PD) with amylose resin, which captures MBP-tagged proteins. (c) Quantitation of interaction of synaptojanin 1 with SH3 proteins in 293T cells shown in (a). (d) Quantitation of in vitro binding of synaptojanin 1 with SH3 proteins shown in (b). (e) Attenuation of synaptojanin 1-endophilin interaction in hippocampal neurons treated with ephrin-B2. (f) Quantification of interaction of synaptojanin 1 with SH3 proteins in ephrin-B2-treated neurons shown in (e). (g) Interaction of the synaptojanin 1 tyrosine mutants with endophilin in 293T cells cotransfected with EphB2. (h) Quantitation of interaction of synaptojanin 1 mutants with endophilin 1 in 293T cells shown in (g).
The 5′-phosphatase domain of synaptojanin 1 catalyzes the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) to phosphatidylinositol 4-monophosphate (PIP)12. Interaction with endophilin plays a role in the activation of the phosphatidylinositol 5′-phosphatase activity of synaptojanin 120. Hence, we examined whether EphB2-dependent tyrosine phosphorylation of synaptojanin 1 has an effect on the 5′-phosphatase activity. In both B35-EphB2 cells stimulated with ephrin-B2-Fc and 293T cells transfected with EphB2, immunoprecipitated synaptojanin 1 showed a significantly lower 5′ phosphatase activity compared with synaptojanin 1 from control cells (Fig. 3a and 3b). No such effects on the 5′-phosphatase activity were observed in 293T cells transfected with a kinase inactive EphB2 (Fig. 3b). We further examined which tyrosine residues are involved in phosphorylation-mediated inhibition of the 5′-phosphatase activity. While the 5′-phosphatase activity of Y1018F was inhibited by transfection of EphB2 (Fig. 3c) as was that of wild-type synaptojanin 1, the activity of the Tyr1059 and/or Tyr1172 mutants (Y1059F, Y1172F, and Y1018/1059/1172F) was not decreased (Fig. 3c). Thus, Tyr1059 and Tyr1172 are important both for EphB2-dependent modulation of the 5′-phosphatase activity of synaptojanin 1 and for the synaptojanin-endophilin interaction (see Fig. 2g and 2h). These results suggest that EphB2 inhibits the 5′-phosphatase activity of synaptojanin 1 by inhibiting the interaction with endophilin through tyrosine phosphorylation. Ephrin-B-EphB signaling not only reduces synaptojanin 5′-phosphatase activity but also increases cellular PIP2 levels. An ELISA assay using an anti-PIP2 antibody demonstrated that PIP2 levels are significantly higher in ephrin-B2-treated cells than in control cells (Fig. 3d). Overall, these results suggest that tyrosine phosphorylation in the proline-rich domain has an important regulatory role on the function of synaptojanin 1.
Figure 3.
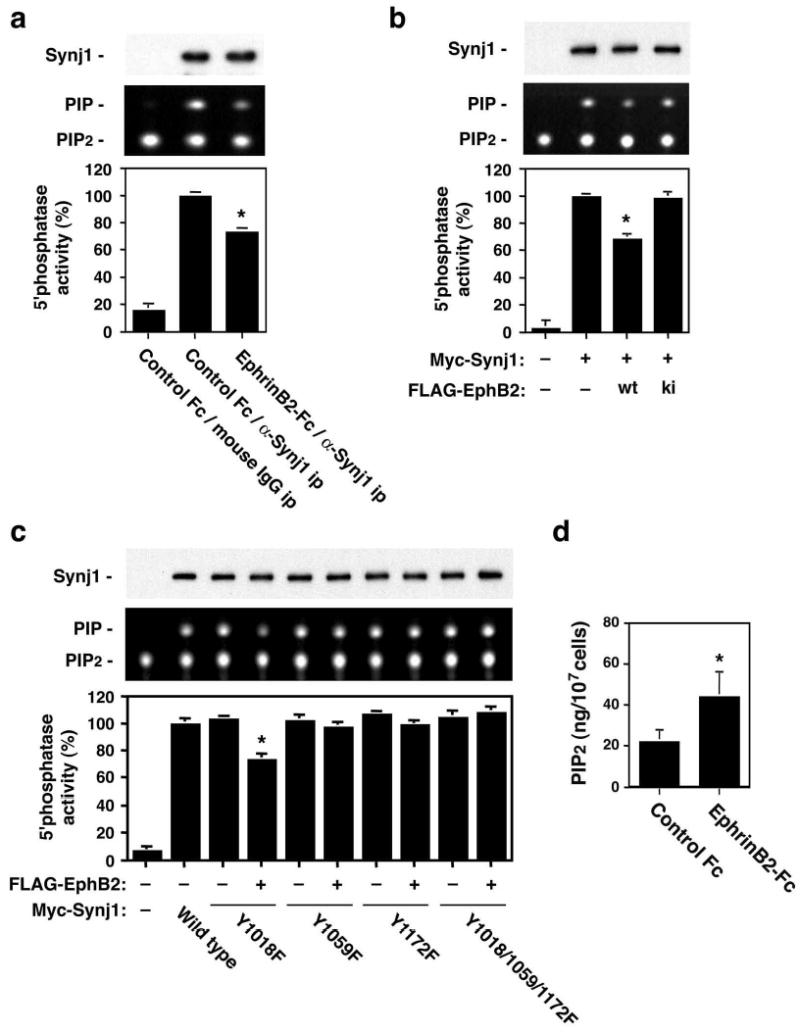
Regulation of PIP2 metabolism by ephrin-B-EphB signaling. (a, b) Effect of EphB2-mediated phosphorylation on the 5′ phosphatase activity of synaptojanin 1. (a) B35-EphB2 cells were treated with ephrin-B2-Fc or control Fc, and lysates from these cells were immunoprecipitated with monoclonal anti-synaptojanin 1 (α -Synj1) or normal mouse IgG (mouse IgG). Immunoprecipitates were subjected to the 5′phosphatase assay using BODIPY FL-PIP2 and analyzed by thin-layer chromatography (middle panel). Amounts of precipitated Myc-Synj1 are depicted in the top panel. Enzymatic activities (bottom panel) are normalized to α -Synj1 immunoprecipitation with control Fc treatment (middle lane) and represented by mean ± SD (n=5, *p<0.001). (b) 293T cells were transfected with Myc-tagged synaptojanin 1 and wild-type or kinase-inactive EphB2. The 5′ phosphatase activity assay was performed on immunoprecipitated synaptojanin 1 and the results are depicted as in (a). (c) Effect of EphB2-mediated phosphorylation on the 5′ phosphatase activity of synaptojanin 1 point mutants. The assay was performed as described in (b) with wild type and Y1018F, Y1059F, Y1172F, and Y1018/1059/1172F mutants of synaptojanin 1. (d) Cellular PIP2 levels in B35-EphB2 cells after treatment with ephrin-B2-Fc or control Fc. Amounts of PIP2 measured by ELISA with anti-PIP2 antibody are represented by mean ± SD (n=5, *p<0.01).
To investigate the effect of ephrin-B-EphB signaling on clathrin-mediated endocytosis, we analyzed uptake of transferrin, which is internalized through a clathrin-dependent pathway, in B35-EphB2 cells in an assay with biotinylated transferrin. Pretreatment of B35-EphB2 cells with ephrin-B2 increased transferrin uptake by approximately two-fold compared with control Fc-treated cells (Fig. 4a and b). An increase in transferrin uptake following ephrin-B2 treatment was also observed in an assay using transferrin conjugated with the fluorescent dye, Alexa 488 (Fig. 4c and 4e). This effect was neutralized by expression of a dominant negative mutant of EphB2 (kinase inactive form) (lower panels in Fig. 4c, and Fig. 4e), indicating that the increase in transferrin uptake by ephrin-B2 is dependent on the tyrosine kinase activity of EphB2. The kinase inactive EphB2 mutant did not affect transferrin uptake in control Fc-treated cells (upper panels in Fig. 4c, and Fig. 4e). Furthermore, transfection with a synaptojanin mutant (Y1059/1172F), in which the two functionally important tyrosine residues (Tyr1059 and Tyr1172) were mutated, inhibited the ephrin-B2-mediated increase in transferrin uptake (lower panel in Fig. 4d, and Fig. 4e), whereas wild type synaptojanin 1 did not affect it (upper panel in Fig. 4d, and Fig. 4e). Taken together, these results confirm that ephrin-B-EphB signaling stimulates internalization through a clathrin-dependent pathway.
Figure 4.
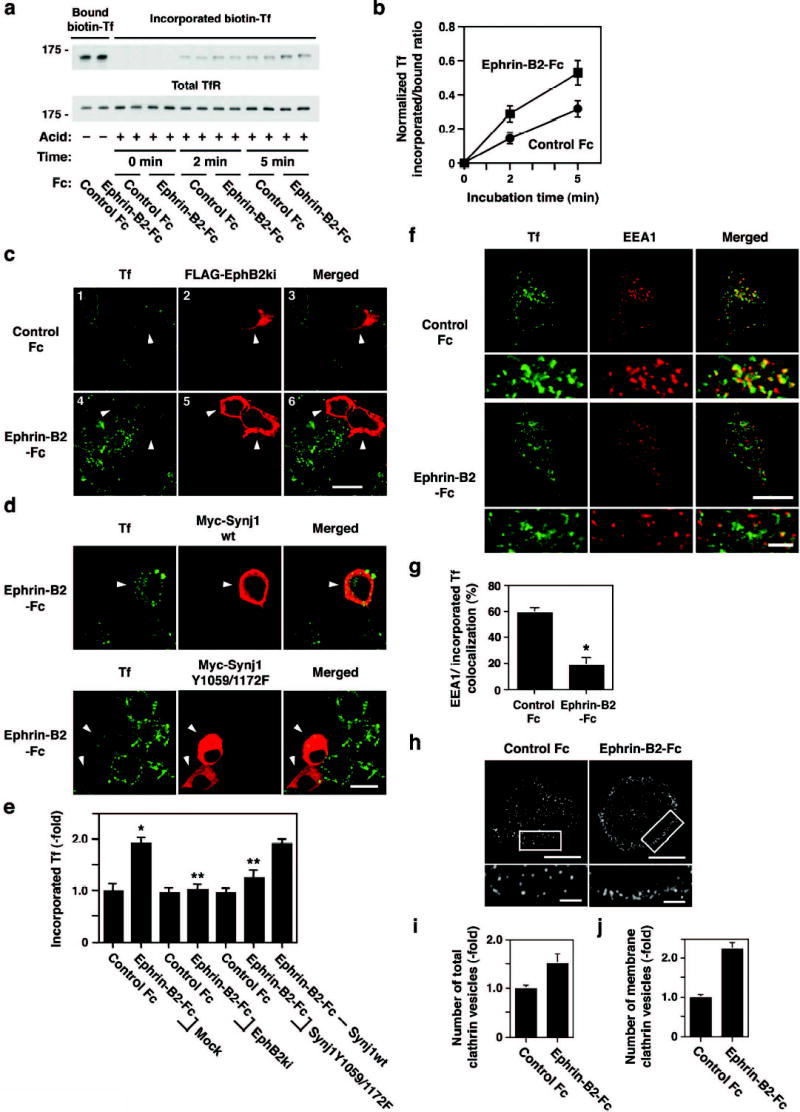
Effect of EphB receptor activation on transferrin uptake and transport. (a) Effect of ephrin-B2-Fc on the internalization of biotinylated transferrin. B35-EphB2 cells were treated with ephrin-B2-Fc or control Fc proteins for 15 min and then incubated with biotinylated transferrin for 15 min at 4°C, followed by incubation without transferrin for the indicated time period at 37°C. After acid stripping of surface-attached transferrin, cell lysates were subjected to overlay assays with HRP-avidin for transferrin (Tf) uptake (upper panel) and anti-transferrin receptor (TfR) antibody for total receptor expression (lower panel). (b) Quantitation of the uptake of biotinylated transferrin, represented by mean ± SD (n=6). (c) Effect of ephrin-B2-Fc on endocytosis as determined by an internalization assay with Alexa488-transferrin combined with kinase-inactive EphB2 transfection. Ephrin-B2-Fc treatment increases transferrin uptake (compare left panels 1 and 4). This increase is neutralized in cells expressing kinase inactive EphB2 (indicated by arrowheads in panels 4 and 5). Scale bar, 10 μm. (d) Neutralization of ephrin-B2-mediated increase in transferrin uptake by transfection with the synaptojanin 1 mutant Y1059/1172F (lower panels). Wild type synaptojanin 1 does not affect transferrin uptake (upper panels). Arrowheads indicate cells expressing the constructs of synaptojanin 1. Scale bar, 10 μm. (e) Quantitative analyses of transferrin incorporation represented by mean ± S.D. (n=10). *p<0.001 compared with mock+control Fc. **p<0.001 compared with mock+ephrin-B2-Fc. (f) Internalized transferrin is not transported into early endosomes in B35-EphB2 cells treated with ephrin-B2. After treatment with Alexa488-transferrin, the cells were incubated without transferrin for 10 min. The cells were then subjected to acid stripping and immunostained with an antibody against EEA1, a marker of early endosomes. (g) Quantitation of colocalization of incorporated transferrin and EEA1 represented by mean ± S.D. (n=6, *p<0.001). (h) Immunocytochemistry of clathrin shows increase in number of clathrin-coated vesicles and their localization at the inner plasma membrane. Lower panels show magnified images of rectangles in the upper panels. Scale bars, 5 μm (upper panel) and 1 μm (lower panel). (i,j) Quantitative analyses of the number of total (i) and membrane associated (j) clathrin vesicles represented by mean ± S.D. (n=4).
Since EphB-dependent phosphorylation of synaptojanin 1 affects its interaction with endophilin (Fig. 2), EphB receptor activation can also have some effects on the fate of internalized clathrin vesicles. In control Fc-treated cells, incorporated transferrin showed a good colocalization with EEA1, an endosomal marker (upper panel in Fig. 4f, and Fig. 4g). In contrast, incorporated transferrin did not colocalize with EEA1 in ephrin-B2-treated cells (lower panel in Fig. 4f, and Fig. 4g), suggesting that transferrin is not transported to endosomes. Furthermore, clathrin-positive vesicles, visualized by immunocytochemistry with an anti-clathrin antibody, accumulated along the plasma membrane in ephrin-B2-treated cells, whereas they were widely distributed in control cells (Fig. 4h). Quantitatively, ephrin-B2 treatment increases the total number of clathrin vesicles by approximately 1.5-fold compared with control (Fig. 4i), and the number of clathrin vesicles in the vicinity of the plasma membrane by 2.2 fold (Fig. 4j). These results suggest that EphB activation inhibits the late phases of endocytosis. This notion is consistent with the results that EphB-mediated phosphorylation inhibits the association of synaptojanin 1 with endophilin, which is thought to be important for clathrin uncoating16,17. Thus, EphB activation appears to exert distinct effects in different phases of clathrin-mediated endocytosis: a promoting effect on the early phase and an inhibitory effect on the late phase.
Finally, we examined the effect of EphB-mediated tyrosine phosphorylation of synaptojanin 1 in a more physiological context. It has been demonstrated that EphB receptors are expressed in the postsynaptic sites in the adult brain and are involved in synaptic plasticity21–23. Endocytosis of AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid)-type glutamate receptors plays a critical role in synaptic long-term depression (LTD), and depends on clathrin-mediated mechanisms24. To investigate the role of EphB-synaptojanin 1 signaling in AMPA receptor endocytosis, we transfected cDNAs encoding kinase inactive EphB2 and the Y1059/1172F synaptojanin 1 mutant into primary hippocampal neurons. Because inhibition of EphB receptor signaling blocks dendritic spine formation in neurons cultured for less than 10 days11, these experiments were performed using more mature cultures, which had already developed spines. In control cultures, stimulation with AMPA ligands promoted internalization of GluR1, a subunit of AMPA receptors, as previously reported25,26 (second column in Fig. 5a, and Fig. 5d). In contrast, transfection with kinase inactive EphB2, which interferes with B-class Eph receptors in a dominant negative manner, inhibited GluR1 endocytosis in both cell body and dendrites (third column in Fig. 5a, and Fig. 5d). Kinase inactive EphB2 did not affect surface expression levels (Supplementary Fig. 1a and 1c) nor total levels (bottom panel in Fig. 5a) of GluR1 in neurons. The Y1058/1172F synaptojanin 1 mutant, but not wild type synaptojanin 1, also prevented AMPA ligand-dependent GluR1 endocytosis (fourth and fifth columns in Fig. 5a, and Fig. 5d) without altering total and surface GluR1 levels (Supplementary Fig. 1a and 1c). Activation of NMDA (N-methyl-D-aspartate)-type glutamate receptors also induces GluR1 endocytosis predominantly in dendrites26. In untransfected neurons, GluR1 was indeed internalized in dendrites in response to NMDA treatment (Fig. 5b, open arrowheads). Transfection with kinase inactive EphB2 (left column in Fig. 5b) or Y1058/1172F synaptojanin 1 mutant (right column in Fig. 5b), but not wild type synaptojanin 1 (middle column in Fig. 5b), significantly attenuated the NMDA-dependent GluR1 endocytosis (closed arrowheads) (Fig. 5e). Neurons transfected with siRNA to inhibit expression of synaptojanin 1 (Fig. 5c, arrowheads; Supplementary Fig. 2), exhibited reduced ligand-induced GluR1 endocytosis following AMPA treatment (Fig. 5c and 5f). Overall, these findings suggest that tyrosine phosphorylation of synaptojanin 1 by EphB receptors controls synaptic activity through the regulation of clathrin-mediated endocytosis in the postsynaptic sites. It has been previously reported that EphB receptors regulate synaptic plasticity through extracellular interaction with NMDA receptors21,22. Our data show that EphB receptors also play a role in AMPA receptor endocytosis.
Figure 5.
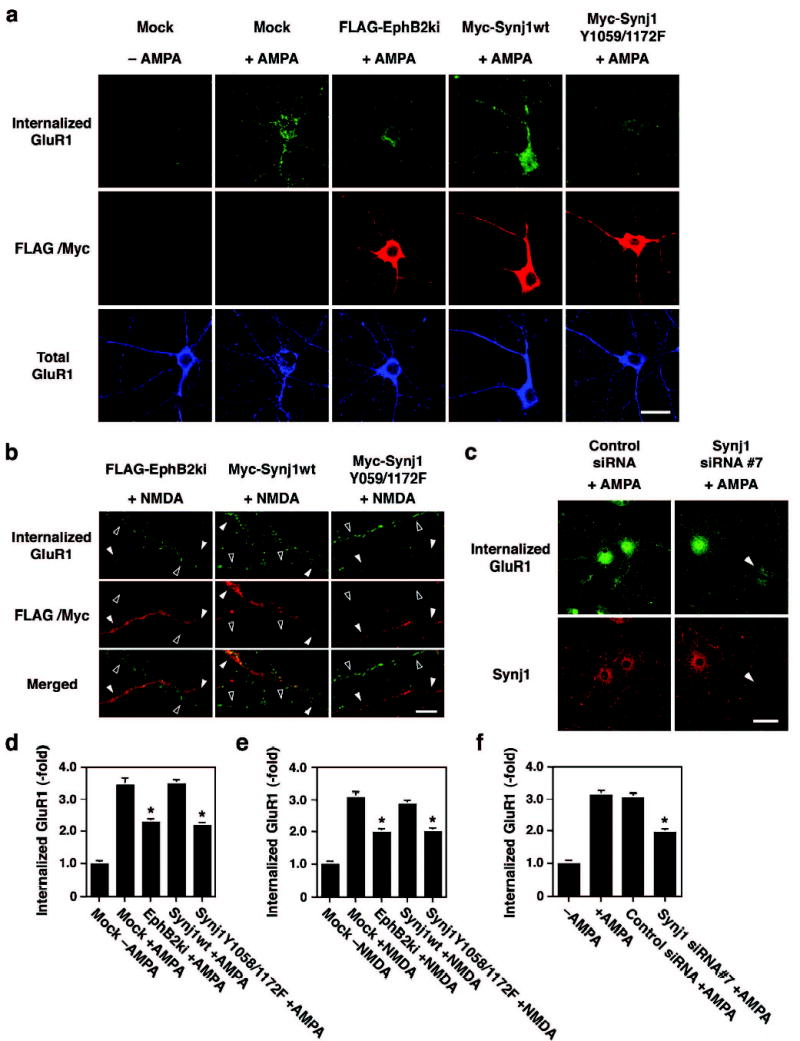
Involvement of EphB receptors and synaptojanin 1 signaling in AMPA glutamate receptor endocytosis in hippocampal neurons. (a) Effect of kinase inactive EphB2 (EphB2ki), synaptojanin 1 wild type (Synj1wt) and synaptojanin 1 mutant (Synj1Y1059/1172F) on AMPA-induced GluR1 endocytosis. Cultures were triple-labeled for internalized GluR1 (top row), expression of transfected expression constructs (middle row), and total GluR1 expression (bottom row). Scale bar, 20 μm. (b) Effect of EphB2ki, Synj1wt and Synj1Y1059/1172F on NMDA-induced GluR1 endocytosis on dendrites. Mutants-transfected neurons show greatly reduced NMDA-dependent GluR1 endocytosis on dendrites (closed arrowheads) compared with dendrites from untransfected neurons (open arrowheads). In contrast, Synj1wt-transfected neuron exhibits the similar level of GluR1 endocytosis as untransfected neuron (middle column). Scale bar, 10 μm. (c) Effect of siRNA against synaptojanin 1 on AMPA-induced GluR1 endocytosis. GluR1 endocytosis is greatly inhibited in siRNA#7-transfected neuron, in which the expression of endogenous synaptojanin 1 is lost (arrowheads in right panels), compared with untransfected neuron. (d–f) Quantitative analyses of GluR1 endocytosis represented by mean ± S.D. (n=20). Effects of mutants of EphB2 and synaptojanin 1 on GluR1 endocytosis induced by AMPA (d) and NMDA (e). *p<0.001 compared with Mock+AMPA (d) or Mock+NMDA (e). Effects of siRNAs on AMPA-induced GluR1 endocytosis (f). *p<0.001 compared with +AMPA.
In this study, we identify a novel ephrin-B-EphB signaling pathway, in which EphB-dependent phosphorylation of synaptojanin 1 modulates the interaction of endocytic proteins and phosphoinositide metabolism. Most of the recent studies concerning downstream signaling of Eph receptors have demonstrated that reorganization of the actin cytoskeleton is a major consequence of ephrin-Eph signaling27,28. Yet a direct regulation of endocytic proteins by ephrin-Eph signaling was not entirely unexpected. For instance, ephrin-mediated growth cone collapse involves not only reorganization of the cytoskeleton but also membrane endocytosis29. In addition, we previously reported that EphB receptor activation regulates dendritic spine formation through intersectin5, a multidomain adaptor protein that also plays a role in clathrin endocytosis30,31. Ephrins and Eph receptors themselves undergo bi-directional endocytosis upon activation32,33. This process does not appear to involve clathrin-mediated mechanisms, suggesting that Eph signaling may regulate both clathrin dependent and independent endocytosis.
Many aspects of the regulation of synaptojanin 1 by phosphorylation remain to be characterized. Cdk5-mediated serine phosphorylation (Ser1142) in the proline-rich domain of synaptojanin 1 also negatively regulates the synaptojanin-endophilin interaction20. It is currently unclear whether there is a functional relationship between this serine phosphorylation and EphB-mediated tyrosine phosphorylation of synaptojanin 1. However, there are differences between serine and tyrosine phosphorylation of synaptojanin 1. For instance, Cdk5 phosphorylates not only synaptojanin 1 but also other endocytic proteins such as amphiphysin34. In contrast, EphB2 does not phosphorylate amphiphysin in 293T cells and tyrosine phosphorylation of amphiphysin was not detected in the synaptosome fraction (Supplementary Fig. 3). These observations suggest that serine phosphorylation may influence additional steps of the clathrin-mediated endocytic process.
One of the critical issues remaining to be addressed is how dephosphorylation of synaptojanin 1 is regulated. While EphB receptor activation increases cellular levels of PIP2 and stimulates clathrin-mediated endocytosis, clathrin vesicles accumulate near the plasma membrane. This is presumably due to the phosphorylation-dependent inhibition of the 5′-phosphatase activity and interaction of synaptojanin 1 with endophilin, which are necessary for clathrin uncoating13,16,17. It is thus likely that the function of synaptojanin 1 is also regulated by a protein tyrosine phosphatase. A similar mechanism has been demonstrated for serine/threonine phosphorylation of endocytic proteins, in which calcineurin plays a critical role35. The interplay between EphB tyrosine kinases and a protein tyrosine phosphatase may play a critical role in regulating the endocytic activity of cells responding to external ephrin stimulation.
Methods
Mammalian expression vectors
Synaptojanin 1 cDNA (U45479) was amplified by RT-PCR from rat brain total RNA and fused with the c-Myc epitope sequence (EQKLISEEDL) at its N-terminus (designated as Myc-Synj1). Myc-Synj1- SAC1 and Myc-Synj1- 5′P were generated by deleting amino acid residues 4–500 and 500–885, respectively, from the full-length cDNA. Myc-Synj1- PR was generated by mutating Glu1012 into a stop codon. Single, double, and triple point mutants of tyrosine residues were generated by mutating Tyr1018, Tyr1059, and Tyr1172, individually or in combination, to Phe. FLAG-tagged EphB2 and its kinase-inactive form (K662R) have been previously described11. Wild-type EphA4 and its kinase-inactive form (K653R) were tagged with the FLAG epitope sequence (DYKDDDDK) at the N-terminus of the mature EphA4 protein. Human amphiphysin 1 (U07616) and mouse endophilin 1 (U58886) were amplified by PCR from human and mouse brain cDNAs, respectively, and fused with the hemagglutinin (HA) epitope (YPYDVPDYA) at their N-terminus. All cDNA inserts were sequenced and ligated to pcDNA3 (Invitrogen).
Cell culture and transfection
A stable EphB2-expressing cell line, EphB2-B35, was established by transfecting rat B35 neuroblastoma cells with chicken EphB2 cDNA. 293T cells were transfected by Lipofectamine 2000 (Invitrogen). Rat hippocampal neurons were cultured in Neurobasal medium containing B27 supplement under 5% CO2 and 10% O2 as described previously11. Transfection of hippocampal neurons was performed at 14 DIV for endocytosis assay by the calcium-phosphate precipitation method11.
Preparation of recombinant proteins
cDNA of synaptojanin 1-proline-rich domain (Synj1-PRD; amino acid residues 1016–1292) was prepared by PCR from Myc-Synj1-pcDNA3 and was ligated to pCAL-n (Stratagene), which is a plasmid for production of calmodulin-binding peptide (CBP)-tagged protein. Recombinant CBP-Synj1-PRD was produced in E. coli (BL21) and was purified by calmodulin-resin (Stratagene) according to manufacture’s protocol. cDNAs of SH3 domains of amphiphysin 1 (622–695) and endophilin 1 (290–352) were generated by PCR and ligated to pMAL-c2X (New England BioLabs). Recombinant maltose-binding protein (MBP)-SH3 domains were isolated from extract of E. coli by amylose resins (Novagen). Tyrosine kinase domains (TKD; 624–889) of EphB2 were fused with glutathione S-transferase (GST) by ligation to pGEX4T-1 (Amersham) and were prepared by glutathione-Sepharose (Amersham).
Phosphorylation analyses
Mass spectrometric analysis
For the analysis of EphB receptor targets, EphB2-B35 cells in five 10-cm dishes were incubated for 15 min in a CO2 incubator with 20 μg/dish of ephrin-B2-Fc (R&D Systems) or control Fc protein (Chemicon), which had been multimerized with anti-human Fc antibody (Sigma). Cells were then lysed in PBS containing 1% Triton X-100, 1μM Na3VO4, and protease inhibitor cocktail (Sigma), and immunoprecipitated with anti-phosphotyrosine antibody (PY-20)-conjugated agarose (Sigma). Precipitated materials were separated by SDS-PAGE and silver stained. Bands that were present specifically in ephrin-B2-treated cells were excised, trypsinized, and subjected to MALDI-TOF analysis as described previously11.
In vitro phosphorylation
CBP-Synj1-PRD (0.2 μg) was incubated with wild type or kinase inactive GST-EphB2-TKD, which had been coupled to glutathione-Sepharose, for 1 h at 37C° in 25 mM Hepes (pH 7.4) containing 0.3 mM MnCl2, 0.2 mM Na3VO4, and 0.2 mM ATP. After reaction, the supernatant was subjected to immunoblotting with monoclonal anti-phosphotyrosine antibody (PY-20; BD Transduction Laboratories). CBP and linker in the Synj1-PRD contain no tyrosine residue. CBP-tagged protein was detected by blotting with biotinylated calmodulin (Calbiochem), followed by HRP-conjugated avidin (Sigma).
Phosphorylation in hippocampal neurons
For the analysis of endogenous synaptojanin 1 phosphorylation, hippocampal neurons at 17DIV were treated with ephrin-B2-Fc and lysed as described above. Cell lysates were immunoprecipitated with monoclonal anti-synaptojanin 1 (gift from Dr. Pietro De Camilli, Yale University) or polyclonal anti-EphB211 antibody immobilized on Protein G-agarose (Zymed). Precipitated materials were immunoblotted with polyclonal anti-synaptojanin 1 (gift from Dr. Peter S. McPherson, McGill University, Canada) and PY-20 antibodies.
Phosphorylation in HEK293T cells
In experiments using 293T cells, cells were transfected with Myc-Synj1 and FLAG-Eph vectors as indicated in the figures. Cell lysates were immunoprecipitated with 9E10 anti-Myc antibody coupled to agarose (Santa Cruz), and immunoblotted with anti-Myc, anti-FLAG (M2; Upstate Biotechnology), and PY-20 antibodies.
Protein-protein interaction
In vitro binding assay
Tyrosine-phosphorylated (by GST-EphB2-TKD) or unphosphorylated CBP-Synj1-PRD (2 μg) was incubated with MBP-amphiphysin 1 or endophilin 1-SH3 domain coupled to amylose resins for 3h at room temperature in TBS containing 0.1% Triton X-100 and 1 mM Na3VO4. After washing in 0.05% Triton X-100-TBS, precipitated materials were subjected to blotting with biotinylated calmodulin and HRP-conjugated avidin to detect bound CBP-Synj1-PRD to SH3 proteins.
Endogenous protein interaction in hippocampal neurons
Primary rat hippocampal neurons (10 DIV) were treated with ephrin-B2-Fc for 20 min as described above, and synaptojanin 1 was immunoprecipitated with monoclonal anti-synaptojanin 1 antibody. Bound amphiphysin and endophilin were detected by immunoblotting with monoclonal anti-amphiphysin (Stressgen) and polyclonal anti-endophilin (Zymed) antibodies, respectively.
Protein interaction in HEK293T cells
Myc-Synj1 was cotransfected with various combination of constructs including HA-amphiphysin 1, HA-endophilin 1, and FLAG-EphB2 and was immunoprecipitated with 9E10 agarose. The bound HA-tagged proteins were evaluated by immunoblotting with anti-HA antibody (3F10; Roche).
Phosphatidylinositol 5′-phosphatase assays
Synaptojanin 1 was immunoprecipitated from ephrin-B2-Fc-treated B35-EphB2 cells with monoclonal anti-synaptojanin 1 antibody (2G10; MBL) or from transfected 293T cells with anti-Myc 9E10 antibody. Immunoprecipitates were incubated with 0.5 μg of BODIPY-FL C6-phosphatidylinositol 4,5-diphosphate (BODIPY-PIP2, Molecular Probes) in 25 μl of Tris-buffered saline containing 4 mM CHAPS, 5 mM MgCl2, 0.5 mM EDTA for 5 min at 37°C. Two μl of reaction mixtures were resolved by thin-layer chromatography (TLC) and developed by 1-propanol/NH4OH/H2O (65:20:15, v/v/v). Reaction products were visualized by UV illuminator and identified by standard lipids, BODIPY-PIP2 and BODIPY-FL C6-phosphatidylinositol 4-phosphate (BODIPY-PIP, Molecular Probes). Hydrolysis of PIP2 to PIP was determined by densitometrical analysis using NIH image and normalized to control Fc-treated cells or cells transfected with wild type Myc-Synj1 (without EphB2).
Measurement of cellular PIP2 by ELISA
B35-EphB2 cells (107 cells/10 cm dish) were treated with ephrin-B2-Fc or control Fc for 15 min as described above. Lipids were extracted with 3.75 volumes of chloroform/methanol/12 N HCl (20/40/1, v/v/v) for 10 min at RT and partitioned by centrifugation after addition of 1.25 volumes of chloroform and H2O. The lower phase, which contains PIP2 was subjected to ELISA assays using monoclonal anti-PIP2 antibody (KT10; Assay Designs), biotinylated anti-mouse IgG antibody, and horseradish peroxidase-conjugated streptavidin-biotin complex (Vector). The immunoreactivity was visualized by 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (Sigma) and the absorbance was measured at 405 nm.
Measurement of clathrin coated vesicles
B35-EphB2 cells were fixed in methanol for 5 min at −20°C after treatment with ephrin-B2-Fc, and were permeabilized by 0.2% Triton X-100 in PBS for 10 min at 4°C. Clathrin-coated vesicles were visualized with monoclonal anti-clathrin heavy chain antibody (X22; Sigma) and Cy2-anti-mouse IgG antibody (Jackson ImmunoResearch). The fluorescence images near the bottom of cells were acquired by a Bio-Rad MRC-1024 confocal microscope. Number of total and membrane associated clathrin vesicles, which are localized along the plasma membrane, were counted by NIH image. Statistical significance was evaluated by Student’s t-test.
Transferrin receptor endocytosis
For biotinylated transferrin uptake experiment, B35-EphB2 cells were plated into 24 well plate. Cells were pretreated with ephrin-B2-Fc as described above, and incubated with 50 μg/ml of biotinylated transferrin (Molecular Probes) in Opti-MEM for 15 min at 4°C. After incubation, cells were washed in Opti-MEM briefly and were further incubated without transferrin for 0, 2, and 5 min in a CO2 incubator. To strip unincorporated transferrin from the cell surface, cells were treated with ice-cold acid solution (0.2 M acetic acid, 0.5 M NaCl) for 10 min on ice. Immediately after this treatment, cells were washed with PBS and lysed in SDS-sample buffer. Incorporated transferrin was detected by blotting with HRP-conjugated avidin (Sigma) and subjected to densitometric quantitation. The level of incorporated transferrin was divided by that of total transferrin bound on the cell surface, which was measured in cells without acid stripping. The units of the Y-axis in bar graphs were obtained by normalization to control cells (control Fc-treated, 0 min). For fluorescent transferrin uptake experiments, cells were cultured on coverslips and incubated with Alexa 488-conjugated transferrin (50 μg/ml, Molecular Probes) for 3 min after pretreatment with ephrin-B2-Fc for 15 min. After acid stripping, cells were fixed and stained with M2 and 9E10 antibodies to identify transfected cells with FLAG-tagged kinase inactive EphB2 and Myc-tagged synaptojanin 1 constructs, respectively. Incorporation of transferrin was examined by a Bio-Rad MRC-1024 confocal microscopy and was quantified by fluorescent intensities of transferrin using NIH image. Statistical significance was evaluated by ANOVA.
AMPA receptor endocytosis
Hippocampal neurons that had been transfected with expression plasmids at 14 DIV were incubated with a rabbit polyclonal antibody against the N-terminal portion of GluR1 (1:20, Oncogene) in conditioned medium containing 2 μM tetrodotoxin (TTX) for 15 min in a CO2 incubator at 16~18 DIV. After a brief washing with Neurobasal medium, neurons were stimulated with 100 μM AMPA or 50 μM NMDA/50 μM CNQX for 1 min and incubated for 10 min without ligands. After fixation with 4% paraformaldehyde/4% sucrose in PBS for 10 min at RT, labeled surface GluR1 was blocked for 30 min at RT by excess non-labeled anti-rabbit antibody Fab fragment (Jackson ImmunoResearch) diluted in 3% normal goat serum in PBS (NGS-PBS). Cells were then permeabilized in 0.2% Triton X-100 in PBS, and internalized GluR1 was detected with Cy2-conjugated anti-rabbit IgG antibody Fab fragment. Expression of dominant negative EphB2 and synaptojanin 1 constructs were verified by labeling with M2 and 9E10 antibodies and Rhodamine Red-X-conjugated anti-mouse IgG antibody (Jackson ImmunoResearch). For detection of total GluR1, cells were incubated with rabbit polyclonal anti-GluR1 antibody against the C-terminal portion (Upstate), followed by Cy5-conjugated anti-rabbit IgG antibody (Jackson ImmunoResearch). Fluorescent images were acquired using a Bio-Rad MRC-1024 confocal microscope. Intensities of immunoreactive puncta of internalized GluR1 in 20~25 dendrites were analyzed using NIH image software in a blinded manner. The units of the Y-axis in bar graphs were obtained by normalization to untreated control cells. Statistical significance was evaluated by ANOVA.
siRNA transfection
siRNAs for synaptojanin 1 (siRNA#7: AAUGUCUCAUGUUCGAGUCUG, 227-248 bp; siRNA#9: AAGGAGGCCAUUAAAGGCACA, 277-298 bp) were designed according to the rat sequence (U45479) and produced by Dharmacon. Scramble siRNA (Scramble I duplex, Dharmacon) was used as a control. Hippocampal neurons were transfected with synaptojanin 1 siRNAs at 16 DIV. Two μl of 20 μM siRNA were mixed with 1 μl of Enhancer R and 2 μl of TransMessenger reagent (Qiagen) as described in the manufacturer’s instructions, and diluted in 300 μl of Neurobasal-B27 medium without antibiotics. At 2.5 h after transfection, transfection medium was replaced with the original conditioned medium.
Supplementary Material
Table I.
Synaptojanin 1-derived tryptic peptides identified by MALDI-TOF analysis of phosphotyrosine-immunoprecipitates from ephrin-B2-Fc treated B35-EphB2 cells.
| M/zmass | Mcalcda | Peptide | Position in rat synaptojanin 1 |
|---|---|---|---|
| 788.54 | 788.50 | TITITLK | 963 – 969 |
| 806.04 | 806.37 | CQSGTVR | 374 – 380 |
| 1129.30 | 1129.62 | TIYAAHKQAK | 191 – 200 |
| 1219.50 | 1219.61 | NEDFVEIARK | 701 – 710 |
| 1620.73 | 1620.85 | TNSVQAFLGLEMLAK | 390 – 404 |
| 1747.81 | 1747.96 | LDPPPFSLIVETRHK | 13 – 27 |
| 1764.71 | 1765.01 | QLEALGLAEKPQLVTR | 405 – 420 |
| 2343.35 | 2343.09 | SRSSQSLPSDSSPQLQQEQPTG | 1271 –1292 |
Monoisotopic mass.
Acknowledgments
We thank Fatima Valencia for establishing the EphB2-B35 cell line; Tristan Williams for MALDI-TOF analysis; Drs. P. De Camilli and P. McPherson for their gift of antibodies; and Dr. W. Stallcup for critical reading of the manuscript. This work was supported by NIH grant P01 HD25938.
Footnotes
Competing interests statement
The authors declare that they have no competing financial interests.
References
- 1.Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signaling. Nat Rev Mol Cell Biol. 2002;3:475–486. doi: 10.1038/nrm856. [DOI] [PubMed] [Google Scholar]
- 2.Kalo MS, Pasquale EB. Signal transfer by Eph receptors . Cell Tissue Res. 1999;298:1–9. [PubMed] [Google Scholar]
- 3.Torres R, et al. PDZ proteins bind, cluster, and synaptically colocalize with Eph receptors and their ephrin ligands. Neuron. 1998;21:1453–1463. doi: 10.1016/s0896-6273(00)80663-7. [DOI] [PubMed] [Google Scholar]
- 4.Shamah SM, et al. EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell. 2001;105:233–244. doi: 10.1016/s0092-8674(01)00314-2. [DOI] [PubMed] [Google Scholar]
- 5.Irie F, Yamaguchi Y. EphB receptors regulate dendritic spine development via intersectin, Cdc42 and N-WASP. Nat Neurosci. 2002;5:1117–1118. doi: 10.1038/nn964. [DOI] [PubMed] [Google Scholar]
- 6.Yu H-H, Zisch AH, Dodelet VC, Pasquale EB. Multiple signaling interactions of Abl and Arg kinases with The EphB2 receptor. Oncogene. 2001;20:3995–4006. doi: 10.1038/sj.onc.1204524. [DOI] [PubMed] [Google Scholar]
- 7.Carter N, Nakamoto T, Hirai H, Hunter T. EphrinA1-induced cytoskeletal reorganization requires FAK and p130cas. Nat Cell Biol. 2002;4:565–573. doi: 10.1038/ncb823. [DOI] [PubMed] [Google Scholar]
- 8.Holland SH, et al. Juxtamembrane tyrosine residues couple the Eph family receptor EphB2/Nuk to specific SH2 domain proteins in neuronal cells. EMBO J. 1997;16:3877–3888. doi: 10.1093/emboj/16.13.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zou JX, et al. An Eph receptor regulates integrin activity through R-Ras. Proc Natl Acad Sci USA. 1999;96:13813–13818. doi: 10.1073/pnas.96.24.13813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Penzes P, et al. Rapid induction of dendritic spine morphogenesis by trans-synaptic ephrinB-EphB receptor activation of the Rho-GEF Kalirin. Neuron. 2003;37:263–274. doi: 10.1016/s0896-6273(02)01168-6. [DOI] [PubMed] [Google Scholar]
- 11.Ethell IM, et al. EphB/syndecan-2 signaling in dendritic spine morphogenesis. Neuron. 2001;31:1001–1013. doi: 10.1016/s0896-6273(01)00440-8. [DOI] [PubMed] [Google Scholar]
- 12.McPherson PS, et al. A presynaptic inositol-5-phosphatase. Nature. 1996;379:353–357. doi: 10.1038/379353a0. [DOI] [PubMed] [Google Scholar]
- 13.Cremona O, et al. Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell. 1999;99:179–188. doi: 10.1016/s0092-8674(00)81649-9. [DOI] [PubMed] [Google Scholar]
- 14.de Heuvel E, et al. Identification of the synaptojanin-binding proteins in brain. J Biol Chem. 1997;272:8710–8716. doi: 10.1074/jbc.272.13.8710. [DOI] [PubMed] [Google Scholar]
- 15.Ringstad N, Nemoto Y, De Camilli P. The SH3p4/SH3p8/SH3p13 protein family: binding partners for synaptojanin and dynamin via Grb2-like Src homology 3 domain. Proc Natl Acad Sci USA. 1997;94:8569–8574. doi: 10.1073/pnas.94.16.8569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verstreken P, et al. Synaptojanin is recruited by endophilin to promote synaptic vesicle uncoating. Neuron. 2003;40:733–748. doi: 10.1016/s0896-6273(03)00644-5. [DOI] [PubMed] [Google Scholar]
- 17.Schuske KR, et al. Endophilin is required for synaptic vesicle endocytosis by localizing synaptojanin. Neuron. 2003;40:749–762. doi: 10.1016/s0896-6273(03)00667-6. [DOI] [PubMed] [Google Scholar]
- 18.Cestra G, et al. The SH3 domains of endophilin and amphiphysin bind to the proline-rich region of synaptojanin 1 at distinct sites that display an unconventional binding specificity. J Biol Chem. 1999;274:32001–32007. doi: 10.1074/jbc.274.45.32001. [DOI] [PubMed] [Google Scholar]
- 19.Ringstad N, Nemoto Y, De Camilli P. Differential expression of endophilin 1 and 2 dimers at central nervous system synapses. J Biol Chem. 2001;276:40424–40430. doi: 10.1074/jbc.M106338200. [DOI] [PubMed] [Google Scholar]
- 20.Lee SY, et al. Regulation of synaptojanin 1 by cyclin-dependent kinase 5 at synapses. Proc Natl Acad Sci USA. 2004;101:546–551. doi: 10.1073/pnas.0307813100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grunwald IC, et al. Kinase-independent requirement of EphB2 receptors in hippocampal synaptic plasticity. Neuron. 2001;32:1027–1040. doi: 10.1016/s0896-6273(01)00550-5. [DOI] [PubMed] [Google Scholar]
- 22.Henderson JT, et al. The receptor tyrosine kinase EphB2 regulates NMDA-dependent synaptic function. Neuron. 2001;32:1041–1056. doi: 10.1016/s0896-6273(01)00553-0. [DOI] [PubMed] [Google Scholar]
- 23.Contractor A, et al. Trans-synaptic Eph receptor-ephrin signaling in hippocampal mossy fiber LTP. Science. 2002;296:1864–1869. doi: 10.1126/science.1069081. [DOI] [PubMed] [Google Scholar]
- 24.Carroll RC, et al. Role of AMPA receptor endocytosis in synaptic plasticity. Nat Rev Neurosci. 2001;2:315–324. doi: 10.1038/35072500. [DOI] [PubMed] [Google Scholar]
- 25.Lin JW, et al. Distinct molecular mechanisms and divergent endocytic pathways of AMPA receptor internalization. Nat Neurosci. 2000;3:1282–1290. doi: 10.1038/81814. [DOI] [PubMed] [Google Scholar]
- 26.Beattie EC, et al. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci. 2000;3:1291–1300. doi: 10.1038/81823. [DOI] [PubMed] [Google Scholar]
- 27.Yamaguchi Y, Pasquale EB. Eph receptors in the adult brain. Curr Opin Neurobiol. 2004;14:288–296. doi: 10.1016/j.conb.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Noren NK, Pasquale EB. Eph receptor-ephrin bidirectional signals that target Ras and Rho proteins . Cell Signal. 2004;16:655–666. doi: 10.1016/j.cellsig.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 29.Jurney WM, et al. Rac1-mediated endocytosis during ephrin-A2- and semaphoring 3A-induced growth cone collapse. J Neurosci. 2002;22:6019–6028. doi: 10.1523/JNEUROSCI.22-14-06019.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koh TW, Verstreken P, Bellen HJ. Dap160/intersection acts a s a stabilizing scaffold required for synaptic development and vesicle endocytosis. Neuron. 2004;43:193–205. doi: 10.1016/j.neuron.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 31.Marie B, et al. Dap160/intersection scaffolds the periactive zone to achieve high-fidelity endocytosis and normal synaptic growth. Neuron. 2004;43:207–219. doi: 10.1016/j.neuron.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Zimmer M, Palmer A, Köhler J, Klein R. EphB-ephrinB bi-directional endocytosis terminates adhesion allowing contact mediated repulsion. Nat Cell Biol. 2003;5:869–878. doi: 10.1038/ncb1045. [DOI] [PubMed] [Google Scholar]
- 33.Marston DJ, Dickinson S, Nobes CD. Rac-dependent trans-endocytosis of ephrinBs regulates Eph-ephrin contact repulsion. Nat Cell Biol. 2003;5:879–888. doi: 10.1038/ncb1044. [DOI] [PubMed] [Google Scholar]
- 34.Floyd SR, et al. Amphiphysin 1 binds the cyclin-dependent kinase (cdk) 5 regulatory subunit p35 and is phosphorylated by cdk5 and cdc2. J Biol Chem. 2001;276:8104–8110. doi: 10.1074/jbc.M008932200. [DOI] [PubMed] [Google Scholar]
- 35.Cousin MA, Robinson PJ. The dephosphins: dephosphorylation by calcineurin triggers synaptic vesicle endocytosis. Trends Neurosci. 2001;24:659–665. doi: 10.1016/s0166-2236(00)01930-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


