Abstract
Objective
To test if transdermal progesterone (P) confers coronary vascular protection in surgically menopausal preatherosclerotic rhesus monkeys.
Methods and Results
Ovariectomized rhesus monkeys fed an atherogenic diet (AD) for 19 months were treated with an investigational transdermal P cream (n=7) or identical placebo cream (n=5) for 4 weeks. Aorta and carotids showed fatty streaks and Oil Red O staining demonstrated lipid deposition. Serum P levels in P-treated rhesus monkeys (0.6 ng/mL) were significantly greater than placebo (0.2 ng/mL). Significant elevation of cholesterol, LDL cholesterol, and HDL cholesterol, was noted in all animals. Lp(a) was significantly attenuated in the AD-fed P-treated monkeys. Coronary angiographic experiments stimulating vasoconstriction by intracoronary injections of serotonin plus U46619 showed exaggerated prolonged actions amplified by AD, but significant protection against severe prolonged vasoconstriction in P-treated monkeys. Immunocytochemistry confirmed co-expression of P and thromboxane prostanoid (TP) receptors in coronaries and aorta. Western blotting demonstrated TP receptor attenuation in vascular muscle after P treatment.
Conclusions
Coronary hyperreactivity, a putative component of coronary artery disease mediated via increased vascular muscle thromboxane prostanoid receptors, can be prevented by subphysiological levels of P, not only in nonatherosclerotic (previously shown) but also in preatherosclerotic primates.
Keywords: coronary occlusion, menopause, progesterone replacement, cardiac catheterization
The production of ovarian steroid hormones (OSH) such as estrogen (E) and progesterone (P) is markedly reduced in postmenopausal women (PMW), with a concomitant increased risk for the development of coronary artery disease (CAD).1 Based on several observational clinical studies, hormone therapy (HT) was suggested to be important in cardiovascular protection in PMW. However, recently reported results from the Women’s Health Initiative (WHI) trial underscore those from the Heart and Estrogen/Progestin Replacement Study (HERS II)2 and indicate that postmenopausal HT using a combination of conjugated equine estrogens (CEE) and medroxyprogesterone acetate (MPA) should not be used for prevention of coronary heart disease (CHD) in women already having CHD.3 Furthermore, the WHI study, evaluating the most commonly used HT (CEE+MPA) for chronic prevention of diseases in healthy PMW, recommended that this regimen not be used for primary prevention of CHD.4 Despite substantial evidence from experimental studies and observational clinical studies indicating a beneficial cardiovascular effect, the failure of a cardioprotective effect of HT in randomized controlled trials was surprising. This outcome has prompted much debate and impetus for further research.
Most HT studies have tested E only or E combined with a synthetic progestin such as MPA, focusing primarily on favorable alteration in blood lipids and endothelial function as primary endpoints of cardiovascular protection. Animal data indicate that administration of MPA adversely affects coronary reactivity7 and might counteract the improvement produced by CEE in atherosclerotic ovariectomized (ovx) primates.5 Despite negative conclusions regarding HT, an important untested possibility of using parenteral administration of bioidentical hormones (chemically identical to endogenous hormones) for primary prevention of CHD is not precluded.
An overlooked component of CHD, beyond the established atherosclerosis model, is the phenomenon of coronary hyperreactivity observed by cardiac catheterization studies in surgically menopausal primates.6 Applying this model, we have previously demonstrated that MPA, in stark contrast to bioidentical P, increases the risk of coronary vasospasm.7 The coronary vascular protective effect of P in nonatherogenic ovx rhesus monkeys appears to be genomically mediated by P-dependent decreases in the thromboxane A2 receptor-mediated exaggeration of late calcium signals in the vascular muscle,8 and by a further direct nongenomic vasodilator action on coronary arteries.9 However, whether P can favorably influence coronary hyperreactivity in the presence of atherosclerotic disease in primates has not been tested. In the present study, we investigated whether low-dose bioidentical P in the form of a transdermal cream (without E replacement) confers coronary vascular protection in surgically menopausal primates fed atherogenic diet (AD), without the confounding influence of soy proteins present in conventional monkey diets.10
Methods
Methods are available in their entirety at http://atvb.ahajournals.org.
Animals
Animal experiments were approved by Institutional Animal Care and Use Committee and performed in compliance with established guidelines for the humane care and euthanization of animals used in research. At the start of the project, 6 adult female rhesus monkeys (Macaca mulatta) were assigned to each group (placebo and P), but because of a procedural error in cream application for 1 animal, there were 7 rhesus in the P cream-treated group and 5 in the placebo cream group. The error resulted in an unmistakable increase (>10-times baseline) in serum P level in this animal (initially assigned as placebo) after treatment. Such high P levels could result only if P instead of placebo cream had been applied. Based on unequivocal evidence of P measured 3 times in 2 different laboratories, this animal was therefore reclassified as a P-treated animal. Monkeys were ovx 84 days before the study began and assigned to an AD based on soy-free high-lipid content, patterned after those described by others10–13 for 19 months, with the last 4 weeks as a P or placebo skin cream treatment period (please see www.ahajournals.org). Average ages (mean ± SD) of rhesus in this study were 14.53 ± 5.02 years (placebo) and 15.3 ± 1.57 years (P). Average weights (mean ± SD) of animals before coronary catheterization studies were 8.14 ± 1.63 kg (placebo) and 8.93 ± 1.48 kg (P). Average weight gain (mean ± SD) after 19 months on AD was 1.33 ± 0.55 kg (placebo) and 2.63 ± 1.11 kg (P).
Statistical Analysis
Results were compared by independent t tests and ANOVA using Origin software (OriginLab Corp, Northampton, Mass). P<0.05 was chosen as the level of statistical significance. Reclassification of the P-treated rhesus that was moved from the placebo group did not change the outcome of statistical testing compared with deletion of this subject. Analysis of both blood and urine clearly showed that P treatment of the subject in question must have occurred as the result of a labeling error. Deletion of data for the reclassified monkey would not change the conclusions. The analysis presented, therefore, includes all 12 rhesuses.
Results
Progesterone Plasma Levels and Urinary Pregnanediol Levels
At the end of cream treatment, P-treated rhesus had serum P levels of 614 ± 126 pg/mL, which is a significant 10-times increase over pretreatment values (60 ± 17 pg/mL). In contrast, placebo-treated rhesus averaged P levels of 248 ± 126 pg/mL after treatment compared with 168 ± 64 pg/mL before treatment, which is an insignificant change (Figure 1A). As a separate measure, pregnanediol, the predominant P metabolite in the urine, was determined. Urinary pregnanediol levels were significantly increased in P-treated compared with placebo-treated monkeys (Figure 1B). Together, these data confirmed absorption and excretion of transdermal P that attained the intended small, but significant, increase to the subphysiological range (≈2 nM).
Figure 1.
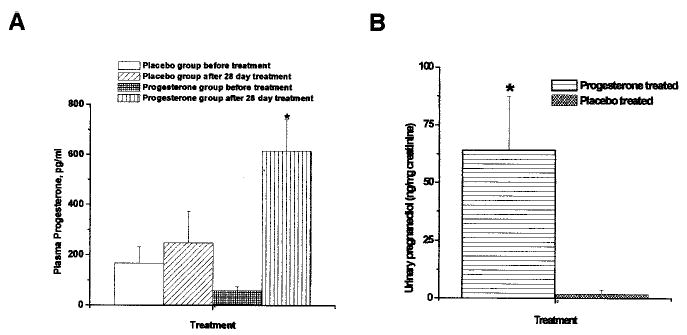
A, Serum P levels before treatment with placebo and P cream were 168 ± 64 pg/mL in placebo-treated monkeys (n=5) and 60 ± 17 pg/mL in P cream-treated monkeys (n=7), respectively. After 4 weeks of P cream treatment, rhesus (n=7) showed a significant 10× increase in P levels to 614 ± 126 pg/mL, whereas the P levels in placebo-treated rhesus (n=5) did not change significantly (248 ± 126 pg/mL). *Significance compared with baseline P. There was a significant difference between placebo and P groups after treatment at P<0.05. B, Urinary pregnanediol levels in P-treated and placebo cream-treated monkeys. Urine obtained after 28-day application of P or placebo cream was analyzed for pregnanediol and creatinine levels. The creatinine levels were comparable in both groups (P-treated=6.49 ± 1.13 mg/dL and placebo-treated=6.52 ± 2.82 mg/dL). The pregnanediol levels were 64.21 ± 23.31 ng/mg creatinine in the P-treated group and 1.82 ± 1.8 ng/mg creatinine in the placebo group. There was a significant difference between placebo and P groups after treatment at P<0.05.
Effects of AD on Serum Lipids and Lp(a)
Feeding the AD for 19 months resulted in significant increases in total cholesterol, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol, in both P-treated and placebo-treated rhesus. Figure 2A shows averages (mean ± SEM) measured before and at the end of cream treatment after feeding the AD in the 12 rhesus. Triglycerides (TG) also tended to increase after AD, although this increase was not statistically significant. All post-AD lipid measurements were performed at the end of treatment with placebo or P. There were no significant differences between the placebo- and P-treated groups (not shown). Although Lp(a) levels were not significantly changed in placebo-treated rhesus (before AD=27 ± 8 mg/dL versus after AD=30 ± 14 mg/dL), P treatment significantly reduced Lp(a) levels (14 ± 3 mg/dL after AD versus 31 ± 7 mg/dL before AD) (Figure 2B).
Figure 2.
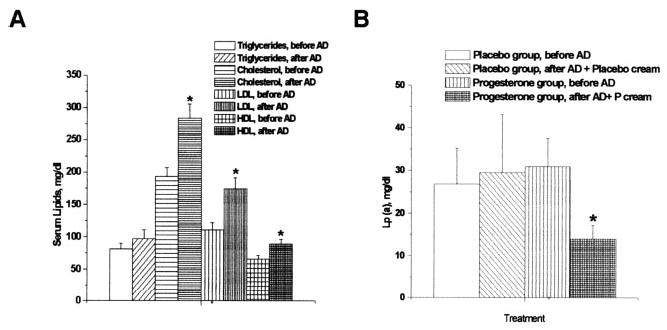
A, Serum lipid concentrations before and after AD. Serum cholesterol, LDL cholesterol, and HDL cholesterol increased significantly after AD. The marginal TG increase after AD was not statistically significant. Data represent mean ± SEM of samples from 12 monkeys (5 placebo-treated and 7 P-treated). *Significance at P<0.05 when comparing levels after AD to before AD. B, Lp(a) levels before and after AD. Before AD, there were no differences in the Lp(a) levels between the placebo- and P-treated groups. P-treated monkeys showed significant attenuation of Lp(a) levels after AD at the end of cream treatment (at 19 months) compared with baseline levels before AD. There was no significant difference in the levels in the placebo-treated group. Data represent mean ± SEM of samples from 5 placebo- and 7 P-treated rhesus monkeys. *Significance at P<0.05 when comparing Lp(a) levels in P- and placebo-treated monkeys after AD and cream treatment compared with before AD.
Pathology
Both gross and histopathology showed preatherogenic fatty streaks typical of early stage atherosclerosis in all 12 monkeys. Fatty infiltration of the coronaries and fatty streaks in the aorta were noted to the same extent in P- and placebo-treated groups. Although fatty streaks were noted during dissection of coronaries at necropsy, there was insufficient coronary tissue for histopathology studies because all available coronaries were used for immunocytochemistry (ICC) and cell culture. Therefore, only aorta and carotids were processed for Oil Red O staining. Fatty deposits in thoracic aorta and carotid arteries were similar. Gross pathology indicated an unequivocal preatherogenic state for all 12 subjects. Figure IA (available online at http://atvb.ahajournals.org) shows an example of the histopathological appearance of cross-sections of aorta with Oil Red O stained lipid deposition indicative of the preatherogenic state in both P-treated and placebo-treated rhesus. Quantitative analysis of staining for lipids with Oil Red O (Figure IB) showed no distinguishable difference between aorta from P-treated and placebo-treated rhesus (please see www.ahajournals.org).
Progesterone in Vivo Coronary Vascular Protection
P treatment for 4 weeks protected against severe or prolonged vasoconstriction to serotonin (S) plus U46619 (U) challenge in AD rhesus. The resilience of P-only–treated monkeys was equivalent to previous studies by this group with subdermal implants of E2 that produced normal follicular levels of E2 either with or without P implants. Thus, transdermal P alone was sufficient to protect against coronary hyperreactivity. In contrast, placebo-treated rhesus had severe prolonged vasoconstrictions indicative of coronary hyperreactivity. Figure 3A illustrates the efficacy of P in protecting against S-plus-U–induced vasospasm. All P-treated rhesuses were similarly protected against S-plus-U–induced vasospasm.
Figure 3.
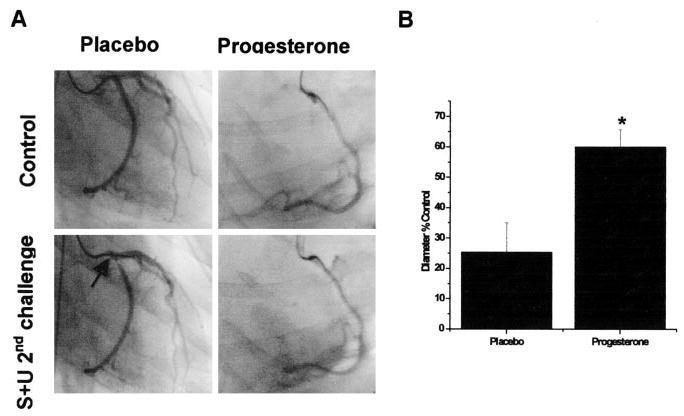
A, Representative control and S+U provoked angiograms are shown at smallest recorded diameters in a P-treated monkey compared with a placebo-treated ovx control. Each angiogram is from 3 minutes after the second S+U injection. The vasospasm that occurred in the placebo ovx is typical of the ovx group. There was prolonged (for >5 minutes) vasoconstriction in 5 of 5 placebo monkeys. In contrast, 7 of 7 DP9-treated monkeys were protected (no vasospasm or sustained vasoconstriction to <33% of control diameter occurred at any point in the protocol). B, Angio-graphically measured minimum diameters (φ) as a percent of control diameter (recorded before vasospasm challenge) in response to intracoronary injections over 30 seconds of the provocative stimulus (S+U successive injections) are shown as means ± SE. The provoking constrictor stimulus of S+U [100 μmol/L serotonin + 1 μmol/L U46619 (syringe concentration)] is estimated to be diluted 15 times by coronary blood flow, ie, to 6.7 μmol/L serotonin + 67 nM U46619, that would appear in the epicardial coronary arteries. Vasospasm-like constrictions were considered to be a reduction to <33% of control diameter for >5 minutes. The average minimum φ in placebo ovx (25.47% ± 9.58%) met the criterion of reduction to <33% of control diameter and are indicative of hyperreactivity and vasospasm-like constrictions that occurred in 5 of 5 ovx monkeys. In contrast, the average minimum φ in P-treated monkeys (60.08% ± 5.62%) are indicative of protection against hyperreactivity in all 7 P-treated monkeys. *Significant differences from placebo (P<0.05).
Dilator capacity of coronary arteries was tested with acetylcholine (ACh) at the beginning of the intracoronary drug injections series. Typically, dilation was 5% to 10% over control diameter in all 12 subjects and was not significantly different between P and placebo groups (data not shown). In the vasoconstrictor injection series (S+U protocol), none of the P-treated rhesus experienced constrictions <33% of control diameter or associated physiological indicators of severe ischemia (cardiogenic shock) at any point in the protocol. However, all placebo-treated rhesus exhibited vasospasm during the same vasoconstrictor series. Angiograms in Figure 3A contrast the lack of hyperreactivity (or vasospasm) in a P-treated compared with a placebo-treated monkey. Vasospasm in the rhesus without P treatment (Figure 3A, arrow) was noted as a severe vasoconstriction to 10% of control diameter persisting for >5 minutes (in fact, 15 minutes). Correlates of the vasospasm were a severe decrease in blood pressure to 40/16 mm Hg and left ventricular akinesia requiring closed chest massage. There was no significant difference in baseline blood pressures or heart rate between the 2 groups. Placebo-treated animals showed more sensitivity by more dramatic decreases in blood preasm provocation. For hemodynamic data before and during vasoconstrictor challenge, please see Table III, available online at http://atvb.ahajournals.org. In sharp contrast, P-treated subjects did not show a reduction in diameter to <50% of control diameter during the entire series of catheterization experiments and were less sensitive to blood pressure fluctuations (data not shown). Angiograms were examined as enlarged digital images to define the point of minimum diameter of a responding epicardial artery (left anterior descending or circumflex before the first branch point) for each monkey studied; the same point was spatially mapped and measured on the corresponding control image to provide average measured diameters as percent of control diameters. Diameters in the predrug control state averaged 0.92 ± 0.12 mm and 0.85 ± 0.09 mm for placebo-treated and progesterone-treated groups, respectively. The average minimum diameters ± standard error as a percent of baseline (control) diameter for each group during the vasospasm protocol are illustrated in Figure 3B. Vasospasm-like constrictions were considered to occur when diameters reached <33% of control diameter for >5 minutes. The average of minimum diameters (φ) in placebo ovx (25.47% ± 9.58%) shows this group met the criteria, indicative of vasospasm-like constrictions that occurred in 5 of 5 placebo-treated ovx monkeys. In contrast, average minimum φ in P-treated monkeys (60.08% ± 5.62%) showed notably less severe vasoconstrictions and consequently no long duration ischemic events. P treatment was protective against hyperreactivity (or vasospasm) in all 7 P-treated monkeys.
Effect of Progesterone Treatment on Thromboxane Prostanoid Receptor Expression in Aorta and Coronary Vascular Muscle Cells
Because P is hypothesized to regulate thromboxane prostanoid (TP) receptor expression via progesterone receptor (PR),8 we examined whether PR are expressed in rhesus artery walls (aorta and coronary) as well as in coronary vascular muscle cells in culture. To our knowledge, this is the first such demonstration of co-expression of TP and PR in rhesus coronaries and aorta. PR was detected in the aorta and coronary vascular muscle cells as an 84 kDa band (Figure 5A). This band closely corresponds to the reported molecular weight of PR-A (92 kDa), which is the dominant form expressed in both vascular muscle cells in culture and in the aorta. Double-labeling ICC studies showed co-expression of PR and TP in vascular muscle in cross-sections of both coronary arteries (Figure 4A) and aorta (Figure II, available online at http://atvb.ahajournals.org). TP receptor analysis demonstrated significant qualitative attenuation in expression in aorta from P-treated compared with placebo-treated subjects (Figure 4B). A dominant role for the TP receptor in mediating coronary hyperreactivity in ovx rhesus fed normal diet has emerged. Because ICC studies provide only a qualitative measure, Western blotting was performed to quantitate differences in TP receptor expression. Western blotting demonstrated the TP receptor as a 56 kDa band, which was significantly attenuated in aorta from P-treated compared with placebo-treated rhesus (Figure 5B and 5C). Because TP receptor expression in homogenates of arteries may not definitively derive from the vascular muscle, we studied in vitro treatment with low-dose P (1 nM) and its effect on TP receptor expression in primary coronary vascular muscle cell by quantitative Western blot analysis (Figure 5B and 5C). In vitro P treatment attenuated TP receptor expression in primary coronary vascular muscle cells (Figure 5B and 5C). Together, these data demonstrating attenuation of TP receptors confirm our previous findings of regulation of TP receptor expression by subdermal P implants in ovariectomized nonatherogenic rhesus.8 Moreover, the data substantiate our hypothesis that low-dose P attenuates primate coronary hyperreactivity via attenuation of TP receptor expression even in the presence of atherosclerosis.
Figure 5.
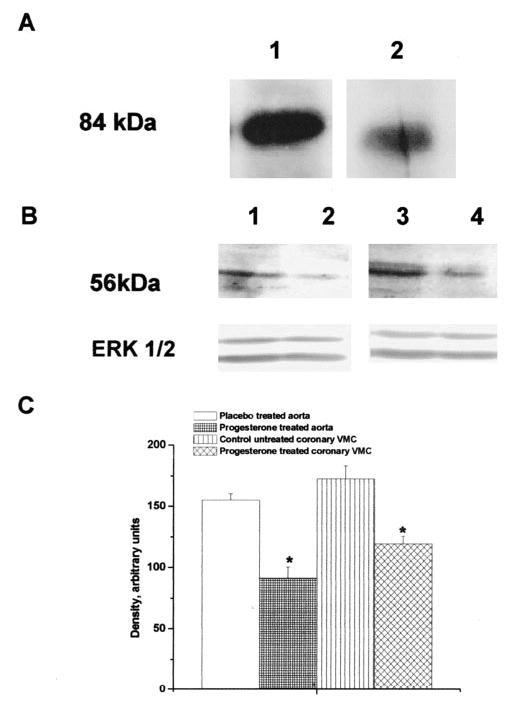
A, PR in aorta and coronary vascular muscle. Immunoblot showing PR expression in aorta from a placebo-treated rhesus1 and coronary vascular muscle cells isolated from a control (untreated monkey and untreated vascular muscle cells).2 The result is representative of 2 independent experiments from 3 animals or coronary VMC cultures. B, Effects of P on TP receptor. TP receptor expression in aorta from placebo control,1 P-treated rhesus monkey,2 primary untreated coronary vascular muscle cells from placebo control3 and primary coronary vascular muscle cells treated with 1 nM P.4 Data are representative of 2 independent experiments each for P-treated or placebo-treated groups1,2 or coronary vascular muscle cells.3,4 Detection of ERK 1/2 was used as an internal loading control. C, Quantitation of TP receptor expression in rhesus aorta and in coronary vascular muscle cells demonstrating effects of P treatment in vivo and in vitro. *Significant attenuation in density compared with respective placebo control for aorta and untreated coronary vascular muscle cells (P<0.05).
Figure 4.
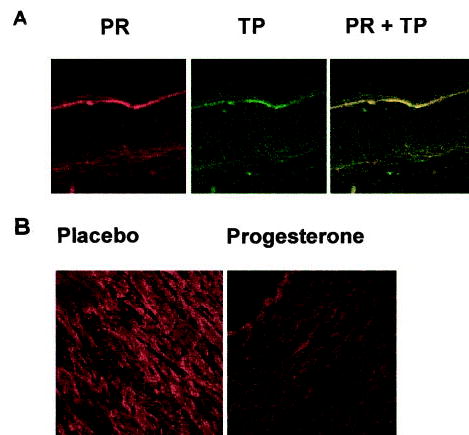
A, Double-labeling ICC by indirect immunofluorescence in 5-μm cross-sections of rhesus coronary arteries for PR (labeled with a rat monoclonal antibody, JZB39) and TP receptors (labeled with a rabbit polyclonal antibody, PH4). Data are representative of results from 3 cross-sections each from 5 placebo monkeys. B, ICC for TP receptor in cross-sections of rhesus aorta from monkeys treated with placebo or P cream. Data are representative results from 3 P-treated and 3 placebo-treated monkeys.
Discussion
CAD occurs in women predominantly after menopause. The primary focus, however, has been limited to estrogens. Other ovarian hormones, their metabolic products, different routes of administration, hormonal exposure patterns, and sensitivity and responsiveness at target organs after a loss of the premenopausal balanced P hormonal milieu have been overlooked.14 Data from recent HRT trials indicate a lack of beneficial cardiovascular effects with a possible increase in cardiovascular risk.3,4 Timing, dose, route of administration, and choice of regimen from the standpoint of beneficial cardiovascular effects of hormone replacement also need to be investigated.
The most important finding of this study is protection of coronary arteries against hyperreactivity in preatherosclerotic rhesus by low-dose transdermal P compared with exaggerated vasoconstriction magnitude and duration in placebo group. Salutary effects of subphysiological blood levels of P on coronary arteries8,15,16 would thus extend to the much larger atherosclerotic population. Furthermore, treatment with P but without E (and specifically excluding soy proteins and isoflavones that occur in monkey chow), were beneficial during the AD. Differences in P levels at baseline (2- to 3-times higher in placebo versus P-treated) were noted. To our knowledge, these are the first high-resolution measurements performed on P levels in surgically menopausal rhesus. Pregnanediol, the predominant urinary metabolite of P, reflects P excretion and documents absorption of transdermal P.
All progestins are not alike,17 as shown by contrasts of P versus MPA effects on consideration of coronary artery reactivity.7,18 MPA, a synthetic progestin distinct from P, has significant androgenic properties19–21 and reverses estrogen’s atheroprotective effects in both primate5,22 and rat models.23 Divergent effects of protective bioidentical P, as contrasted with the synthetic MPA, are grossly underappreciated.7,16,18,22
Inability of coronary arteries to relax after combined serotonin and thromboxane receptor stimulation leads to persistent coronary blood flow restriction, causing myocardial ischemia and infarction, which defines coronary hyperreactivity.6,7,8,15 Although the clinical significance of hyperreactivity remains to be firmly established, women with angiographically proven CAD experiencing stable exertional angina pectoris benefit from combined, parenteral E plus P, as shown by increases in treadmill exercise test symptom-limited duration compared with E plus MPA treatment.24 The severe coronary contractions corresponding with elevated late Ca2+ signals suggest that hyperreactivity occurs because of differential effects of P versus MPA on TP receptors and downstream Ca2+ signals.7,8,15 In support of the coronary hyperreactivity hypothesis, contrasting effects of MPA compared with E2 and P have been demonstrated in rat hippocampal neuronal cells where P and E2 are neuroprotective against glutamate excitotoxicity, whereas MPA is not.25 Under the influence of MPA, the phospho-ERK signal did not reach the nucleus, unlike the signal translocation seen after P treatment. This lack of phospho–extracellular regulated kinase (ERK) signal transduction may thus underlie the failure of MPA to protect.26
In the nonhuman primate surgical menopause model, if E therapy is delayed for 2 years, there is little effect on the extent of atherosclerosis,14,22 although immediate administration of E significantly reduces the extent of atherosclerosis.5,13,27 Our study is the first to examine the effects of delayed (>19 months after ovx) P treatment for 28 days on coronary hyperreactivity in preatherosclerotic, nonhuman primates. Soy-free AD for 19 months significantly and equivalently increased total cholesterol, LDL cholesterol, and HDL cholesterol, in both P-treated and placebo-treated rhesus. Despite early atherosclerosis and elevated cholesterol and LDL, P protected against provoked hyperreactivity. Furthermore, P treatment significantly lowered Lp(a) levels below pre-AD measurements. Increased serum Lp(a) with accumulation of Lp(a) in atherosclerotic coronary arteries has previously been demonstrated in diet-induced atherosclerosis in male primates.28 Human studies have shown that Lp(a) is deposited at sites of vascular injury. However, the pathogenic role of Lp(a) in atherosclerosis remains undefined,29 and the significance of P to suppression of Lp(a) levels is not known.
A limitation of this study is that effects of P on the atherosclerotic process and in vivo vascular muscle cells proliferation were not tested because the primary focus was on dynamic hyperreactivity. In vitro studies suggest that P inhibits VMC proliferation.30,31 Controversy exists, however, regarding the exact role of P in regulating vascular, atherosclerotic, proliferative responses. In vivo, placebo-treated PR knockout mice show significantly more medial hypertrophy and vascular muscle cell proliferation in response to vascular injury than wild-type. Although P did not cause adverse effects in PR knockout mice, P (at an unspecified dose) unexpectedly worsened the response to injury in wild-type mice.32
Reasons for the surprising divergence of in vivo and in vitro effects of P on vascular proliferation are unclear. Removal of transrepression by unliganded PR, consequently increasing expression of genes promoting proliferation, has been implicated.32 Such biological effects of various hormone ligands are likely species-dependent.33 The combination of species differences, timing of intervention, dose and route of administration, and promiscuity of steroid hormones likely account for the varied outcomes seen when different hormones from the same class (MPA versus P) or even the same hormone (P) are tested. Mechanisms of both proliferation and reactivity should be targets of investigation.
During the atherosclerotic process, functional abnormalities associated with coronary artery remodeling may lead to a more hyperreactive artery. Such functional changes warrant consideration in addition to anatomical and structural changes increasing vessel stiffness.34 Data from the present study demonstrate that a functional abnormality in the form of hyperreactivity results in profound, sustained (for >5 minutes) constriction of coronaries in atherogenic P-deficient primates. More significantly, transdermal P treatment dramatically attenuates this abnormal, prolonged vasomotor response. In early stages, atherosclerotic changes include increased vascular reactivity. Early hormone therapy, when the vascular wall is less reactive and more responsive to steroids, would be more effective in slowing the progression of atherosclerosis.14
Frequently, changes in vascular reactivity are discussed in terms of attenuation of endothelial-dependent dilation, focusing on inadequate vasodilator function. However, a dilator-only model of altered vascular reactivity ignores important contractile vascular muscle changes. In a previous investigation8 and in this study, we have focused on TP receptors as a putative mechanism of coronary hyperactivity, with serotonergic receptors contributing as well. Previous work35,36 indicates that hyperresponsiveness to putative pathophysiological vasoconstrictors, particularly serotonin and TxA2 (known to be released in locally high concentrations by activated blood platelets), likely underlies the observed exaggerated vasoconstriction. Specific contributions of serotonergic receptors were not examined in this study because of the complexity inherent in the many subtypes of serotonergic receptors.
Reactivity studies are an important component in the investigation of CAD. In cynomolgus monkeys, hypercholesterolemia augments responses of small vessels to vasoconstrictor during early hypercholesterolemia. Although responses to norepinephrine return to normal later, vasoconstrictor effects of serotonin continue to be greatly potentiated.35,36 Dietary treatment of atherosclerosis can abolish increased vasoconstrictor responses to serotonin, and thus treatment of atherosclerosis may be beneficial, even if vasodilator responses fail to improve, by reducing susceptibility to serotonin-induced vasospasm.37 Reducing dietary cholesterol reverses abnormal vascular responses to serotonin in atherosclerotic monkeys without structural CAD.38 In conjunction with reabsorption of lipid from the arterial wall, these early functional improvements in vasoconstriction occur well before changes in the mass of the atherosclerotic lesion can be detected.39 Similarly, despite little reduction in size of intimal lesions after regression of atherosclerosis, attenuation of carotid and ocular artery vasoconstrictor hyperresponsiveness to serotonin and platelet activation has been observed.40
Hyperreactivity of vascular contractile function must now be considered as an additional contributor to initiation and progression of CAD. Studies cited highlight the significance of hyperresponsiveness to vasoconstrictors in atherosclerosis. Results from our previous studies6–8,15,16,18 combined with data presented here support the notion of a “reactivity component” to CAD, attributable to hyperreactivity of blood vessels. Furthermore, the hyperreactivity aspect of CAD warrants investigation as a potential risk factor in future clinical studies, especially in women. In principle, attenuating vascular hyperreactivity early in atherosclerosis could minimize prolonged ischemic constrictions, reducing the incidence of cardiovascular events.
Acknowledgments
The authors thank the many people who provided expertise in development of this manuscript. Supported by grants 2R44AG16159 to R.K.H. and RR00163-ONPRC from the National Institutes of Health.
Footnotes
Dimera is pursuing development of prescription transdermal progesterone for treatment of angina pectoris. Both R.K.H. and R.G.M. are employees of Dimera.
References
- 1.Oparil S. Hormones and vasoprotection (Corcoran Lecture) Hypertension. 1999;33:170–176. doi: 10.1161/01.hyp.33.1.170. [DOI] [PubMed] [Google Scholar]
- 2.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) research group. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 3.Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, Hsia J, Hulley S, Herd A, Khan S, Newby LK, Waters D, Vittinghoff E, Wenger N. Cardiovascular disease outcomes during 6.8 years of hormone therapy - Heart and Estrogen/progestin Replacement Study Follow-up (HERSII) JAMA-Express. 2002;288:49–57. doi: 10.1001/jama.288.1.49. [DOI] [PubMed] [Google Scholar]
- 4.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 5.Clarkson TB. Effects of estrogens, progestins, and androgens on coronary vasomotion and atherosclerosis. J Reprod Med. 1998;43:741–745. [PubMed] [Google Scholar]
- 6.Hermsmeyer K, Miyagawa K, Kelley ST, Rösch J, Hall AS, Axthelm MK, Greenberg B. Reactivity based coronary vasospasm independent of atherosclerosis in rhesus monkeys. J Am Coll Cardiol. 1997;29:671–680. doi: 10.1016/s0735-1097(96)00524-4. [DOI] [PubMed] [Google Scholar]
- 7.Miyagawa K, Rösch J, Stanczyk F, Hermsmeyer K. Medroxyprogesterone interferes with ovarian steroid protection against coronary vasospasm. Nat Med. 1997a;3:324–327. doi: 10.1038/nm0397-324. [DOI] [PubMed] [Google Scholar]
- 8.Minshall RD, Pavcnik D, Halushka PV, Hermsmeyer K. Progesterone regulation of vascular thromboxane A2 receptors in rhesus monkeys. Am J Physiol. 2001;281:H1498–H1507. doi: 10.1152/ajpheart.2001.281.4.H1498. [DOI] [PubMed] [Google Scholar]
- 9.Minshall RD, Pavcnik D, Browne DL, Hermsmeyer K. Nongenomic vasodilator action of progesterone on primate coronary arteries. J Appl Physiol. 2002;92:701–708. doi: 10.1152/japplphysiol.00689.2001. [DOI] [PubMed] [Google Scholar]
- 10.Anthony MS, Clarkson TB, Bullock BC, Wagner JD. Soy protein versus soy phytoestrogens in the prevention of diet-induced coronary artery atherosclerosis of male cynomolgus monkeys. Arterioscler Thromb Vasc Biol. 1997;17:2524–2531. doi: 10.1161/01.atv.17.11.2524. [DOI] [PubMed] [Google Scholar]
- 11.Adams MR, Anthony MS, Manning JM, Golden DL, Parks JS. Low-dose contraceptive estrogen-progestin and coronary artery atherosclerosis of monkeys. Obstet Gynecol. 2000;96:250–255. doi: 10.1016/s0029-7844(00)00891-7. [DOI] [PubMed] [Google Scholar]
- 12.Williams JK, Anthony MS, Herrington DM. Interactive effects of soy protein and estradiol on coronary artery reactivity in atherosclerotic, ovariectomized monkeys. Menopause. 2001;8:307–313. doi: 10.1097/00042192-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Clarkson TB, Anthony MS, Morgan TM. Inhibition of postmenopausal atherosclerosis progression: a comparison of the effects of conjugated equine estrogens and soy phytoestrogens. J Clin Endocrinol Metab. 2001;86:41–47. doi: 10.1210/jcem.86.1.7151. [DOI] [PubMed] [Google Scholar]
- 14.Hodis HN, Mack WJ, Azen SP, Lobo RA, Shoupe D, Mahrer PR, Faxon DP, Cashin-Hemphill L, Sanmarco ME, French WJ, Shook TL, Gaarder TD, Mehra AO, Rabbani R, Sevanian A, Shil AB, Torres M, Vogelbach KH, Selzer RH. Women’s Estrogen-Progestin Lipid-Lowering Hormone Atherosclerosis Regression Trial Research Group. Hormone therapy and the progression of coronary-artery atherosclerosis in postmenopausal women. N Engl J Med. 2003;349:535–545. doi: 10.1056/NEJMoa030830. [DOI] [PubMed] [Google Scholar]
- 15.Minshall RD, Stanczyk FZ, Miyagawa K, Uchida B, Axthelm MK, Novy M, Hermsmeyer K. Ovarian steroid protection against coronary artery hyperreactivity in rhesus monkeys. J Clin Endocrinol Metab. 1998a;83:649–659. doi: 10.1210/jcem.83.2.4576. [DOI] [PubMed] [Google Scholar]
- 16.Minshall RD, Miyagawa K, Chadwick CC, Novy MJ, Hermsmeyer K. In Vitro modulation of primate coronary vascular muscle cell reactivity by ovarian steroid hormones. FASEB J. 1998b;12:1419–1429. doi: 10.1096/fasebj.12.13.1419. [DOI] [PubMed] [Google Scholar]
- 17.Stanczyk FZ. All progestins are not created equal. Steroids. 2003;68:879–890. doi: 10.1016/j.steroids.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Miyagawa K, Vidgoff J, Hermsmeyer K. Ca2+ release mechanism of primate reactivity based coronary vasospasm. Am J Physiol. 1997b;272:H2645–H2654. doi: 10.1152/ajpheart.1997.272.6.H2645. [DOI] [PubMed] [Google Scholar]
- 19.Bentel JM, Birrell SN, Pickering MA, Holds DJ, Horsfall DJ, Tilley WD. Androgen receptor agonist activity of the synthetic progestin, medroxyprogesterone acetate, in human breast cancer cells. Mol Cell Endocrinol. 1999;154:11–20. doi: 10.1016/s0303-7207(99)00109-4. [DOI] [PubMed] [Google Scholar]
- 20.Sitruk-Ware R. Progestins and cardiovascular risk markers. Steroids. 2000;65:651–658. doi: 10.1016/s0039-128x(00)00174-4. [DOI] [PubMed] [Google Scholar]
- 21.Wakatsuki A, Okatani Y, Ikenoue N, Fukaya T. Effect of medroxyprogesterone acetate on vascular inflammatory markers in postmenopausal women receiving estrogen. Circulation. 2002;105:1436–1469. doi: 10.1161/hc1202.105945. [DOI] [PubMed] [Google Scholar]
- 22.Williams JK, Honoré EK, Washburn SA, Clarkson TB. Effects of hormone replacement therapy on reactivity of atherosclerotic coronary arteries in cynomolgus monkeys. J Am Coll Cardiol. 1994;24:1757–1761. doi: 10.1016/0735-1097(94)90184-8. [DOI] [PubMed] [Google Scholar]
- 23.Levine RL, Chen SJ, Durand J, Chen YF, Oparil S. Medroxyprogesterone attenuates estrogen-mediated inhibition of neointima formation after balloon injury of the rat carotid artery. Circulation. 1996;94:2221–2227. doi: 10.1161/01.cir.94.9.2221. [DOI] [PubMed] [Google Scholar]
- 24.Rosano GM, Webb CM, Chierchia S, Morgani GL, Gabraele M, Sarrel PM, de Ziegler D, Collins P. Natural progesterone, but not medroxyprogesterone acetate, enhances the beneficial effect of estrogen on exercise-induced myocardial ischemia in postmenopausal women. J Am Coll Cardiol. 2000;36:2154–2159. doi: 10.1016/s0735-1097(00)01007-x. [DOI] [PubMed] [Google Scholar]
- 25.Nilsen J, Brinton RD. Impact of progestins on estrogen-induced neuroprotection: synergy by progesterone and 19-norprogesterone and antagonism by medroxyprogesterone acetate. Endocrinology. 2002;143:205–212. doi: 10.1210/endo.143.1.8582. [DOI] [PubMed] [Google Scholar]
- 26.Nilsen J, Brinton RD. Divergent impact of progesterone and medroxyprogesterone acetate (Provera) on nuclear mitogen-activated protein kinase signaling. Proc Natl Acad Sci U S A. 2003;100:10506–10511. doi: 10.1073/pnas.1334098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adams MR, Register TC, Golden DL, Wagner JD, Williams JK. Medroxyprogesterone acetate antagonizes inhibitory effects of conjugated equine estrogens on coronary artery atherosclerosis. Arterioscler Thromb Vasc Biol. 1997;17:217–221. doi: 10.1161/01.atv.17.1.217. [DOI] [PubMed] [Google Scholar]
- 28.Nachman RL, Gavish D, Azrolan N, Clarkson TB. Lipoprotein(a) in diet-induced atherosclerosis in nonhuman primates. Arterioscler Thromb. 1991;11:32–38. doi: 10.1161/01.atv.11.1.32. [DOI] [PubMed] [Google Scholar]
- 29.Marcovina SM, Koschinsky ML, Albers JJ, Skarlatos S. Report of the National Heart, Lung, and Blood Institute Workshop on Lipoprotein(a) and Cardiovascular Disease: Recent Advances and Future Directions. Clin Chem. 2003;49:1785–1796. doi: 10.1373/clinchem.2003.023689. [DOI] [PubMed] [Google Scholar]
- 30.Lee WS, Harder JA, Yoshizumi M, Lee ME, Haber E. Progesterone inhibits arterial smooth muscle cell proliferation. Nat Med. 1997;3:1005–1008. doi: 10.1038/nm0997-1005. [DOI] [PubMed] [Google Scholar]
- 31.Carmody BJ, Arora S, Wakefield MC, Weber M, Fox CJ, Sidawy AN. Progesterone inhibits human infragenicular arterial smooth muscle cell proliferation induced by high glucose and insulin concentrations. J Vasc Surg. 2002;36:833–838. [PubMed] [Google Scholar]
- 32.Karas RH, van Eickels M, Lydon JP, Roddy S, Kwoun M, Aronovitz M, Baur WE, Conneely O, O’Malley BW, Mendelsohn ME. A complex role for the progesterone receptor in the response to vascular injury. J Clin Invest. 2001;108:611–618. doi: 10.1172/JCI11374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris HA, Bapat AR, Gonder DS, Frail DE. The ligand binding profiles of estrogen receptors alpha and beta are species dependent. Steroids. 2002;67:379–384. doi: 10.1016/s0039-128x(01)00194-5. [DOI] [PubMed] [Google Scholar]
- 34.McLeod AL, Newby DE, Northridge DB, Fox KA, Uren NG. Influence of differential vascular remodeling on the coronary vasomotor response. Cardiovasc Res. 2003;59:520–526. doi: 10.1016/s0008-6363(03)00396-1. [DOI] [PubMed] [Google Scholar]
- 35.Heistad DD, Armstrong ML, Marcus ML, Piegors DJ, Mark AL. Augmented responses to vasoconstrictor stimuli in hypercholesterolemic and atherosclerotic monkeys. Circ Res. 1984;54:711–718. doi: 10.1161/01.res.54.6.711. [DOI] [PubMed] [Google Scholar]
- 36.Heistad DD, Armstrong ML, Marcus ML, Piegors DJ, Mark AL. Potentiation of vasoconstrictor responses to serotonin in the limb of atherosclerotic monkeys. J Hypertens Suppl. 1986;4:S17–S21. [PubMed] [Google Scholar]
- 37.Heistad DD, Mark AL, Marcus ML, Piegors DJ, Armstrong ML. Dietary treatment of atherosclerosis abolishes hyperresponsiveness to serotonin: implications for vasospasm. Circ Res. 1987;61:346–351. doi: 10.1161/01.res.61.3.346. [DOI] [PubMed] [Google Scholar]
- 38.Lamping KG, Piegors DJ, Benzuly KH, Armstrong ML, Heistad DD. Enhanced coronary vasoconstrictive response to serotonin subsides after removal of dietary cholesterol in atherosclerotic monkeys. Arterioscler Thromb. 1994;14:951–957. doi: 10.1161/01.atv.14.6.951. [DOI] [PubMed] [Google Scholar]
- 39.Benzuly KH, Padgett RC, Kaul S, Piegors DJ, Armstrong ML, Heistad DD. Functional improvement precedes structural regression of atherosclerosis. Circulation. 1994;89:1810–1818. doi: 10.1161/01.cir.89.4.1810. [DOI] [PubMed] [Google Scholar]
- 40.Sobey CG, Faraci FM, Piegors DJ, Heistad DD. Effect of short-term regression of atherosclerosis on reactivity of carotid and retinal arteries. Stroke. 1996;27:927–933. doi: 10.1161/01.str.27.5.927. [DOI] [PubMed] [Google Scholar]


