Summary
The objective is to evaluate the efficacy of galantamine when a slow titration regimen is employed in Thai Alzheimer's disease (AD) patients with or without cerebrovascular disease and vascular dementia (VaD).
A 6-month, multicentre, open-label, uncontrolled trial was undertaken in 75 AD patients. Eligible patients received an initial galantamine dose of 8 mg/day and escalated over 5–8 weeks to maintenance doses of 16 or 24 mg/day. Primary efficacy measures were AD Assessment Scale-cognitive subscale (ADAS-cog) and the Clinician's Interview-Based Impression of Change-Plus version (CIBIC-plus). The Behavioural Pathology in AD Rating Scale (BEHAVE AD), the AD Cooperative Study Activities of Daily Living Inventory and Pittsburgh Sleep Quality Index were the secondary efficacy variables. Analyses were based on the intent-to-treat population.
Treatment with galantamine showed significant improvement in cognition on the ADAS-cog and CIBIC-plus at month 6. Galantamine showed favourable effects on activities of daily living. Behavioural symptoms and sleep quality were also significantly improved (p < 0.05). Galantamine was well tolerated. The adverse events were mild-to-moderate intensity. The most frequent adverse events commonly reported were nausea (16.4%), dizziness (9.6%) and vomiting (6.8%).
The results of this study may be consistent with galantamine being an effective and safe treatment for mild-to-moderate AD patients with or without cerebrovascular disease and VaD. Flexible dose escalation of galantamine was well tolerated. The daily maintenance dose of galantamine was 16 mg/day, followed by a back up dose of 24 mg/day.
Keywords: Alzheimer's disease, galantamine, acetylcholinesterase inhibitors, efficacy, tolerability
Introduction
Alzheimer's disease (AD), a progressive brain disorder, is the most common cause of dementia among the elderly. It is characterised by a progressive decline of memory and intellectual abilities, which eventually becomes severe enough to interfere with functioning in daily living, the overall quality of life, and ultimately leads to death (1). Cerebrovascular disease and vascular dementia (VaD) is a chronic condition which results from reduced blood flow to the brain nerve cells and can occur together with AD in a condition called ‘mixed dementia’.
During recent years, both epidemiological and neuropathological studies have suggested an association between AD and several vascular-risk factors, such as hypertension, coronary heart disease, diabetes mellitus, ischaemic white matter lesions and generalised atherosclerosis (2–4). Further possibilities include that AD may increase the risk of vascular disease or that vascular disease may stimulate the AD process (5). Interestingly, there is considerable overlap between AD and VaD in terms of both risk factors (vascular-risk factors) and vascular pathology in the brain (e.g. lacunae and white-matter lesion) (6, 7). There is considerable evidence indicating that, as in AD, the central cholinergic system is impaired in VaD (8). In these commonalties, it is reasonable to consider the same treatment strategies for both AD and VaD (9). Therefore, increasing brain nicotinic functions to a level sufficient to improve synaptic plasticity and neuronal survival emerges as a promising therapeutic approach for treatment of these patients (10).
Galantamine, a novel treatment for AD, has a dual mechanism of action, combining allosteric modulation of nicotinic acetylcholine receptors with reversible, competitive inhibition of acetylcholinesterase (11). On the basis of these studies, galantamine provides a broad spectrum of benefits in cognition, global function and activities of daily living in AD patients, but no studies have been conducted in Thai patients before (12–15).
Because differentiation between AD and VaD on clinical grounds can be difficult, a treatment that provides benefits to both the groups of patients would be valuable. The study was designed to determine the therapeutic potential on cognitive and neuropsychiatric response of galantamine when a slow titration regimen is employed in Thai Alzheimer's patients with or without cerebrovascular disease and VaD. In clinical practice, slow dose escalation is advocated as a means of improving the tolerability of cholinergic agents (16). The current study is to further explore the maximum tolerable dose of galantamine, using slow dose escalation schedule of up to 8 weeks in Thai patients.
Methods
Patients
Men and women with a diagnosis of possible AD who met the clinical criteria of National Institute of Neurological and Communicative Disorders and Stroke and AD and Related Disorders Association (NINCDS/ADRDA) (17) or with possible VaD according to the National Institute of Neurological Disorders and Stroke and the Association Internationale pour la Recherche et l'Enseignement en Neurosciences (NINDS-AIREN) (18) with the modified Hachinski scale given a score of 4 or higher were included in the study. They also documented on a CT or MRI scan less than 12 months before entry into the study. Eligible patients also showed presence of mild-to-moderate dementia as evidenced by a Thai Mental State Examination (TMSE) (19) score of 10–24 and a score of ≥12 on the standard cognitive subscale of the AD Assessment Scale (ADAS-cog) (20). The onset of disease had to be between ages 40 and 90. In addition, patients had to have the opportunity to perform certain activities of daily living.
Patients were excluded if they had evidence of other neurodegenerative disorders other than AD, cognitive impairment resulting from acute cerebral trauma, hypoxic cerebral damage, vitamin deficiency, infection, cerebral neoplasia, metabolic disease, mental retardation and oligophrenia, or coexisting medical condition that would limit the patient's ability to complete a study. Patients who had received an investigational medication within the previous 30 days were also excluded. Any other antidementia medication had to be discontinued before entry to the study. The use of drugs for concomitant conditions was permitted during the study, with the exception of sedative-hypnotics and sedating cough and cold remedies, which were discontinued 48 h before cognitive evaluation.
All eligible patients (or a legal representative) and the caregiver provided written informed consent to participate in the study, which was conducted according to the Declaration of Helsinki and its subsequent amendments and approved by institutional review boards at each participating site.
Study Design
The study was a multicentre, open-label, uncontrolled trial undertaken in Thailand that ran from January 2002 until December 2003. Patients received flexible dose of galantamine 16 or 24 mg/day. During dose escalation, patients received galantamine 4 mg twice daily for weeks 1–4 and 8 mg twice daily for weeks 5–8. At week 8, the investigator could then increase the dosage to 12 mg twice daily if the change of patient's ADAS-cog score is less than 4 points at the evaluation of week 8 and based on patient tolerance.
Psychometric evaluations, physical and neurological examinations, laboratory determinations and measurements of vital signs were performed at screening, baseline and (together with checks for medication compliance and adverse events) at weeks 8, 12 and 24. Patients also underwent a CT or MRI scan at screening if this had not been performed within the previous 6 months.
Assessments
The primary efficacy outcome measures were ADAS-cog to assess cognitive function and the Clinician's Interview-Based Impression of Change–plus caregiver input (CIBIC-plus) to assess overall clinical response.
Secondary efficacy endpoints included the Behavioural Pathology in AD Rating Scale (BEHAVE AD), which was composed of two parts (symptomatology and global rating) that covers seven domains of behaviours reported in patients with AD: paranoid and delusional ideation, hallucinations, activity disturbances, aggressiveness, diurnal rhythm disturbances, affective disturbance and anxieties and phobias; the AD Cooperative Study Activities of Daily Living Inventory (ADCS/ADL) with scale is a 23-item informant-based assessment scale measuring widely applicable daily activities; Pittsburgh Sleep Quality Index (PSQI) as a measure of average sleep quality (21).
Safety and tolerability of study medication were assessed by rates of discontinuation and treatment-emergent adverse events as well as changes from baseline in laboratory test values and vital signs, ECG abnormalities and changes on physical examination.
Statistical Analysis
Data from an earlier validation study of galantamine indicated that 64 patients were needed in the study, to achieve 80% power (α = 0.05) for differences in the assessment scores between patients treated with galantamine at baseline and 6 months (22).
All galantamine-treated patients were included in the analysis of safety, demographic and baseline characteristic data. Changes in outcome variables, vital signs and bodyweight from baseline were assessed using two-tailed, paired t-tests. Analysis of efficacy was based on the intent-to-treat population (ITT), which included all patients who took at least one dose of the study medication and had at least one postbaseline efficacy assessment. The repeated measure anova model and paired t-tests were also used in the analysis of change from baseline score to week 8, 12 and 24 of all categorical efficacy assessments. All statistical tests were interpreted at the 5% significance level.
Results
The baseline characteristics are shown in Table 1. In total, 75 patients were enrolled and randomised to treatment and 59(79%) patients completed the study (Figure 1). Premature withdrawal was due to loss of follow-up, nausea, vomiting, weight loss, dizziness and rash. At baseline, patients had a mean TMSE score and ADAS-cog score of 19.7 ± 4.2 and 21.8 ± 1.1, respectively (Table 1). Fifty-two patients (69%) were classified as mild severity, which is defined by the TMSE score >18, and the rest 23 (31%) patients were classified as moderate severity (TMSE ≤ 18). The mean daily dose of galantamine was 21.01 ± 3.9 mg/day. There were 28 (47%) patients maintained on galantamine 16 mg/day and 31 (53%) patients maintained on galantamine 24 mg/day at end point.
Table 1.
Baseline characteristics
| Characteristics | |
|---|---|
| Demography | |
| Male (%) | 32 (42.3%) |
| Female (%) | 43 (57.7%) |
| Age (mean ± SE, years) | 74.5 ± 0.9 |
| Bodyweight (mean ± SE, kg) | 53.6 ± 9.9 |
| Cognitive function | |
| ADAS-cog (mean ± SE) | 21.78 ± 1.1 |
| TMSE (mean ± SE) | 19.7 ± 4.2 |
| Diagnosis, n (%) | |
| Possible AD | 37 (50%) |
| Possible AD with cerebrovascular disease | 32 (42.1%) |
| Vascular dementia | 6 (7.9%) |
ADAS-cog, Alzheimer's Disease Assessment Scale-cognitive subscale; TMSE, Thai Mental State Examination; AD, Alzheimer's disease.
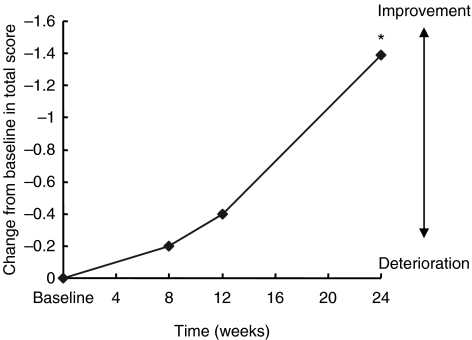
Figure 1.
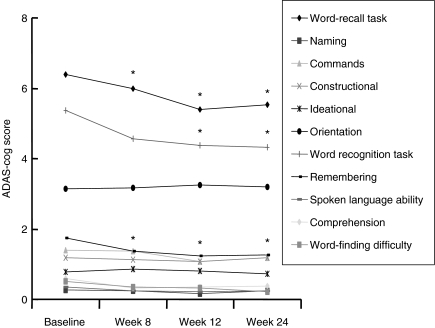
Mean subset scores of Alzheimer's Disease Assessment Scale-cognitive subscale (ADAS-cog) over 6 months (intent-to-treat population analysis). *p < 0.01 vs. baseline
Primary Efficacy Analyses
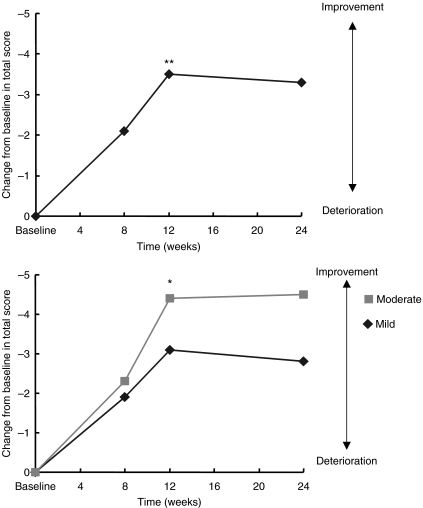
Improvements in ADAS-cog score over baseline were statistically significant at weeks 8, 12 and 24 (−2.10 ± 5.0, −3.53 ± 5.4 and −3.34 ± 6.8 points, respectively; p < 0.05) (Figure 1 and Table 2). Significant improvements in cognitive function from baseline were seen within 3 months after initiating treatment with galantamine (p < 0.05; Figure 1 and Table 3), especially words recall task, word recognition task and remembering test instruction (−0.9 ± 0.1, −1.1 ± 0.5 and −0.5 ± 0.2 points, respectively). Subgroup analysis of patients as classified the disease severity by TMSE demonstrate that there was a significant advantage for mild-severity patients compared with moderate-severity patients (mean change from baseline score at end point: mild, −2.8 ± 5.7; moderate, −4.6 ± 9.6, Figure 2). Using the CIBIC-plus as a measure of overall global function response to galantamine therapy, both groups of patient with mild and moderate severity could maintain or improve their CIBIC-plus score at the end of study. At study endpoint, two-thirds of the patients (67.8%) reported improvement, 25.4% reported no change and the remaining 6.8% reported worsened (Table 2).
Table 2.
Efficacy outcomes in the total population after 6 months
| Assessment | Mean change from baseline |
|---|---|
| Primary efficacy outcomes | |
| ADAS-cog (mean ± SE) | −3.34 ± 0.9 |
| CIBIC-plus [number (%) patients] | |
| Improved | 11 (18.6%) |
| No change | 2 (3.4%) |
| Worsened | 9 (15.3%) |
| Secondary efficacy outcomes | |
| BEHAVE-AD† | −2.8 ± 7.5* |
| ADCS/ADL‡ | 2.4 ± 17.9* |
| PSQI† | 1.2 ± 2.4 |
ADAS-cog, Alzheimer's Disease Assessment Scale-cognitive subscale; CIBIC-plus, Clinician's Interview-Based Impression of Change–plus caregiver input; BEHAVE-AD, Behavioural Pathology in AD Rating Scale; ADCS/ADL, Activities of Daily Living Inventory; PSQI, Pittsburgh Sleep Quality Index.
p < 0.05
Negative changes indicate improvement
Positive changes indicate improvement.
Table 3.
Mean score in each domain of ADAS-cog score throughout the study (intent-to-treat population analysis)
| Mean ± SD | ||||
|---|---|---|---|---|
| ADAS-cog domain | Baseline | Week 8 | Week 12 | Week 24 |
| Word-recall task | 6.4 ± 0.2 | 6.0 ± 0.2* | 5.4 ± 0.3* | 5.5 ± 0.2* |
| Naming | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 |
| Commands | 1.4 ± 0.1 | 1.4 ± 0.2 | 1.1 ± 0.1 | 1.2 ± 0.1 |
| Constructional | 1.2 ± 0.1 | 1.1 ± 0.1 | 1.1 ± 0.1 | 1.2 ± 0.1 |
| Ideational | 0.8 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.1 | 0.7 ± 0.1 |
| Orientation | 3.1 ± 0.3 | 3.2 ± 0.3 | 3.2 ± 0.8 | 3.2 ± 0.3 |
| Word recognition task | 5.4 ± 0.5 | 4.6 ± 0.4 | 4.3 ± 0.5* | 4.3 ± 0.5* |
| Remembering | 1.7 ± 0.2 | 1.4 ± 0.2* | 1.2 ± 0.2* | 1.3 ± 0.2* |
| Spoken language ability | 0.4 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 |
| Comprehension | 0.6 ± 0.1 | 0.3 ± 0.1* | 0.4 ± 0.1* | 0.4 ± 0.1* |
| Word-finding difficulty | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.1 |
ADAS-cog, Alzheimer's Disease Assessment Scale-cognitive subscale
p < 0.05 vs. baseline.
Figure 2.
Mean change from baseline in cognitive abilities (as assessed with Alzheimer's Disease Assessment Scale-cognitive subscale) over 6 months (intent-to-treat population analysis); results in the total population and in patients with moderate and mild groups. **p < 0.01, *p < 0.05 vs. baseline
Secondary Efficacy Analyses
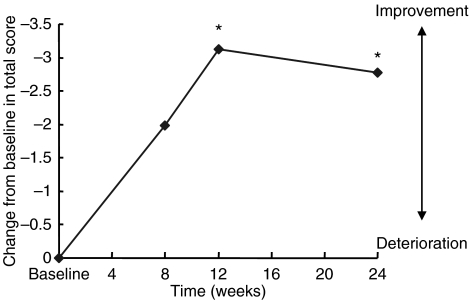
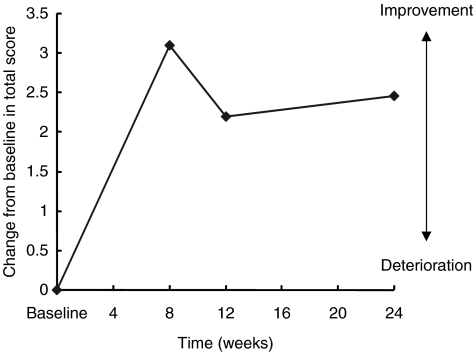
Significant improvements on the BEHAVE-AD were observed at week 12 evaluations and end point (Table 2; Figure 3). Results on the ADCS/ADL inventory scores demonstrated that overall activities of daily living were improved throughout the study (Table 2; Figure 4). Significant benefits of galantamine in the quality of sleep were also observed on the PSQI at week 24 (Table 2; Figure 5).
Figure 3.
Mean change from baseline in Behavioural Pathology in AD Rating Scale over 6 months (intent-to-treat population analysis). *p < 0.05 vs. baseline
Figure 4.
Mean change from baseline in Activities of Daily Living inventory scores over 6 months (intent-to-treat population analysis)
Figure 5.
Mean change from baseline in global Pittsburgh Sleep Quality Index scores over 6 months (intent-to-treat population analysis). *p < 0.05 vs. baseline
Safety Analyses
Generally, adverse events were mild to moderate in intensity and were transient. The adverse events most commonly reported were those affecting the gastrointestinal system, the musculoskeletal system and the nervous system (Table 4). No clinically meaningful changes from baseline were observed in vital signs, physical examination findings or ECG status. There were also no clinically meaningful changes from baseline in clinical chemistry, haematology or urinalysis tests in any of the treatment groups.
Table 4.
Number (%) of patients with adverse events during treatment with galantamine
| Number of Severity | ||||
|---|---|---|---|---|
| Adverse event | Total number (%) | Mild | Moderate | Severe |
| Nausea | 12 (16.4) | 6 | 3 | 3 |
| Vomiting | 5 (6.8) | 2 | 1 | 2 |
| Abdominal pain | 3 (4.1) | 2 | 1 | – |
| Diarrhoea | 2 (2.7) | 2 | – | – |
| Muscle cramp | 2 (2.7) | 2 | – | – |
| Fatigue | 2 (2.7) | 2 | – | – |
| Headache | 2 (2.7) | 2 | – | – |
| Dizziness | 7 (9.6) | 6 | 1 | – |
| Weight loss | 11 (15.1) | 9 | 2 | – |
Discussion
This open-label, uncontrolled study suggests that Thai patients with mild-to-moderately severe AD with or without cerebrovascular disease and VaD receiving galantamine experienced benefits in cognition and global function after 24-week treatment. The clinical relevance of these findings was emphasised by the improvements seen in both the ADAS-cog scores and the CIBIC-plus on observed case and ITT analyses. These broad benefits are desirable in dementia, with potential favourable effects on the burden of careers and health-care resources.
Acetylcholinesterase inhibitors have consistently shown improvements in the cognitive symptoms of AD (23–26), and available data suggest that galantamine also benefit daily activities and ameliorate behavioural symptoms (6, 26). The results of ADAS-cog indicated significant beneficial effects of galantamine on the items associated with attention and executive function than other items. These observations support the hypothesis that the allosteric modulation of neuronal nicotinic receptors by galantamine effectively enhances attention and executive function. Galantamine enhances cholinergic function by moderating nicotinic acetylcholine receptors (27, 28) and by competitively and reversibly inhibiting acetylcholinesterase (29, 30), that is the therapeutic value in patients with AD (31, 32). Subgroup analysis revealed that patients with both mild and moderate severity had significant changes on ADAS-cog as compared with baseline (Figure 1). Thus, moderately severe patients seem to gain the benefit of treatment more than those with mild severity. This may be explained by the evidences supporting that severity of AD was closely correlated to the loss of nicotinic receptors, decrease in choline acetyltransferase and acetylcholinesterase enzymatic activity (33–35). Previous studies have demonstrated that modulation of specific nicotinic receptors in the prefrontal cortex can lead to increased release of serotonin, glutamate and dopamine, resulting in improvements in attention, concentration and cognition as well as alleviation of aggression and depression (36, 37). Indeed, direct agonists or allosteric modulators of presynaptic nicotinic acetylcholine receptors may activate not only the cholinergic system but also other noncholinergic pathway that are impaired in AD. This raises the possibility that, compared with currently available treatment for AD, galantamine may produce additional clinical benefits (22).
Concerning global assessment, CIBIC-plus was used in this study. We found that the majority of patients were assessed to have improved at the end of study period. No difference on treatment effect between patients with mild and moderate severity was observed in this study. The patients had better outcome on the ADCS/ADL relative to baseline; even the difference in mean change between baseline and end point was not statistically significant. A larger sample size might have detected clearer differences in efficacy of this outcome. Over the 6-month study period, galantamine also significantly improved behaviour and quality of sleep from baseline.
The adverse events associated with galantamine in this study were generally those expected from cholinergic stimulation. The adverse event was mild-to-moderate severity, occurred primarily during the dose escalation phase and may be reduced further using a slower dose escalation (38). The main objectives of the study were to investigate whether the tolerability of galantamine was improved with slow dose escalation. The slow introduction of galantamine was well tolerated. Patients in the low-dose galantamine (16 mg/day) group experienced fewer adverse events than those receiving the 24 mg/day dose of galantamine. This finding suggests that the maintenance dose of galantamine should be 16 mg/day while 24 mg/day was used as a back-up dose for those patients who did not respond to 16 mg/day.
Long-term, placebo-controlled studies are the ideal way to assess the duration of benefit of treatments in AD. However, such studies are difficult to conduct because of the ethical reasons and high drop out rates. An alternative method is to conduct an open-label study (25, 39). In conclusion, the results of this long-term, open-label study suggest that galantamine may be effective in the treatment of dementia due to AD and in dementia due to cerebrovascular disease with tolerable adverse effects. These evidences may provide a treatment option to a broader range of patients and therapeutic effects that will give important benefits to patients with dementia.
Acknowledgments
The authors acknowledge the study investigators and staff at each centre as well as the participating patients and caregivers. We also thank Weerawat Meekaew and Orawan Kijkuan for their help with trial coordination. Jaturaporn Chagkutip, PhD, was involved in writing, editing and revising the manuscript. Activities related to study design, data collection and analysis as well as preparation and review of the manuscript were funded by Janssen-Cilag Ltd, Thailand.
Appendix
The GAL-THAI-1 study group includes the following clinical investigators: N Thavichachart, K Phanthumchinda, S Chankrachang, R Praditsuwan, S Nidhinandana, V Senanarong, N Poungvarin, S Tangwongchai, S Suppapitiporn, B Kanchanatawan, S Hemrungrojn, P Worakul, K Tongprasert, R Kaewline, H Ling, W Chotinaiwattarakul, A Sriboonruang, V Srinontprasert, C Udommongkol, P Sithinamsuwan, N Sontayanont, W Pongsuwan, P Tiemdao and K Phitiwattana.
References
- 1.Chung JA, Cummings JL. Neurobehavioral and neuropsychiatric symptoms in Alzheimer's disease: characteristics and treatment. Neurol Clin. 2000;18:829–46. doi: 10.1016/s0733-8619(05)70228-0. [DOI] [PubMed] [Google Scholar]
- 2.Gorelick PB. Risk factors for vascular dementia and Alzheimer disease. Stroke. 2004;35:2620–2. doi: 10.1161/01.STR.0000143318.70292.47. [DOI] [PubMed] [Google Scholar]
- 3.Luchsinger JA, Mayeux R. Cardiovascular risk factors and Alzheimer's disease. Curr Atheroscler Rep. 2004;6:261–6. doi: 10.1007/s11883-004-0056-z. [DOI] [PubMed] [Google Scholar]
- 4.Skoog I, Kalaria RN, Breteler MM. Vascular factors and Alzheimer disease. Alzheimer Dis Assoc Disord. 1999;13:106–14. doi: 10.1097/00002093-199912003-00016. [DOI] [PubMed] [Google Scholar]
- 5.Skoog I. Vascular aspects in Alzheimer's disease. J Neural Transm Suppl. 2000;59:37–43. doi: 10.1007/978-3-7091-6781-6_6. [DOI] [PubMed] [Google Scholar]
- 6.Erkinjuntti T, Kurz A, Gauthier S, Bullock R, Lilienfeld S, Damaraju V. Efficacy of galantamine in probable vascular dementia and Alzheimer's disease combined with cerebrovascular disease: a randomized trial. Lancet. 2002;359:1283–90. doi: 10.1016/S0140-6736(02)08267-3. [DOI] [PubMed] [Google Scholar]
- 7.de la Torre JC. Vascular basis of Alzheimer's pathogenesis. Ann N Y Acad Sci. 2002;977:196–215. doi: 10.1111/j.1749-6632.2002.tb04817.x. [DOI] [PubMed] [Google Scholar]
- 8.Kalaria RN. Comparison between Alzheimer's disease and vascular dementia: implications for treatment. Neurol Res. 2003;25:661–4. doi: 10.1179/016164103101201968. [DOI] [PubMed] [Google Scholar]
- 9.Kurz AF, Erkinjuntti T, Small GW, Lilienfeld S, Damaraju CR. Long-term safety and cognitive effects of galantamine in the treatment of probable vascular dementia or Alzheimer's disease with cerebrovascular disease. Eur J Neurol. 2003;10:633–40. doi: 10.1046/j.1468-1331.2003.00677.x. [DOI] [PubMed] [Google Scholar]
- 10.Woodruff-Pak DS, Vogel RW, Wenk GL. Galantamine effect on nicotinic receptor binding, acetylcholinesterase inhibition, and learning. Proc Natl Acad Sci USA. 2001;98:2089–94. doi: 10.1073/pnas.031584398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lilienfeld S, Parys W. Galantamine: additional benefits to patients with Alzheimer's disease. Dement Geriatr Cogn Disord. 2000;11:19–27. doi: 10.1159/000051228. [DOI] [PubMed] [Google Scholar]
- 12.Kewitz H, Wilcock G, Davis B. Galantamine in Alzheimer's disease. In: Giacobini E, Becker R, editors. Alzheimer's Disease: Therapeutic Strategies. Boston, MA: Birkhauser; 1994. [Google Scholar]
- 13.Dal-Bianco P, Maly Wober J, Lind C, et al. Galantamine treatment in Alzheimer's disease. J Neural Transm. 1991;33:59–63. doi: 10.1007/978-3-7091-9135-4_10. [DOI] [PubMed] [Google Scholar]
- 14.Thomsen T, Bickel U, Fischer JP, Kewitz H. Galantamine hydrobromide in a long-term treatment of Alzheimer's disease. Dementia. 1990;1:46–51. [Google Scholar]
- 15.Wilcock GK, Scott M, Pearsall T, Neubauer K, Boyle M, Razay G. Galantamine and the treatment of Alzheimer's disease. Int J Geriatr Psychiatry. 1993;8:781. [Google Scholar]
- 16.Farlow MR, Evans RM. Pharmacological treatment of cognition in Alzheimer's dementia. Neurology. 1998;51:36–44. doi: 10.1212/wnl.51.1_suppl_1.s36. [DOI] [PubMed] [Google Scholar]
- 17.Blacker D, Albert MS, Bassett SS. Reliability and validity of NINDCS-ADRDA criteria for Alzheimer's Disease. The National Institute of Mental Health Geriatrics Initiative. Arch Neurol. 1994;51:1198–204. doi: 10.1001/archneur.1994.00540240042014. [DOI] [PubMed] [Google Scholar]
- 18.Roman GC, Tatemichi TC, Erkinjuntti T. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS- AIREN International Workshop. Neurology. 1993;43:250–60. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 19.Folstein M, Folstein S, McHugh P. Mini-mental state. A practical method for grading the cognitive status of patients for the clinician. J Psychiatric Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Galasko D. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. Alzheimer Dis Assoc Disord. 1997;11(Suppl. 2):33–9. [PubMed] [Google Scholar]
- 21.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 22.Wilcock G, Howe I, Coles H, et al. Members of the GAL-GBR-2 Study Group. A long-term comparison of galantamine and donepezil in the treatment of Alzheimer's disease. Drug Aging. 2003;10:777–89. doi: 10.2165/00002512-200320100-00006. [DOI] [PubMed] [Google Scholar]
- 23.Rosler M, Anand R, Cicin-Sain A on behalf of the B303 Exelon Study Group. Efficacy and safety of rivastigmine in patients with Alzheimer's disease: international randomized controlled trial. BMJ. 1999;318:633–8. doi: 10.1136/bmj.318.7184.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT the Donepezil Study Group. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer's disease. Neurology. 1998;50:136–45. doi: 10.1212/wnl.50.1.136. [DOI] [PubMed] [Google Scholar]
- 25.Rogers SL, Friedhoff LT. Long-term efficacy and safety of donepezil in the treatment of Alzheimer's disease: an interim analysis of the results of a US multicentre open label extension study. Eur Neuropharmacol. 1998;8:67–75. doi: 10.1016/s0924-977x(97)00079-5. [DOI] [PubMed] [Google Scholar]
- 26.Raskind MA, Peskind ER, Wessel T, Yuan W the Galantamine USA-1 Study Group. Galantamine in AD: a 6 month randomized, placebo-controlled trial with a 6 month extension. Neurology. 2000;54:2261–8. doi: 10.1212/wnl.54.12.2261. [DOI] [PubMed] [Google Scholar]
- 27.Schrattenholz A, Pereira ER, Roth U, Weber K, Albuquerque EX, Maelick A. Agonist responses of neuronal acetylcholine receptors are potentiated by a novel class of allosterically acting ligands. Mol Pharmacol. 1996;49:1–6. [PubMed] [Google Scholar]
- 28.Albuquerque EX, Alkondon M, Pereira ER. Properties of neuronal nicotinic acetylcholine receptors: pharmacological characterization and modulation of synaptic function. J Pharmacol Exp Ther. 1997;280:1117–36. [PubMed] [Google Scholar]
- 29.Vasilenko ET, Tonkopii VD. Characteristics of galantamine as a reversible inhibitor of cholinesterase. Biokhimiia. 1974;39:701–3. [PubMed] [Google Scholar]
- 30.Bores GM, Huger FP, Petko W. Pharmacological evaluation of novel Alzheimer's disease therapeutics: acetylcholinesterase inhibitors related to galantamine. J Pharmacol Exp Ther. 1996;277:728–38. [PubMed] [Google Scholar]
- 31.Levin ED, Simon BB. Nicotinic acetylcholine involvement in cognitive function in animals. Psychopharmacology. 1998;138:217–30. doi: 10.1007/s002130050667. [DOI] [PubMed] [Google Scholar]
- 32.Newhouse PA, Potter A, Levin ED. Nicotinic system involvement in Alzheimer's and Parkinson's diseases. Implications for therapeutics. Drugs Aging. 1997;11:206–28. doi: 10.2165/00002512-199711030-00005. [DOI] [PubMed] [Google Scholar]
- 33.Giacobini E. Do cholinesterase inhibitors have disease modifying effects in Alzheimer's disease? CNS Drugs. 2001;1:61–9. doi: 10.2165/00023210-200115020-00001. [DOI] [PubMed] [Google Scholar]
- 34.Maelicke A, Samochocki M, Jostock R, Fehrenbacher A, Ludwig J, Albuquerque EX. Allosteric sensitization of nicotinic receptors by galantamine, a new treatment strategy for Alzheimer's disease. Biol Psychiatry. 2001;49:279–88. doi: 10.1016/s0006-3223(00)01109-4. [DOI] [PubMed] [Google Scholar]
- 35.Nordberg A. Nicotinic receptors abnormalities of Alzheimer's disease: therapeutic implication. Biol Psychiatry. 2001;49:200–10. doi: 10.1016/s0006-3223(00)01125-2. [DOI] [PubMed] [Google Scholar]
- 36.Granon S, Passetti F, Thomas KL. Enhanced and impaired attentional performance after infusion of D1 dopaminergic receptor agents into rat prefrontal cortex. J Neurosci. 2000;20:1208–15. doi: 10.1523/JNEUROSCI.20-03-01208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou FM, Liang Y, Dani JA. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat Neurosci. 2001;4:1224–9. doi: 10.1038/nn769. [DOI] [PubMed] [Google Scholar]
- 38.Rockwood K, Mintzer J, Truyen L, Wessel T, Wilkinson D. Effects of a flexible galantamine dose in Alzheimer's disease: a randomized, controlled trial. J Neurol Neurosurg Psychiatry. 2001;71:589–95. doi: 10.1136/jnnp.71.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Imbimbo BP, Verdelli G, Martelli P, Marchesini D the Eptastigmine Study Group. Two-year treatment of Alzheimer's disease with eptastigmine. Dement Geriatr Cogn Disord. 1999;10:139–47. doi: 10.1159/000017114. [DOI] [PubMed] [Google Scholar]