Abstract
Coronary hyperreactivity (CH), characterized by persistent severe vasoconstrictions in response to vasoconstrictor challenge, is oppositely influenced by progesterone (P) and medroxyprogesterone acetate (MPA) treatment in surgically menopausal primates. In this study we tested whether multiweek MPA or dihydrotestosterone (DHT) exposure induced CH in intact male rhesus monkeys. Coronary angiographic experiments with intracoronary serotonin and the thromboxane A2 analog U46619 stimulated brief vasoconstriction (for 1–3 min) in large epicardial coronaries in untreated male monkeys. In contrast, MPA- and DHT-treated monkeys displayed long-duration constrictions (>5 min), with significantly greater reductions in the minimal diameters of epicardial coronaries. Immunocytochemistry demonstrated androgen receptors (AR) and P receptors in aorta and coronary arteries, and immunocytochemistry and Western blotting showed AR and P receptors in rhesus coronary vascular muscle cells. In vivo, MPA or DHT increased thromboxane prostanoid (TP) receptor expression in the aorta. In vitro, MPA or DHT increased, whereas P did not change, TP receptor expression in primary coronary vascular muscle cell. This MPA- or DHT-mediated increase in TP receptor expression was attenuated by the AR antagonist flutamide. MPA or DHT induction of CH in intact adult male primates, hypothesized to occur via androgenic up-regulation of vascular muscle TP receptor expression, could predispose to CH-mediated myocardial ischemia.
Abbreviations: ACh, Acetylcholine; AR, androgen receptor; CAD, coronary artery disease; CEE, conjugated equine estrogen; CH, coronary hyperreactivity; DHT, dihydrotestosterone; E, estrogen; ICC, immunocytochemistry; MPA, medroxyprogesterone acetate; ovx, ovariectomized; P, progesterone; φ, minimal diameter; PR, P receptor; RM, rhesus monkey; S, serotonin; T, testosterone; TP, thromboxane prostanoid; TxA2, thromboxane A2; U, U46619; VMC, vascular muscle cell
THE HALLMARK OF coronary hyperreactivity (CH) is persistent, severe vasoconstrictions, which are the consequence of vascular muscle cell (VMC) hyperreactivity, as evidenced not only by the extent, but also, more essentially, by the duration of reduced artery diameter. Recent studies have shown that CH provoked by the combination of serotonin (S) and the thromboxane A2 (TxA2) analog U46619 (U) can be induced in menopausal rhesus monkeys (RM) with normal (1) as well as early atherosclerotic arteries (2).
Synthetic progestins have been used in conjunction with estrogen (E) in hormone therapy regimens to counteract adverse proliferative effects of unopposed E. Nonhuman primate studies suggest that the blood lipid effects of the synthetic progestin, medroxyprogesterone acetate (MPA), do not explain the observed attenuation of E replacement benefits (3). However, MPA dramatically and consistently induced CH in surgically menopausal monkeys (4) and antagonized the inhibitory effects of conjugated equine estrogens (CEE) on coronary atherosclerosis (5). Treatment of ovariectomized (ovx) monkeys for 2 wk with physiological levels of progesterone (P) in the absence of added exogenous E reduced CH in vivo and Ca2+ responses of isolated VMC in vitro (6–8). These data suggest that, like E, P can lower CH and thus lower the risk of coronary artery disease (CAD) whereas MPA, the most commonly used synthetic progestin in the United States, essentially negates any beneficial effect of E on CH.
The negative outcomes of recently reported randomized clinical trials (9, 10) have fulfilled the predictions (4, 5) of potential cardiovascular pitfalls resulting from continuous use of MPA combined with CEE (Prempro, Wyeth, Madison, NJ). MPA is a potent progestogen with significant androgenic activity (11). However, progestogens other than MPA, most notably bioidentical P, do not negate the beneficial cardiovascular effects of E (8, 12). P prevents CH not only in nonatherogenic (1, 8), but also in preatherogenic, surgically menopausal primates (2).
Interestingly, MPA reduces aggression and sexual behavior in nonhuman primates (13) and has been used for the treatment of hypersexuality in male sex offenders (14, 15). More recently, a depot contraceptive containing testosterone (T) and MPA has been proposed as an effective contraceptive in men (16). However, the coronary reactivity effects of increased androgenicity (by exogenous administration of an androgenic progestin such as MPA) in the presence of circulating endogenous androgens remain unexplored. Whether MPA induces CH in intact male RM has not been previously tested and must be considered in light of the outcome of Women’s Health Initiative (WHI) and Heart and Estrogen-Progestin Replacement Study (HERS). In the present study, therefore, we tested whether MPA could increase CH in the intact male RM. We also tested corollary adverse effects of MPA on CH via known androgenic actions (4, 11) by parenteral treatment with a potent androgen agonist, dihydrotestosterone (DHT). Applying our validated standard protocol, consisting of incremental intracoronary vasoconstrictor challenges in the cardiac catheterization laboratory, we explored susceptibility to CH after subdermal implantation with either MPA or DHT for 2 wk in gonadally intact male RM. Additionally, we performed in vitro immunocytochemistry (ICC) and Western blot experiments with RM coronary VMC from ovx female RM to determine a possible role for androgen receptor (AR)-mediated increased thromboxane prostanoid (TP) receptor expression in increasing susceptibility to CH. Our studies suggest that augmented androgenicity may have important cardiovascular consequences in both genders.
Materials and Methods
Animals
Animal experiments were approved by the institutional animal care and use committee and were performed in compliance with established guidelines for the humane care and euthanasia of animals used in research. CH was assessed in 11 adult nonatherosclerotic intact male RM [fed standard Purina monkey chow (Ralston Purina Co., St. Louis, MO), not atherogenic diets], randomized to three groups: control (n = 4), MPA (n = 4), and DHT (n = 3). CH was defined as long-duration (>5-min) epicardial coronary vasoconstrictions to 33% or less of the control (before vasoconstrictor challenge) diameter that sometimes included hourglass vasospasm patterns of focal constrictions (resembling human vasospasm). In the MPA group, four RM (weight, 11.1 ± 1.1 kg; age, 10.7 ± 0.2 yr) received subdermal SILASTIC brand (Dow Corning Corp., Midland, MI) implants containing MPA for 2 wk (MPA blood level, 3.6 ± 1.8 nm). In the DHT group, three monkeys (12.1 ± 1.8 kg; 14.5 ± 2.5 yr old) received SILASTIC brand implants containing DHT for 2 wk (DHT blood level, 31 ± 13.7 nm). Four intact male RM (9.2 ± 1.3 kg; 9.6 ± 1.1 yr old) received no treatment (DHT blood level, 5.9 ± 2.1 nm) and served as controls.
Hormone assay
Intact male RM show a distinct diurnal variation in T levels, characterized by lower values during the day, followed by a marked increase in the early evening, with levels remaining high throughout most of the lights-off period (17). In this study blood was collected in the morning between 0800 and 1000 h (in the early period after lights on) for all animals. MPA and DHT were determined at the Endocrine Services Laboratory, Oregon National Primate Research Center, by specific RIAs using previously validated methods (18–20). These methods have been applied in previously reported measurements of DHT (18), MPA (4), and P (2, 8)
Test of reactivity and provocation of coronary vasospasm
Monkeys were subjected to the coronary artery combined stimulus protocol (described previously) in the catheterization laboratory (1, 2, 4, 6, 8). The intracoronary injection protocol is designed to test the ability of endogenous (platelet) vasoconstrictor substances to provoke prolonged (>5-min), severe (to ≤33% of control diameter) ischemic vasoconstriction with a time course relevant to human coronary ischemia and vasospasm. In the first steps of the protocol, endothelium-dependent vasodilation was tested with intracoronary acetylcholine (ACh) injection via the angiographic catheter. Interventional drugs were each diluted in saline, then slowly and continuously injected into the coronary artery (as 1 ml over a 30-sec interval). Under these conditions, it is estimated (based on monkey coronary blood flow) that an immediate 15-fold dilution of the syringe concentration occurs during the 30-sec slow continuous injection (1, 2, 4, 6, 8).
Briefly stated, the protocol is as follows. Male RM were sedated with 10 mg/kg ketamine, im. Before beginning catheterization, 1–1.5% isoflurane in 100% O2 was continuously administered by inhalation to induce and maintain a surgical plane of anesthesia. Blood pressure, electrocardiogram, heart rate, core temperature, end-tidal CO2, respiratory rate, and percent O2 saturation of the blood were continuously monitored and recorded. Before coronary catheterization, 1000 U heparin were administered iv. Control angiograms were acquired to establish baseline diameter and define the preprotocol pattern of the epicardial coronary arteries using a 1- to 2-ml injection of Hexabrix (Tyco-Mallinckrodt, St. Louis, MO), a radioopaque contrast medium that minimally perturbs coronary diameter. Angiograms were acquired within 15 sec from the end of the stimulus infusion, 3 min after the first injection, and at 5, 10, or 15 min when a persistent epicardial vasoconstriction could be detected. Endothelial integrity was tested by a vasodilation (vs. a constriction) response to 1 μm ACh. After a minimum interval of 7 min and a return to control heart rate and blood pressure ±15% (the interinjection criteria throughout this protocol), the coronary arteries were stimulated with the S plus U protocol (1, 2, 4, 6, 8).
The protocol included a series of five 30-sec vasoconstrictor injections that constituted the main vasospasm challenges. Three separate sequential infusions of combined S (100 μm) and U (1 μm; a nonmetabolizable TxA2 analog), spaced at least 7 min apart, were followed by the fourth challenge, the triple combination of S (100 μm), U (1 μm), and endothelin 1 (1 nm). Finally, as the fifth provoking challenge, 100 μm S with a higher concentration (3 μm) of U was infused. The cumulative U dose was 2455 ng at this point. When constrictions that meet defined ischemia criteria (persisting for >5 min, to ≤33% of control diameter) were not evident after this sequential challenge with S plus U or cardiogenic shock was not evident (diastolic blood pressure <30 mm Hg for at least 30 sec), the animal was termed protected. Analysts blinded to the treatment groups examined enlarged number-coded digital images of angiograms to define the point of minimum diameter during vasoconstriction of a responding epicardial artery for each monkey. The same defined point was spatially mapped and measured at each corresponding time point, including control images, to determine internal diameter as a percentage of the control diameter that was recorded before the challenge with the vasoconstrictor stimulus (1, 2, 4, 6, 8). Upon completion of the in vivo protocol, the RM were immediately taken to necropsy and euthanized with an overdose of pentobarbital sodium, pneumothorax, and exsanguination. Hearts were immediately removed to allow rapid dissection and enzymatic dispersion of the epicardial coronaries for study in highly differentiated (1% horse serum) primary cell culture (6–8) and for gross and histological examinations of coronary arteries.
Coronary VMC culture
Cell culture studies were performed from cultures prepared from three separate preparations and tissue from intact untreated male RM. Additional cell cultures from at least three ovx RM were used for biochemical experiments, reported in the online supplement data (published as supplemental data on The Endocrine Society’s Journals Online web site at http//jcem.endojournals.org). Coronary VMC were cultured as described previously (7, 8, 21). After plating in 10% horse serum (for 72 h), primary cultures were switched to 5% horse serum for 48 h, then maintained in 1% horse serum medium to minimize exposure to growth factors (e.g. fibroblast growth factor, endothelial growth factor, hepatic growth factor, IGF, and vascular epithelial growth factor, which are abundant in fetal calf serum) that would otherwise be prominent in the usual fibroblast type medium, which was optimized for growth, rather than function (6–8). Under these conditions, VMC retained membrane contractile properties and receptor function as in vivo (6, 7). VMC were grown on ultraclean glass 9 × 22-mm coverslips and used for cellular immunocytochemistry studies. VMC, grown to 80% or greater coverage in 100 × 20-mm Falcon tissue culture dishes and treated appropriately for 72 h with test steroids (P, DHT, and MPA), or untreated parallel time-matched control VMC were harvested for preparation of total cell lysates for Western blotting.
Immunocytochemistry
ICC was performed by indirect immunofluorescence, adapting the method of Yu et al. (22) as described previously (2). Immunolabeling for P receptor A (PR-A) was performed using 1 μg/ml JZB39 Greene, a rat anti-PR monoclonal antibody (23). TP receptor labeling was performed using a custom-made polyclonal chicken antibody against the ligand-binding domain of the TP receptor (Aves Laboratories, Inc., Tigard, OR) (2) based on the reported amino acid sequence for this domain (CFL TLG AES GD) (24). Secondary labeling of TP receptor was performed with a secondary, rhodamine-tagged, antichicken IgY. AR labeling was performed with a rabbit anti-AR antibody (PG-21) provided by Dr. Gail Prins (University of Chicago, Chicago, IL). The specificity of antibodies was tested by omission of the primary antibodies and, where available, preincubation of samples with specific blocking peptide. Appropriate species-specific secondary antibodies conjugated with fluorescein or rhodamine were used for secondary labeling of AR and PR. A minimum of three coverslips from each treatment group were examined. Images were recorded using a Axiovert 200M (Zeiss, Oberkochen, Germany) and VIM or EB-CCD camera (Hamamatsu Photonics, Hamamatsu City, Japan) using Image Proplus (Mediacybernetics, Silver Spring, MD) and Compix software (C-imaging systems, Cranberry Township, PA) and digitally stored for qualitative analysis of differences in TP receptor expression.
Western blotting
For biochemistry experiments, coronary vascular muscle cells plated in 10% horse serum for the first 72 h were then grown in 5% horse serum until reaching 80% area coverage, which usually takes about 1 wk, and were then switched to 1% horse serum for 48 h before in vitro treatments with test agents. Primary cultures of VMC were treated with P (1 nm), MPA (10 nm), or DHT (10 nm) for 72 h. Parallel, time-matched, untreated VMC from the same preparation served as controls. The duration of in vitro VMC treatment (72 h) used in this study was based on previous work from our laboratory, which demonstrated that this duration of treatment is sufficient for in vitro modulation of both expression of the TP receptors and Ca2+ signals that characterize CH (2, 8, 9). Cells were harvested for preparing whole cell lysates, and Western blotting was performed as described previously (2). Immunodetection of proteins was performed using appropriate secondary antibodies conjugated to horseradish peroxidase and detected with enhanced chemiluminescence. Membranes were probed with total ERK1/2 to confirm equal loading of proteins. After enhanced chemiluminescence, the developed blots were scanned using a Hewlett-Packard scanner (Palo Alto, CA), and images were stored digitally. Densitometric quantitation was performed using NIH-Scion Image analysis software (National Institutes of Health, Bethesda, MD).
Drugs, antibodies, and reagents
Secondary antibodies were purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA). Steroids used were from Steraloids (Newport, RI). All buffers and solutions used in the coronary catheterization studies followed the protocol reported previously (4, 7–9). Unless otherwise specified, all chemicals and reagents were obtained from Sigma-Aldrich Corp. (St. Louis, MO).
Statistical analysis
Data are expressed as the mean ± sem. Results were compared by independent Student’s t test and ANOVA, using statistical and plotting elements of Origin software (OriginLab, Northampton, MA). P < 0.05 was chosen as the level of statistical significance.
Results
MPA and DHT treatment increased susceptibility to provoked coronary vasoconstriction
There were no differences in responses to ACh vasodilation among the three groups. Representative angiograms from each of the treatment groups after the second S plus U challenge are shown in Fig. 1A, and average minimal diameter ± se for each treatment group are illustrated in the bar graph in Fig. 1B (asterisks indicate a significant difference compared with the control). The bar graphs represent the average minimum diameter for each animal in response to any of the three separate S plus U challenges for each monkey. Untreated eugonadal male (DHT, 5.9 nM) controls did not exhibit CH during any of the S plus U challenge steps. In the four untreated animals, minimal diameters (φ) of large epicardial coronary in response to S plus U stimulation occurred in the first 3 min after the provocation and averaged 85.5 ± 2.9% of the control diameter. In sharp contrast, MPA treatment for 2 wk (3.6 nM) decreased φ for more than 5 min to 24.5 ± 4.5% of the control diameter. In three monkeys treated with DHT for 2 wk (31 nM), intracoronary injection of S plus U resulted in focal constrictions of more than 5 min to φ to 38 ± 8% of the control diameter.
Fig. 1.
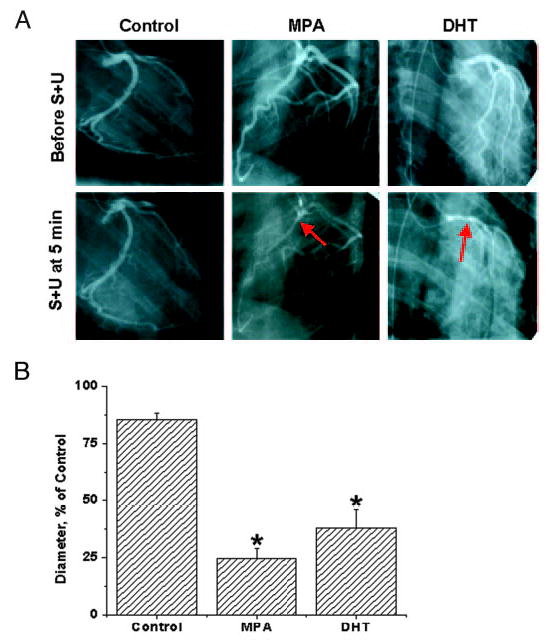
Effects of MPA and DHT on coronary hyperreactivity. A, Representative control and S- plus U-provoked angiograms are shown at the smallest diameters for an MPA- and DHT-treated gonadally intact male RM compared with an untreated control male RM. Each angiogram is from 5 min after the second S plus U injection. The arrows indicate points of vasospasm in MPA- and DHT-treated, gonadally intact, male RM. B, Angiographically measured φ as a percentage of control diameter (recorded before vasospasm challenge) in response to intracoronary injections over 30 sec of the provocative stimulus (S and U successive injections) are shown as the average minimal diameter ± se. The provoking constrictor stimulus of S plus U [100 μm S and 1 μm U (syringe concentration)] is estimated to be diluted 15 times by coronary blood flow, i.e. to 6.7 μm S and 67 nm U, which would exist at the blood vessel wall in the epicardial coronary arteries. Hyperreactivity, with or without vasospasm-like constrictions, was considered to be a reduction to 33% or less of the control diameter for more than 5 min. Intact control monkeys did not show significant hyperreactivity or vasospasm; rather, there was an average minimum φ of 85.5 ± 2.9% of the control value (baseline diameter taken as 100% before vasospasm challenge). In contrast, MPA-treated (3.6 nm at 14 d) RM showed prolonged (>5 min) vasoconstriction, with an average minimum φ of 24.5 ± 4.5%, meeting the hyperreactivity criteria of reduction to 33% or less of the control diameter for more than 5 min in four of four monkeys. DHT-treated (31 nm at 14 d) RM also showed severe persistent reduction of coronary artery diameters. DHT-treated animals (two of three) showed vasospasm (<33% for >5 min), and there was an average minimum φ for all three RM of 38 ± 8%. Data are expressed as the mean ± se. *, Significant differences from the intact control animals (P < 0.05).
AR and PR expressions in aorta, coronary artery, and coronary VMC
In addition to its effects on PR, MPA has androgenic actions (11, 25, 26). We therefore determined the expression of both AR and PR in cross-sections of aorta and coronary arteries as well as in primary cultures of coronary VMC from intact male RM. Double-labeling ICC showed coexpression of AR and PR in aorta and coronary arteries (Fig. 2, A and B). Single-labeling ICC studies in cultured VMC showed expression of AR and PR (Fig. 2C), with a predominantly perinuclear and nuclear localization. AR have been reported in rat VMC (27, 28), and AR, PR, and ER have been demonstrated in baboon aorta (29). In this study we demonstrated both AR and PR in aorta and coronary arteries (as well as primary coronary VMC) of male RM. Expression of these receptors was substantiated by Western blotting of cultured coronary VMC (Fig. 2D). PR was noted as an 84-kDa band representing the PR-A isoform. The PR-B isoform (120-kDa band) was very weakly expressed (data not shown). This is similar to our findings reported on the expression of PR in female RM coronary VMC (2). Thus, PR-A appears to be the dominant form expressed in the RM coronary VMC. AR expression was observed as a 120-kDa band.
Fig. 2.
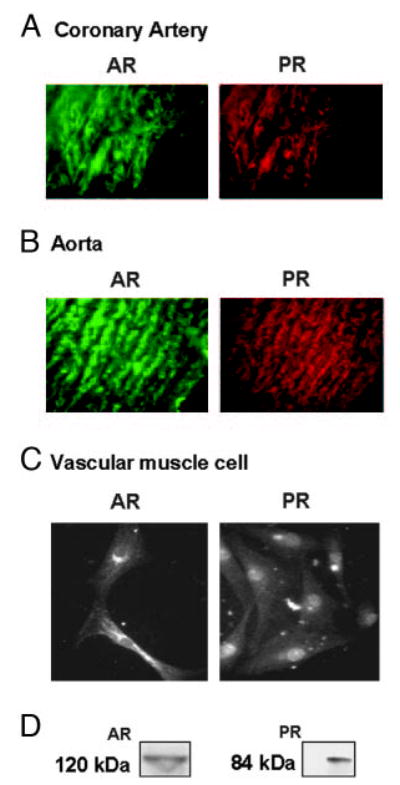
PR and AR expression in coronary arteries, aorta, and primary coronary VMC from untreated intact male RMs. A, Double-labeling ICC showing the expression of AR and PR in 5-μm cross-sections of coronary artery from intact male RM. B, Double-labeling ICC showing the expression of AR and PR in 5-μm cross-sections of aorta from intact male RM. C, Single-labeling ICC showing the expression of AR and PR in coronary VMC. The data in A and B are representative results from three cross-sections from untreated intact male RM, and the data in C are representative of results from untreated (control) male RM coronary VMC from three coverslips. D, Western blotting showed the expression of AR and PR in coronary VMC from male RM. PR was detected as a 84-kDa band using the rat monoclonal antibody (JZB39 Greene), whereas AR was detected as a 120-kDa band using the rabbit polyclonal antibody (PG-21). The data shown represent two independent experiments.
MPA and DHT increased expression of TP receptors in arteries and primary male RM coronary VMC
ICC studies performed on aortic cross-sections from intact male RM showed increased expression of TP receptors in RM treated with DHT or MPA compared with untreated control RM (Fig. 3). ICC studies using primary coronary VMC from intact male RM demonstrated that 72-h treatment with 10 nM MPA or 10 nM DHT increased TP receptor expression, and 1 nM P slightly decreased TP receptor expression (Fig. 4). Western blot analysis demonstrated an increase in the expression density of the 56-kDa TP receptor band in coronary VMC treated with MPA or DHT (10 nM) and showed that TP receptors were not significantly changed after treatment with P (Fig. 5).
Fig. 3.
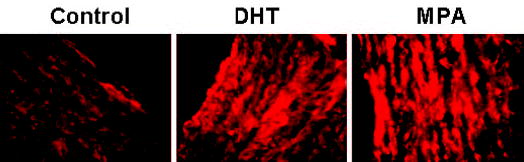
Effects of MPA and DHT on TP receptor expression in aorta. ICC showing expression of TP receptors in 5-μm cross-sections of aorta from untreated (control), MPA-treated, and DHT-treated intact male RM. TP receptor was labeled with a custom primary polyclonal chicken antibody, and secondary labeling was performed with a secondary, rhodamine-tagged, antichicken IgY. Imaging of immunolabeled coronary VMC was performed with a C-Apo ×40/1.2 water immersion on a Zeiss Axiovert 200M microscope with EB-CCD camera (Hamamatsu Photonics). The data shown are representative results from three cross-sections sampled in two monkeys in each of the three groups.
Fig. 4.
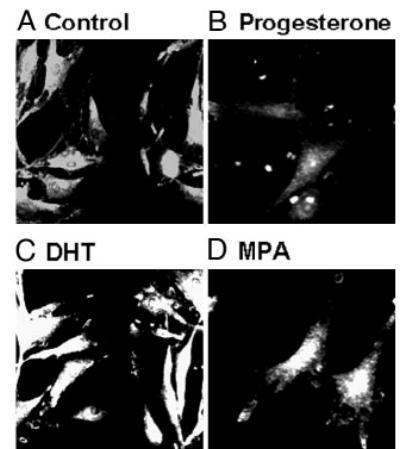
Effects of P, MPA, and DHT on TP receptor expression in primary coronary VMC. ICC by indirect immunofluorescence showing expression of TP receptors in untreated and treated coronary VMC: A, untreated control; B, P treatment (1 nm); C, DHT treatment (10 nm); D, MPA treatment (10 nm). Imaging of immunolabeled coronary VMC was performed with a C-Apo ×40/1.2 water immersion on a Zeiss Axiovert 200M microscope with an EB-CCD camera (Hamamatsu Photonics). The data shown are representative of results from three independent experiments.
Fig. 5.
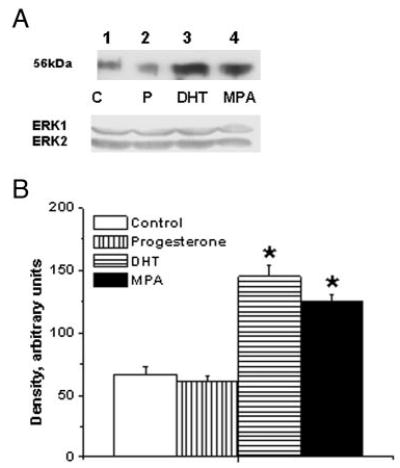
Effects of MPA and DHT on TP receptor expression in male rhesus coronary VMC. A, TP receptors in primary coronary VMC from gonadally intact, male RM treated for 72 h as follows: C, untreated control (lane 1); P, P treatment (1 nm; lane 2); DHT, DHT treatment (10 nm; lane 3); and MPA, MPA treatment (10 nm; lane 4). ERK1/2 detection was used as an internal loading control. B, Quantitation of TP receptor expression in RM coronary VMC was performed by densitometric analysis using NIH-Scion Image analysis software. MPA and DHT treatments increased the expression of TP receptor, whereas P did not significantly change the expression of TP receptor. Data are expressed as the mean ± sem and represent results from two independent experiments performed in duplicate. *, Significant increase compared with respective untreated control coronary VMC (P < 0.05).
MPA, DHT, and P regulation of TP receptor expression in female RM coronary VMC
We performed Western blot experiments for detailed investigation of MPA- and DHT-mediated regulation of TP receptor using VMC from ovx female RM for two reasons. First, there was a lack of availability of adequate male monkey coronary VMC cultures for detailed mechanistic studies. Second, based on our observations when investigating in vitro effects of P on TP receptor expression (Fig. 5 and discussed above), we reasoned that in euhormonal male coronary VMC, the influence of persistent effects of other endogenous steroids on TP receptor expression would probably complicate addressing the question of TP receptor regulation by MPA and DHT.
We first tested the effects of 6 and 10 nM DHT on in vitro TP receptor expression in coronary VMC. The 6-nM concentration was chosen to reflect the in vivo concentration of 5.9 nM in control monkeys. Although there was a slight increase in TP receptor expression with the lower concentration of 6 nM, this was not statistically significant (Fig. 6). The higher concentration of 10 nM DHT caused a significant increase in TP receptor expression (Fig. 6 and online supplemental Figs. S1 and S2). Dose-response experiments demonstrated that DHT at 10 and 30 nM caused a similar increase in TP receptor expression (online supplemental Fig. S2). Additionally, VMC treated with the combination of 6 nM DHT and 4 nM MPA or with 4 nM MPA alone (Fig. 6) showed a significant increase in TP receptor, whereas P treatment (Figs. 6 and 7, and supplemental Fig. S1) attenuated TP receptor expression.
Fig. 6.
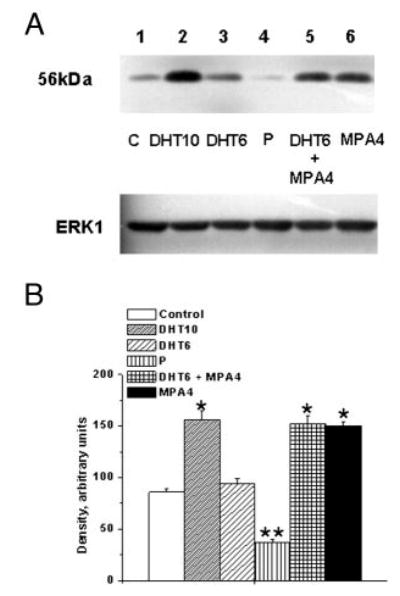
Effects of MPA and DHT on TP receptor expression in female rhesus coronary VMC. A, TP receptor expression in primary coronary VMC from ovx RM treated for 72 h as follows: C, untreated control (lane 1); DHT10, DHT (10 nm) treatment (lane 2); DHT6, DHT (6 nm) treatment (lane 3); P, P (1 nm) treatment (lane 4); DHT6 ± MPA4, DHT (6 nm) and MPA (4 nm) treatment (lane 5); and MPA4, MPA (4 nm) treatment (lane 6). ERK1 detection was used as an internal loading control. B, Quantitation of TP receptor expression. Data are expressed as the mean ± sem and represent results from three independent experiments. *, P < 0.05; **, P < 0.05 (significant decrease compared with untreated control VMC).
Fig. 7.
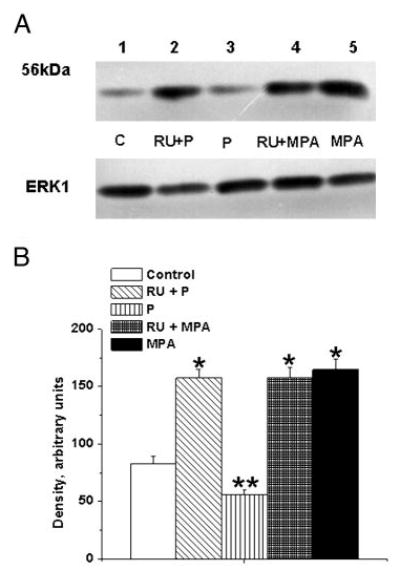
Effects of PR antagonist on the P-mediated decrease and MPA-mediated increase in TP receptor expression in female rhesus coronary VMC. A, TP receptors in primary coronary VMC from ovx RM treated for 72 h as follows: C, untreated control (lane 1); RU+P, RU486 (1 μm) plus P (1 nm) treatment (lane 2); P, P (1 nm) treatment (lane 3); RU+MPA, RU486 (1 μm) and MPA (10 nm) treatment (lane 4); and MPA, MPA (10 nm) treatment (lane 5). ERK1 detection was used as an internal loading control. B, Quantitation of TP receptor expression. Data are expressed as the mean ± sem and represent results from three independent experiments. *, Significant increase compared with control (P < 0.05); **, significant decrease vs. control VMC (P < 0.05).
The P-mediated decrease in TP receptor expression was significantly blocked by pretreatment with the PR antagonist RU486 (Fig. 7). However, RU486 did not block the MPA-mediated increased TP receptor expression (Fig. 7). Pretreatment with the AR antagonist flutamide blocked both DHT-and MPA-mediated increases in TP receptor expression (Fig. 8). RU486 had no effect on the DHT-mediated increase in TP receptor expression, and treatment of coronary VMC with RU486 or flutamide alone did not change TP receptor expression (data not shown).
Fig. 8.
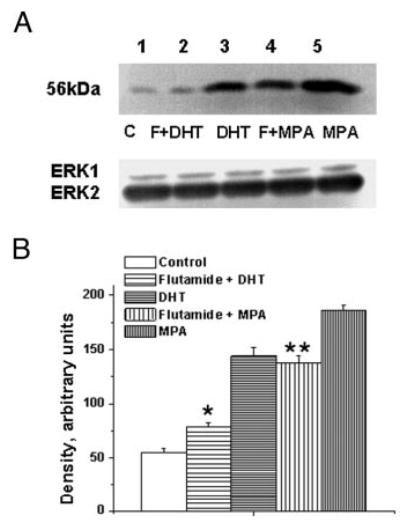
Effect of AR antagonist on the MPA- and DHT-mediated increase in TP receptor expression in female rhesus coronary VMC. A, TP receptors in primary coronary VMC from ovx RM treated for 72 h as follows: C, untreated control (lane 1); F + DHT, flutamide (1 μm) and DHT (10 nm) treatment (lane 2); DHT, DHT (10 nm) treatment (lane 3); F + MPA, flutamide (1 μm) and MPA (10 nm) treatment (lane 4); and MPA, MPA (10 nm) treatment (lane 5). ERK1/2 detection was used as an internal loading control. B, Quantitation of TP receptor expression. Data are expressed as the mean ± sem and represent results from three independent experiments. *, Significant decrease comparing flutamide plus DHT VMC vs. DHT-treated VMC; **, significant decrease comparing flutamide plus MPA-treated VMC vs. MPA-treated VMC (P < 0.05).
Discussion
Contrasting P vs. MPA actions have been evident since the first direct comparisons of coronary artery reactivity effects (4, 21, 30) and antiatherosclerotic effects (5, 12) in nonhuman primates. The principal distinguishing feature has been abnormal inability of hyperreactive coronary arteries to relax within 5 min after combined S and TP receptor stimulation, leading to coronary blood flow restriction severe and persistent enough to cause myocardial ischemia and infarction (1, 2, 4, 6, 8). Our previously reported data on adverse coronary vascular effects of MPA (as opposed to beneficial effects of P) in ovx RM correctly anticipated (4) adverse cardiovascular outcomes with MPA in human trials (9, 10). The first such substantiation of the prediction was in women with angiographically proven CAD experiencing stable exertional angina pectoris, in whom the combination of parenteral E plus P increased treadmill exercise time to reach myocardial ischemia in a direct comparison with E plus MPA (31). Therefore, based on consistent data from randomized, well controlled primate studies, the negative outcome of the HERS (32) and the WHI (9), both based on MPA as the progestagen, had been foreshadowed 5 yr previously (4, 5).
Data from the present study clearly demonstrate that adverse consequences of treatment with low dose MPA (4, 5) extend to male rhesus coronary arteries and thereby underscore the added Prempro risk attributable to the synthetic progestin, MPA. Previous results from surgically menopausal RM strongly suggest a potentially important beneficial role for P via repression of coronary artery TP receptors and emphasize the importance of the TP receptor mechanism, with or without atherosclerosis (2, 8). In the present study, in vitro treatment for more than 72 h of male rhesus coronary VMC with modest concentrations of either MPA or DHT increased TP receptor expression, whereas only 1 nM bioidentical P either reduced (determined by ICC) or did not significantly change (determined by Western blotting) TP receptor expression. This observation supports the hypothesis that a progestin with androgenic potential, such as MPA or the AR agonist DHT, increases TP receptor expression and consequently the downstream signaling that underlies hyperreactivity in intact male RM similar to that seen in surgically menopausal rhesus (4). Although P has been shown to attenuate TP receptor expression in coronary VMC from ovx female RM (2), and this was confirmed in our study, interestingly in the case of coronary VMC from intact male RM, in vitro P did not significantly reduce TP receptor expression. There could be several reasons for the lack of effect of P on TP receptor expression in VMC from male rhesus. We speculate that in intact male RM, the balance of endogenous steroids may result in a relatively lower endogenous TP receptor expression in euhormonal male coronary VMC, as noted. If so, the lower expression will be consistent with difficulty in detecting any attenuation of TP receptor after in vitro P treatment.
Although in vitro treatment with 6 nM DHT (equivalent to the in vivo trough DHT concentration in control male monkeys) in coronary VMC from female RM showed a trend toward increasing TP receptors, this concentration of DHT did not significantly increase TP receptor expression. The higher concentration of 10 nM DHT significantly increased TP receptor expression. The requirement for the higher in vitro concentration of DHT (≥10 nM) that produced significant increases in TP receptor expression probably parallels the higher in vivo concentrations of DHT occurring during daily peaks that would produce dominant DHT effects. During much of the 24-h day, DHT levels in control monkeys could be expected to exceed 6 nM, probably reaching 10 nM or more. Yet control male monkeys were not significantly susceptible to provoked CH, whereas DHT- and MPA-treated monkeys showed increased susceptibility to CH. The exact reason for the relatively protected state of control male monkeys against provoked CH despite possibly high levels of circulating DHT is currently unknown. We speculate that in vivo regulation of TP receptor is probably dependent on the net effect of several circulating endogenous steroids acting over at least 72 h in eugonadal male RM. It is plausible that up to a certain threshold level of DHT, opposing regulatory actions of other steroids could offset actions of higher concentrations of circulating DHT in the control male RM. The higher concentration of DHT (31 nM) provided exogenously via the subdermal implant was probably high enough to favor dominant DHT effects on inducing susceptibility to provoked CH in vivo.
The P-mediated decrease in TP receptor expression was significantly blocked by pretreatment with the PR antagonist RU486, whereas RU486 did not block the MPA- or DHT-mediated increase in TP receptor expression. This suggests that unblocked AR actions of MPA probably contribute to the MPA-induced increases in TP receptor expression. DHT- and MPA-mediated increased TP receptor expression was significantly attenuated by pretreatment with the AR antagonist flutamide. The attenuation of TP receptor expression in the presence of AR antagonism with flutamide was more dramatic in the case of DHT treatment. Together, these data support the hypothesis that there is a major role for AR-mediated regulation of TP receptor expression that could contribute to in vivo CH induced by MPA and DHT.
TP receptors have been reported to mediate both vaso-constrictor and proliferative effects (33). Vasoconstrictor prostanoids, such as TxA2, have been implicated in abnormal vascular responses in atherosclerosis (34) and hypertension (35). Increased TP receptor gene expression and augmented vasoconstriction were reported in diet-induced obese mice (36), implying that increased risk for development of vascular disease, hypertension, and thrombosis may have one or more common mechanisms. In human cardiovascular disease, both increased TP receptor density and a concomitant increase in plasma TxA2 levels have been noted and observed to be benefited by losartan treatment (37), underscoring the significance of TP receptors as a potential target for therapeutic intervention.
Although evidence supporting a potential role for the TP receptor in the pathophysiology of cardiovascular disease continues to grow, the mechanisms regulating expression and signaling via TP are not well understood. The pathophysiological significance of increased TP receptor expression and downstream signaling in vascular muscle has begun to be recognized. TP receptor density and the associated downstream phospholipase C pathway probably play an important role in CH. We previously demonstrated the inverse relationship of TP and PR in ovx RM coronary arteries and VMC (2, 6). This study provides the first demonstration of coexpression of TP, PR, and AR expression in male RM coronary arteries and VMC.
Androgenic factors may contribute to men being at a greater risk of cardiovascular disease compared with pre-menopausal women (38). Although gender risk differential to cardiovascular disease before the onset of menopause may be conferred by physiological levels of circulating ovarian steroid hormones in premenopausal women, results from both HERS and WHI trials demonstrate a lack of beneficial effects of Prempro (9, 10). The failure to detect a beneficial cardiovascular outcome has prompted reexamination of the literature regarding the effects of estrogens, progestins, and androgens on the cardiovascular system in both women (39) and men (40). These clinical data provide a strong rationale for accelerating research focused on the poorly understood genomic contributions of androgens and progestins to the increased risk of cardiovascular disease.
The predominant male hormone T is suggested to have beneficial cardiovascular effects (41). Although we showed that MPA and the T metabolite DHT increased the susceptibility of male RM to CH in this study, we did not test the direct effects of T on the occurrence of CH in intact male RM. T increased flow-mediated vasodilation in men with CAD (42). Short-term intracoronary administration of physiological levels of T dilated human coronaries (43), and iv T attenuated exercise-induced myocardial ischemia in men with CAD (44). Transdermal T treatment for 3 months improved the angina threshold in men with chronic stable angina (45). T may be converted to estradiol in the blood vessel wall, and the balance of formation of the two direct T metabolites, DHT and estradiol, must be explored. Although T per se appears beneficial, the principal active metabolite of T, DHT, appears to be detrimental to the cardiovascular system (46), hinting at the importance of this balance. Acute myocardial infarction may be the consequence of androgenic actions of MPA and has been reported in a woman within 4 h of administration of 10 mg MPA and 0.625 mg CEE (47). The net effect of androgens on the cardiovascular system may be a function of the balance of localized T vs. DHT actions and is probably influenced by the relative local levels of other modulating steroids, especially E and P.
Gender differences attributable to the effects of androgens have been observed in male spontaneously hypertensive rats. In rats, castration significantly attenuated hypertension (48). The male rat aorta was more sensitive to the TxA2 analog than was the female rat aorta; such a gender difference is consistent with higher expression of AR in male rat aortic smooth muscle cells than in those from females (28). Like-wise, multiday systemic T increased TxA2-induced coronary vasoconstriction in male guinea pigs (49). Increased androgen levels (both T and DHT) have been shown to be associated with increased TP receptor density in human erythroleukemia cells (50). An androgen-mediated increase in TP receptor density in smooth muscle and platelets has been hypothesized to contribute to an increased risk of cardiovascular disease (51).
Long-term use of MPA as a contraceptive in young women is associated with impairment of arterial endothelial function, as demonstrated by cardiovascular magnetic resonance imaging (52). Additional complexity is apparent with increasing recognition that both genomic and nongenomic effects, with possibly receptor-dependent and independent actions, are determined by local levels formed in key organs (e.g. brain, heart, and blood vessel). The data reported in this study thus add to the rationale for studies addressing the effects of steroid hormones that appear important for therapeutic advances (53). Rational preventive and therapeutic endocrine strategies should be investigated to allow normalization of the unbalanced (overly androgenic) endocrine milieu during aging. Moreover, recognizing the differences among progestins, especially synthetic vs. bioidentical (54, 55), will greatly enhance these preventive and therapeutic strategies.
Acknowledgments
We kindly thank Drs. Josef Rösch, Art Hall, Stephen Kelly, and Richard Minshall for contributions to this project, and Dr. Lori Thrun for discussions of the data and critiquing the manuscript.
Footnotes
This work was supported by National Institutes of Health Grants HL-51723, HL-73690, AG-16159, HD-18185, and RR00163 (to Oregon National Primate Research Center) .
References
- 1.Hermsmeyer RK, Miyagawa K, Kelley ST, Rösch J, Hall AS, Axthelm MK, Greenberg B. Reactivity based coronary vasospasm independent of atherosclerosis in rhesus monkeys. J Am Col Cardiol. 1997;29:671–680. doi: 10.1016/s0735-1097(96)00524-4. [DOI] [PubMed] [Google Scholar]
- 2.Hermsmeyer RK, Mishra RG, Pavcnik D, Uchida B, Axthelm MK, Stanczyk FZ, Burry KA, Illingworth R, Kaski JC, Nordt FJ. Prevention of coronary hyperreactivity in pre-atherogenic menopausal rhesus monkeys by transdermal progesterone. Arterioscler Thromb Vasc Biol. 2004;24:955–961. doi: 10.1161/01.ATV.0000126372.14332.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarkson TB, Appt SE. MPA and postmenopausal artery atherosclerosis revisited. Steroids. 2003;68:941–951. doi: 10.1016/s0039-128x(03)00123-5. [DOI] [PubMed] [Google Scholar]
- 4.Miyagawa K, Rösch J, Stanczyk F, Hermsmeyer RK. Medroxyprogesterone interferes with ovarian steroid protection against coronary vasospasm. Nat Med. 1997a;3:324–327. doi: 10.1038/nm0397-324. [DOI] [PubMed] [Google Scholar]
- 5.Adams MR, Register TC, Golden DL, Wagner JD, Williams JK. Medroxyprogesterone acetate antagonizes inhibitory effects of conjugated equine estrogens on coronary artery atherosclerosis. Arterioscler Thromb Vasc Biol. 1997;17:217–221. doi: 10.1161/01.atv.17.1.217. [DOI] [PubMed] [Google Scholar]
- 6.Minshall RD, Stanczyk FZ, Miyagawa K, Uchida B, Axthelm MK, Novy M, Hermsmeyer RK. Ovarian steroid protection against coronary artery hyperreactivity in rhesus monkeys. J Clin Endocrinol Metab. 1998;83:649–659. doi: 10.1210/jcem.83.2.4576. [DOI] [PubMed] [Google Scholar]
- 7.Minshall RD, Miyagawa K, Chadwick CC, Novy MJ, Hermsmeyer RK. In vitro modulation of primate coronary vascular muscle cell reactivity by ovarian steroid hormones. FASEB J. 1998;12:1419–1429. doi: 10.1096/fasebj.12.13.1419. [DOI] [PubMed] [Google Scholar]
- 8.Minshall RD, Pavcnik D, Halushka PV, Hermsmeyer RK. Progesterone regulation of vascular thromboxane A2 receptors in rhesus monkeys. Am J Physiol. 2001;281:H1498–H1507. doi: 10.1152/ajpheart.2001.281.4.H1498. [DOI] [PubMed] [Google Scholar]
- 9.Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, Hsia J, Hulley S, Herd A, Khan S, Newby LK, Waters D, Vittinghoff E, Wenger N. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/Progestin Replacement Study follow-up (HER-SII) JAMA. 2002;288:49–57. doi: 10.1001/jama.288.1.49. [DOI] [PubMed] [Google Scholar]
- 10.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 11.Wakatsuki A, Okatani Y, Ikenoue N, Fukaya T. Effect of medroxyprogesterone acetate on vascular inflammatory markers in postmenopausal women receiving estrogen. Circulation. 2002;105:1436–1469. doi: 10.1161/hc1202.105945. [DOI] [PubMed] [Google Scholar]
- 12.Adams MR, Kaplan JR, Manuck SB, Koritnik DR, Parks JS, Wolfe MS, Clarkson TB. Inhibition of coronary artery atherosclerosis by 17-β estradiol in ovariectomized monkeys. Lack of an effect of added progesterone. Arteriosclerosis. 1990;10:1051–1057. doi: 10.1161/01.atv.10.6.1051. [DOI] [PubMed] [Google Scholar]
- 13.Zumpe D, Bonsall RW, Kutner MH, Michael RP. Medroxyprogesterone acetate, aggression, and sexual behavior in male cynomolgus monkeys (Macaca fascicularis) Horm Behav. 1991;25:394–409. doi: 10.1016/0018-506x(91)90010-f. [DOI] [PubMed] [Google Scholar]
- 14.Cooper AJ. Progestogens in the treatment of male sex offenders: a review. Can J Psychiatry. 1986;31:73–79. doi: 10.1177/070674378603100116. [DOI] [PubMed] [Google Scholar]
- 15.Kiersch TA. Treatment of sex offenders with Depo-Provera. Bull Am Acad Psychiatry Law. 1990;18:179–187. [PubMed] [Google Scholar]
- 16.Turner L, Conway AW, Jimenez M, Liu PY, Forbes E, McLachlan RI, Handelsman DJ. Contraceptive efficacy of a depot progestin and androgen combination in men. J Clin Endocrinol Metab. 2003;88:4659–4667. doi: 10.1210/jc.2003-030107. [DOI] [PubMed] [Google Scholar]
- 17.Perachio AA, Alexander M, Marr LD, Collins DC. Diurnal variations of serum testosterone levels in intact and gonadectomized male and female rhesus monkeys. Steroids. 1977;29:21–33. doi: 10.1016/0039-128x(77)90106-4. [DOI] [PubMed] [Google Scholar]
- 18.Resko JA, Jackson GL, Huckins C, Stadelman H, Spies HG. Cryptorchid rhesus macaques: long term studies on changes in gonadotropins and gonadal steroids. Endocrinology. 1980;107:1127–1136. doi: 10.1210/endo-107-4-1127. [DOI] [PubMed] [Google Scholar]
- 19.Hess DL, Spies HG, Hendrickx AG. Diurnal steroid patterns during gestation in the rhesus macaque: onset, daily variation, and the effects of dexamethasone treatment. Biol Reprod. 1981;24:609–616. doi: 10.1095/biolreprod24.3.609. [DOI] [PubMed] [Google Scholar]
- 20.Hiroi M, Stanczyk FZ, Goebelsmann U, Brenner PF, Lumkin ME, Mishell DR., Jr Radioimmunoassay of serum medroxyprogesterone acetate (Provera) in women following oral and intravaginal administration. Steroids. 1975;26:373–386. doi: 10.1016/0039-128x(75)90082-3. [DOI] [PubMed] [Google Scholar]
- 21.Miyagawa K, Vidgoff J, Hermsmeyer RK. Ca2+ release mechanism of primate reactivity based coronary vasospasm. Am J Physiol. 1997b;272:H2645–H2654. doi: 10.1152/ajpheart.1997.272.6.H2645. [DOI] [PubMed] [Google Scholar]
- 22.Yu JG, O’Brien WE, Lee TJ. Morphologic evidence for l-citrulline conversion to l-arginine via the argininosuccinate pathway in porcine cerebral perivascular nerves. J Cereb Blood Flow Metab. 1997;17:884–893. doi: 10.1097/00004647-199708000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Press MF, Greene GL. Localization of progesterone receptor with monoclonal antibodies to the human progestin receptor. Endocrinology. 1988;122:1165–1175. doi: 10.1210/endo-122-3-1165. [DOI] [PubMed] [Google Scholar]
- 24.Turek JW, Halmos T, Sullivan NL, Antonakis K, Le Breton GC. Mapping of a ligand-binding site for the human thromboxane A2 receptor protein. J Biol Chem. 2002;277:16791–16797. doi: 10.1074/jbc.M105872200. [DOI] [PubMed] [Google Scholar]
- 25.Bentel JM, Birrell SN, Pickering MA, Holds DJ, Horsfall DJ, Tilley WD. Androgen receptor agonist activity of the synthetic progestin, medroxyprogesterone acetate, in human breast cancer cells. Mol Cell Endocrinol. 1999;154:11–20. doi: 10.1016/s0303-7207(99)00109-4. [DOI] [PubMed] [Google Scholar]
- 26.Sitruk-Ware R. Progestins and cardiovascular risk markers. Steroids. 2000;65:651–658. doi: 10.1016/s0039-128x(00)00174-4. [DOI] [PubMed] [Google Scholar]
- 27.Fujimoto R, Morimoto I, Morita E, Sugimoto H, Ito Y, Eto S. Androgen receptors, 5α-reductase activity and androgen-dependent proliferation of vascular smooth muscle cells. J Steroid Biochem Mol Biol. 1994;50:169–174. doi: 10.1016/0960-0760(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 28.Higashiura K, Mathur RS, Halushka PV. Gender-related differences in androgen regulation of thromboxane A2 receptors in rat aortic smooth-muscle cells. J Cardiovasc Pharmacol. 1997;29:311–315. doi: 10.1097/00005344-199703000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Lin AL, Gonzalez R, Jr, Carey KD, Shain SA. Gender and baboon aortic steroid hormone receptors. Arteriosclerosis. 1987;7:248–255. doi: 10.1161/01.atv.7.3.248. [DOI] [PubMed] [Google Scholar]
- 30.Williams JK, Honore EK, Washburn SA, Clarkson TB. Effects of hormone replacement therapy on reactivity of atherosclerotic coronary arteries in cynomolgus monkeys. J Am Coll Cardiol. 1994;24:1757–1761. doi: 10.1016/0735-1097(94)90184-8. [DOI] [PubMed] [Google Scholar]
- 31.Rosano GM, Webb CM, Chierchia S, Morgani GL, Gabraele M, Sarrel PM, de Ziegler D, Collins P. Natural progesterone, but not medroxyprogesterone acetate, enhances the beneficial effect of estrogen on exercise-induced myocardial ischemia in postmenopausal women. J Am Coll Cardiol. 2000;36:2154–2159. doi: 10.1016/s0735-1097(00)01007-x. [DOI] [PubMed] [Google Scholar]
- 32.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) research group. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 33.Uruno A, Sugawara A, Kudo M, Sato M, Sato K, Ito S, Takeuchi K. Transcription suppression of thromboxane receptor gene expression by retinoids in vascular smooth muscle cells. Hypertens Res. 2003;26:815–821. doi: 10.1291/hypres.26.815. [DOI] [PubMed] [Google Scholar]
- 34.Mehta JL, Lawson D, Mehta P, Saldeen T. Increased prostacyclin and thromboxane A2 biosynthesis in atherosclerosis. Proc Natl Acad Sci USA. 1988;85:4511–4515. doi: 10.1073/pnas.85.12.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao WG, Richardson JS. Prostacyclin, thromboxane A2, and hypertension. Clin Invest Med. 1990;13:343–352. [PubMed] [Google Scholar]
- 36.Traupe T, Lang M, Goettsch W, Munter K, Morawietz H, Vetter W, Barton M. Obesity increases prostanoid-mediated vasoconstriction and vascular thromboxane receptor gene expression. J Hypertens. 2002;20:2239–2245. doi: 10.1097/00004872-200211000-00024. [DOI] [PubMed] [Google Scholar]
- 37.Katugampola SD, Davenport AP. Thromboxane receptor density is increased in human cardiovascular disease with evidence for inhibition at therapeutic concentrations by the AT1 receptor antagonist losartan. Br J Pharmacol. 2001;134:1385–1392. doi: 10.1038/sj.bjp.0704416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mercuro G, Zoncu S, Dragoni F. Gender differences in cardiovascular risk factors. Ital Heart J. 2003;4:363–366. [PubMed] [Google Scholar]
- 39.Barrett-Connor E. Clinical review 162: cardiovascular endocrinology 3: an epidemiologist looks at hormones and heart disease in women. J Clin Endocrinol Metab. 2003;88:4031–4042. doi: 10.1210/jc.2003-030876. [DOI] [PubMed] [Google Scholar]
- 40.Muller M, van der Schouw YT, Thijssen JH, Grobbee DE. Endogenous sex hormones and cardiovascular disease in men. J Clin Endocrinol Metab. 2003;88:5076–5086. doi: 10.1210/jc.2003-030611. [DOI] [PubMed] [Google Scholar]
- 41.Channer KS, Jones TH. Cardiovascular effects of testosterone: implications of the “male menopause? Heart. 2003;89:121–122. doi: 10.1136/heart.89.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ong PJ, Patrizi G, Chong WC, Webb CM, Hayward CS, Collins P. Testosterone enhances flow-mediated brachial artery reactivity in men with coronary artery disease. Am J Cardiol. 2000;85:269–272. doi: 10.1016/s0002-9149(99)00630-x. [DOI] [PubMed] [Google Scholar]
- 43.Webb CM, McNeill JG, Hayward CS, de Zeigler D, Collins P. Effects of testosterone on coronary vasomotor regulation in men with coronary heart disease. Circulation. 1999;100:1690–1696. doi: 10.1161/01.cir.100.16.1690. [DOI] [PubMed] [Google Scholar]
- 44.Rosano GM, Leonardo F, Pagnotta P, Pelliccia F, Panina G, Cerquetani E, della Monica PL, Bonfigli B, Volpe M, Chierchia SL. Acute anti-ischemic effect of testosterone in men with coronary artery disease. Circulation. 1999;99:1666–1670. doi: 10.1161/01.cir.99.13.1666. [DOI] [PubMed] [Google Scholar]
- 45.English KM, Steeds RP, Jones TH, Diver MJ, Channer KS. Low-dose transdermal testosterone therapy improves angina threshold in men with chronic stable angina: a randomized, double-blind, placebo-controlled study. Circulation. 2000;102:1906–1911. doi: 10.1161/01.cir.102.16.1906. [DOI] [PubMed] [Google Scholar]
- 46.Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocr Rev. 2003;24:313–340. doi: 10.1210/er.2003-0005. [DOI] [PubMed] [Google Scholar]
- 47.Sarrel PM 1997 Clinical findings related to arterial effects of ovarian steroids. In: Forte TM, ed. Hormonal metabolic and cellular influences on cardiovascular disease in women. American Heart Association Monograph Series. Malden, MA: Blackwell Futura; 325–338
- 48.Reckelhoff JF, Zhang H, Srivastava K, Granger JP. Gender differences in hypertension in spontaneously hypertensive rats: role of androgens and androgen receptor. Hypertension. 1999;34:920–923. doi: 10.1161/01.hyp.34.4.920. [DOI] [PubMed] [Google Scholar]
- 49.Schror K, Morinelli TA, Masuda A, Matsuda K, Mathur RS, Halushka PV. Testosterone treatment enhances thromboxane A2 mimetic induced coronary artery vasoconstriction in guinea pigs. Eur J Clin Invest. 1994;24(Suppl 1):50–52. doi: 10.1111/j.1365-2362.1994.tb02428.x. [DOI] [PubMed] [Google Scholar]
- 50.Matsuda K, Mathur RS, Duzic E, Halushka PV. Androgen regulation of thromboxane A2/prostaglandin H2 receptor expression in human erythroleukemia cells. Am J Physiol. 1993;265:E928–E934. doi: 10.1152/ajpendo.1993.265.6.E928. [DOI] [PubMed] [Google Scholar]
- 51.Halushka PV, Matsuda K, Masuda A, Ruff A, Morinelli TA, Mathur RS. Testosterone regulation of platelet and vascular thromboxane A2 receptors. Agents Actions. 1995;45(Suppl):19–26. doi: 10.1007/978-3-0348-7346-8_3. [DOI] [PubMed] [Google Scholar]
- 52.Sorensen MB, Collins P, Ong PJ, Webb CM, Hayward CS, Asbury EA, Gatehouse PD, Elkington AG, Yang GZ, Kubba A, Pennell DJ. Long-term use of contraceptive depot medroxyprogesterone acetate in young women impairs arterial endothelial function assessed by cardiovascular magnetic resonance. Circulation. 2002;106:1646–1651. doi: 10.1161/01.cir.0000030940.73167.4e. [DOI] [PubMed] [Google Scholar]
- 53.Minshall RD, Pavcnik D, Browne DL, Hermsmeyer RK. Nongenomic vasodilator action of progesterone on primate coronary arteries. J Appl Physiol. 2002;92:701–708. doi: 10.1152/japplphysiol.00689.2001. [DOI] [PubMed] [Google Scholar]
- 54.Stanczyk FZ. All progestins are not created equal. Steroids. 2003;68:879–890. doi: 10.1016/j.steroids.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 55.Wiegratz I, Kuhl H. Progestogen therapies: differences in clinical effects? Trends Endocrinol Metab. 2004;15:277–285. doi: 10.1016/j.tem.2004.06.006. [DOI] [PubMed] [Google Scholar]


