Abstract
Objective
To develop a simplified clinical scale defining risk categories for development of advanced age-related macular degeneration (AMD).
Methods
Following development of a detailed scale for individual eyes based on gradings of fundus photographs in the Age-Related Eye Disease Study, rates of progression to advanced AMD were assessed in cross-tabulations of presence or absence in each eye of 2 easily identified retinal abnormalities, drusen and pigment abnormalities. Large drusen and any pigment changes were particularly predictive of developing advanced AMD.
Results
The scoring system developed for patients assigns to each eye 1 risk factor for the presence of 1 or more large (≥125 μm, width of a large vein at disc margin) drusen and 1 risk factor for the presence of any pigment abnormality. Risk factors are summed across both eyes, yielding a 5-step scale (0–4) on which the approximate 5-year risk of developing advanced AMD in at least one eye increases in this easily remembered sequence: 0 factors, 0.5%; 1 factor, 3%; 2 factors, 12%; 3 factors, 25%; and 4 factors, 50%. For persons with no large drusen, presence of intermediate drusen in both eyes is counted as 1 risk factor.
Conclusion
This simplified scale provides convenient risk categories for development of advanced AMD that can be determined by clinical examination or by less demanding photographic procedures than used in the Age-Related Eye Disease Study.
In the companion report in this issue of the Archives,1 gradings of 30° stereoscopic color fundus photographs collected at baseline, 2 years later, and annually thereafter in the Age-Related Eye Disease Study (AREDS) were used to develop a severity scale for age-related macular degeneration (AMD). The photographs were graded with the AREDS classification of AMD, in which the graded characteristics of nonadvanced AMD (drusen size, area and type, and pigment abnormalities), their definitions, and the steps in the severity scales for each characteristic are very similar to those used in the Wisconsin Age-Related Maculopathy Grading System and in the international classification and grading system for age-related maculopathy and AMD.2–4 The AREDS Severity Scale combines select baseline characteristics to provide 9 steps of increasing overall severity of nonadvanced AMD based on the risk of developing advanced AMD, either neovascular AMD or geographic atrophy (GA) involving the center of the macula, within 5 years. Neovascular AMD was defined as retinal pigment epithelial detachment, serous sensory retinal detachment, subretinal hemorrhage, or subretinal fibrosis observed in color photographs within 2 disc diameters of the center of the macula, or the application of photocoagulation for this disorder. The scale may be useful in research to identify baseline risk categories, to track disease progression, and perhaps as a surrogate outcome measure. In the companion report, no attempt was made to adapt the eye scale for use in persons (as was done, for example, in the Early Treatment Diabetic Retinopathy Study5). Also, no attempt was made to simplify the scale for clinical use, although examples were given to suggest how this might be done.
See also pages 1484 and 1598
In this report, we describe a clinically useful, simplified scale for persons. Although the scale is based on the same photographic gradings used in the companion report, in this scale the principal baseline characteristics considered are maximum drusen size and presence or absence of any pigmentary abnormality in one or both eyes, characteristics that we suggest can be assessed easily clinically with ophthalmoscopy and/or slitlamp biomicroscopy, or in less optimal photographs using less complex grading procedures than those used in AREDS.
METHODS
The methods used to develop the research scale presented in the companion report are given in detail in that report and summarized herein. The photographs were taken by certified photographers at 11 clinical centers and graded centrally by trained graders using a detailed protocol and measuring grids.2 The principal outcome used in developing the scale was the occurrence of advanced AMD, defined as either neovascular AMD or GA involving the center of the macula, within 5 years after the baseline examination. The principal baseline characteristics assessed were maximum drusen size, drusen area, and the presence and extent of each of 3 pigmentary abnormalities: increased pigment (thought to be related to AMD), retinal pigment epithelial depigmentation, and noncentral GA. Initial development of the scale used baseline characteristics and outcomes in the right eyes of 3212 patients in whom both eyes were free of advanced AMD at baseline. The scale was then applied to the left eyes of these patients and to the eye that was free of advanced AMD in patients who had advanced AMD in one eye at baseline.
The format of the simplified clinical scale was suggested by 3 observations: (1) the strong association of maximum drusen size and drusen area shown in Table 2 of the companion report, (2) the low frequency of retinal pigment epithelial depigmentation and/or GA in the absence of increased retinal pigment (Table 4 of the companion report), and (3) the finding in a previous AREDS report that the presence of large drusen in both eyes was a stronger risk factor for progression to advanced AMD than presence in only one eye.6 Furthermore, we assumed that eye care professionals can more effectively assess maximum drusen size than drusen area. Large drusen are defined as those (within 2 standard disc diameters of the center of the macula) with (shortest) diameter greater than or equal to that of an average normal retinal vein at the disc margin, considered to be approximately one-twelfth disc diameter or approximately 125 μm, when the average disc diameter is taken as 1500 μm (Figure 1); intermediate drusen are those with a disc diameter greater than or equal to one half that of large drusen (63 μm). To test the hypothesis that these 2 risk factors (drusen size and the presence of pigmentary abnormalities) could be used for a simple scale, data from 3211 persons in the data set used to develop the detailed scale (who were free of advanced AMD in both eyes at baseline and had complete baseline and 5-year information as of May 2001) were cross-classified by maximum drusen size and by absence or presence of any pigmentary abnormality (hyperpigmentation, hypopigmentation, noncentral GA) in each eye at baseline, and 5-year rates of advanced AMD in one or both eyes were calculated for each cell as presented in Table 1.
Table 2.
Five-Year Rates of Advanced AMD (In One or Both Eyes for Patients With Both Eyes at Risk)
|
Patients Without Advanced AMD in Either Eye at Baseline* |
Patients with Advanced AMD in One Eye at Baseline † |
|||||
|---|---|---|---|---|---|---|
| Risk Factors | No. at Risk | No. | % | No. at Risk | No. | % |
| 0 | 1466 | 6 | 0.4 | |||
| 1 | 635 | 20 | 3.1 | |||
| 2 | 455 | 55 | 11.8 | 149 | 22 | 14.8 |
| 3 | 328 | 85 | 25.9 | 178 | 63 | 354 |
| 4 | 317 | 150 | 47.3 | 273 | 145 | 53.1 |
Abbreviation: AMD, age-related macular degeneration.
Assign 1 risk factor for each eye with large drusen.
Assign 1 risk factor for each eye with pigment abnormalities.
Assign 1 risk factor if neither eye has large drusen and both eyes have intermediate drusen (Table 1).
Assign 2 risk factors for the eye that has neovascular AMD.
Assign 1 additional risk factor if the eye at risk has large drusen.
Assign 1 additional risk factor if the eye at risk has pigment abnormalities.
Figure 1.
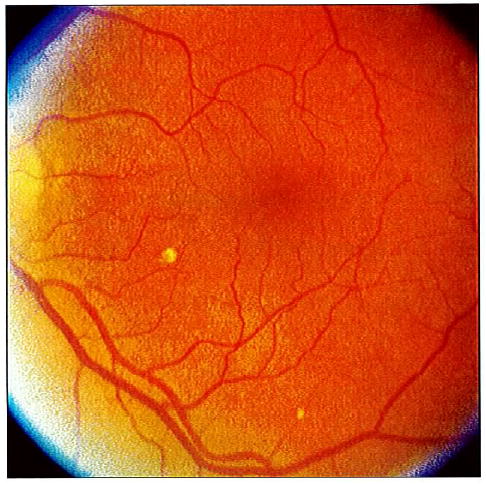
Fundus photograph to illustrate drusen size. In the 8-o’clock meridian, about 1 disc diameter from the center of the macula, there is a large druse, slightly larger than the minimum required to meet the greater than or equal to 125 μm (width of an average large vein at the disc margin) definition. At 5:30, near the edge of the photograph, there is an intermediate druse, defined as greater than or equal to 63 μm but less than 125 μm in diameter.
Table 1.
Number and Percentage of Patients Developing Advanced AMD in One or Both Eyes at or Before the 5-Year Follow-up Visit for 3211 Patients Free of Advanced AMD in Both Eyes at Baseline
|
Pigment Abnormalities |
||||||
|---|---|---|---|---|---|---|
|
None |
1 Eye |
Both Eyes |
||||
| Drusen Size and No. of Eyes | No. of Events/No. of Patients at Risk | % | No. of Events/No. of Patients at Risk | % | No. of Events/No. of Patients at Risk | % |
| Small, only one or both eyes (or none) | 4/1017 | 0.4 | 0/64 | 0 | 1/8 | 12.5 |
| Intermediate, one eye (no large) | 2/449 | 0.5 | 5/101 | 5.0 | 4/31 | 12.9 |
| Intermediate, both eyes (no large) | 4/187 | 2.1 | 6/50 | 12.0 | 7/35 | 20.0 |
| Large, one eye | 11/283 | 3.9 | 17/168 | 10.1 | 30/117 | 25.6 |
| Large, both eyes | 27/208 | 13.0 | 48/176 | 27.3 | 150/317 | 47.3 |
Abbreviation: AMD, age-related macular degeneration.
No. of Risk Factors: 0 1 2 3 4
Subsequently, right and left eyes were categorized separately by these factors and cross-classified to determine whether the small subgroups contained in each scale step were combined appropriately. Tables similar to Table 1 were then produced using each of the 3 pigmentary abnormalities separately) and each of the 3 possible combinations of 2 abnormalities, and each of these iterations was compared with Table 1. Another table, similar to Table 1, was produced in which total drusen area was substituted for drusen size. Finally, the scale was applied to the eye that was free of advanced AMD in patients who had neovascular AMD in one eye at baseline. These tables are available at the AREDS Web site as an appendix to this report (http://spitfire.emmes.com/study/areds/). We compared the various subgroups by inspection, without formal statistical testing but with consideration given to the numbers of persons in each category and the confidence intervals of these estimates.
The cross-tabulations included in this report and in the appendix are for the data set used to develop the detailed scale, which included follow-up through 5 years. To evaluate the simplified risk factor scale using all follow-up visits, repeated-measures logistic regression models were used to estimate risk of progression to advanced AMD through 10 years of follow-up, first for all participants and then separately for the AREDS groups randomly assigned to placebo or to antioxidants and zinc combined.
RESULTS
Five-year rates of advanced AMD in one or both eyes are shown in Table 1 for patients classified by maximum drusen size and presence or absence of any pigmentary abnormality in one or both eyes. A convenient way to summarize the findings in Table 1 is to designate presence of large drusen and of any pigment abnormality as risk factors for development of advanced AMD and to sum their presence across both eyes of an individual as illustrated in Figure 2. A patient with large drusen and pigmentary abnormalities in each eye (both eyes similar to the example shown in Figure 3) would have 4 risk factors. In addition, to take into account the increased risk for persons with no large drusen in either eye but with intermediate drusen in both eyes, the bilateral presence of intermediate drusen is counted as 1 risk factor. The rates in cells with each level of risk factors are combined and presented in the first section of Table 2. Estimates of 5-year risks for the development of advanced AMD for each of the steps from 0 to 4 are 0.4%, 3.1%, 11.8%, 25.9%, and 47.3%, respectively. Retinal features seem to be the most important risk factors when assessing a person’s risk, and many cross-tabulations of these characteristics were performed. In general, if one takes the perspective that this system is identifying persons at small (≤12%), medium (approximately 25%), and large (approximately 50%) risk, the classification scheme seems quite robust. However, there are some specific subgroups where the risk might be different than these global estimates. For example, the presence of noncentral GA is a strong predictor of advanced AMD. Of the 56 patients who had noncentral GA in one (50) or both (6) eyes at baseline, 39 (69.6% [95% confidence interval, 57.6%–81.7%]) developed advanced AMD (in most cases this was GA that involved the center of the macula). Data on the specific risks in various subgroups are available as an appendix to this report at the AREDS Web site (http://spitfire.emmes.com/study/areds/). Caution should be used when interpreting the specific rates because many are based on very small sample sizes and the estimates have broad confidence intervals.
Figure 2.
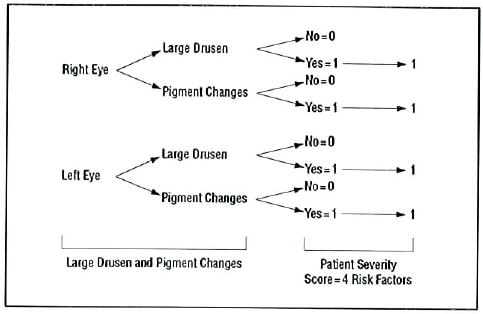
Risk factor scoring for patient with large drusen and pigment abnormalities in both eyes.
Figure 3.
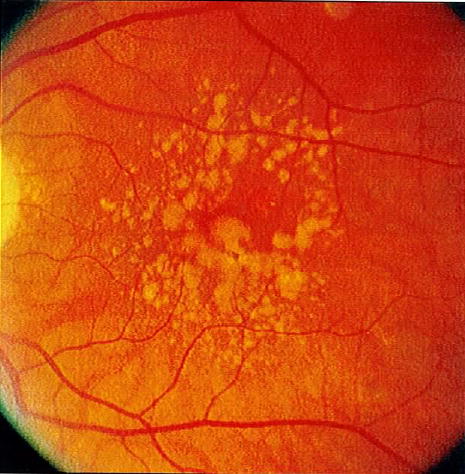
Fundus photograph of an eye with 2 risk factors (large drusen and pigment abnormalities).
For persons who already have advanced AMD in one eye, the second section of Table 2 provides 5-year rates of advanced AMD in the contralateral eye: Two risk factors are assigned for presence of advanced AMD in the first eye and additional risk factors are added for presence of large drusen and/or pigment abnormalities in the eye at risk. Within each risk factor category, risks are similar to those in the first section of the table, but somewhat higher. Again, there may be some specific examples where the estimates may be different than the global estimates for the risk groups. For example, among persons with 3 risk factors, those with advanced AMD in one eye have a 35.4% 5-year risk for developing advanced AMD in the second eye, compared with a 25.9% risk of advanced AMD in one or both eyes for persons without advanced AMD in either eye. In addition, the 57 persons with GA involving the center of the macula in one eye at baseline had approximately the same 40% to 50% 5-year risk of developing advanced AMD in the second eye, whether large drusen were present in it or not.
The rates in these tables are from the partial AREDS data set used in developing the detailed scale, in which follow-up only through 5 years was considered. The event rate estimates using the simplified risk factor scale for all AREDS participants through 10 years of follow-up, assessed by repeated-measures logistic regression, are shown for all treatment groups combined in Figure 4 and separately for the AREDS groups randomly assigned to placebo and to the combination of zinc and antioxidants in Figure 5. These results are consistent with those seen using the scale building data set and also indicate how some risk factors, in this case the use of AREDS supplements, can modify the risks somewhat.
Figure 4.
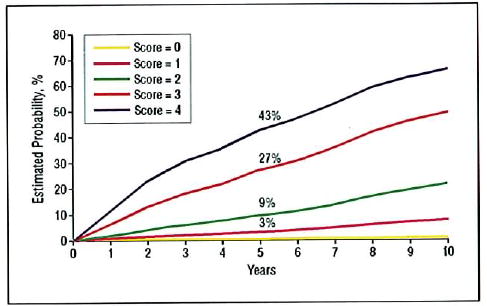
Risk of progression to advanced age-related macular degeneration (in at least one eye in patients with both eyes at risk) based on repeated-measures logistic repression analysis of 4710 Age-Related Eye Disease Study participants, by simplified risk score assigned at baseline.
Figure 5.
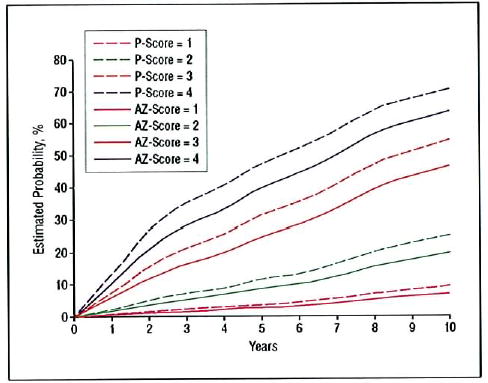
Risk of progression to advanced age-related macular degeneration (in at least one eye in patients with both eyes at risk) based on repeated-measures logistic regression analysis of Age-Related Eye Disease Study (AREDS) participants randomized to the AREDS combined treatment group (antioxidants plus zinc [AZ]) or placebo (P), by simplified risk score assigned at baseline.
COMMENT
The goal of the analyses described in this report was to use AREDS photographic gradings to construct an AMD severity scale that would provide clinically useful risk categories for the development of advanced AMD in persons with earlier stages of AMD. Defining risk factors and counting them provides a convenient way to define risk categories. These categories may be useful in discussing with patients their risk of progression to vision-threatening AMD and in developing inclusion criteria for future clinical studies of AMD. The simplest scheme counts the presence of at least 1 large druse (diameter greater than or equal to that of a large vein at the disc margin) and the presence of any pigment abnormality as 1 risk factor each and sums their presence across both eyes when both are free of advanced AMD. One risk factor is assigned to patients who have no large drusen in either eye but intermediate-sized drusen (diameter ≥ one half that of a large druse) in both eyes. For clinical purposes, as the number of risk factors increases from 0 to 4, the 5-year risk of advanced AMD in at least one eye increases in the easily remembered approximate sequence of 0.5%, 3%, 12%, 25%, and 50% (Figure 6). For the medium- and high-risk levels (approximately 25% and 50% 5-year risk), the risk is cut in half for each decrease in the number of risk factors. These are global risk factors and should be used to provide global estimates for the 5-year risk of developing advanced AMD for patients and their doctors. Other risk factors, such as smoking, family history, or supplement use, may modify these risks somewhat. However, these other factors have relatively small effects compared with the 100-fold change in risk between those with a score of 0 and those with a score of 4 based on fundus characteristics.
Figure 6.
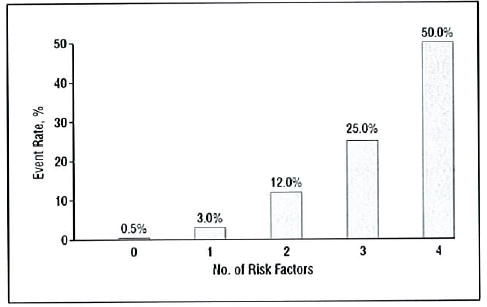
Approximate 5-year rates of progression to advanced age-related macular degeneration.
For persons who already have advanced AMD in one eye, the scale can be used to predict the 5-year rate of advanced AMD in the second eye by assigning 2 risk factors for the first eye and additional risk factors for the presence of large drusen and/or pigment abnormalities in the eye at risk (Table 2).
As is well known and demonstrated in Table 2 of the companion report, drusen size and area are closely associated. This simplified scale uses drusen size, rather than area, because we believe it is easier clinically to estimate the size of the largest druse in an eye than to estimate total drusen area. When at least 1 large druse was present in each eye, drusen area was greater than or equal to 0.5 disc area in at least one eye in 71% of AREDS participants, while in participants with large drusen in only one eye, drusen area was greater than or equal to 0.5 disc area in that eye in only 10%.
Large drusen, presence of pigmentary abnormalities, presence of noncentral GA, and presence of advanced AMD in the fellow eye are all well-recognized risk factors for progression to advanced AMD.7–13 Findings regarding the importance of these characteristics from several recent population-based studies8–11 are reviewed in the companion report.1
The simplified scale shares the limitations of the more complex scale described in the companion report: the patient group studied was not population based; many of the subgroups that were combined to produce the steps in the scale were small and outcome rates low; and treatment was not considered in scale building, nor was age, sex, race, or “softness” of drusen. In addition, use of the simple scale clinically requires estimation of the size of the largest druse present in the posterior pole of each eye. In many cases these estimates will be easy, although in borderline cases or in eyes with retinal vessels or optic discs that are larger or smaller than average, they may not be. Fortunately, the sometimes difficult task of distinguishing between depigmentation and GA is avoided by defining “pigmentary abnormalities” as any of the 3 pigment disturbances that are characteristic of AMD and by the rarity of depigmentalion and/or GA in the absence of increased pigment (67 of the 1067 persons with any pigmentary abnormality among the 3211 persons included in these analyses). However, pigment abnormalities in the retina and retinal pigment epithelium can be related to many diseases other than AMD. The rates published herein are related to pigment changes thought to be associated with AMD, but errors in making this judgment may have occurred. Finally, although similar results were found when the scale was applied to the left eyes of the 3211 participants whose right eyes were used in developing it and to 600 different participants who had advanced AMD in one eye at baseline, its predictive power has not yet been examined in population-based cohorts or in other large groups of people with non-advanced AMD.
CONCLUSIONS
Gradings of stereoscopic color photographs collected during 5 years of follow-up in more than 3000 AREDS participants were used to develop a detailed grading scale discussed in the companion article in this issue of the Archives.1 A simplified scale was developed based on the presence or absence of 2 features characteristic of AMD that were easily identified clinically (drusen size and pigment abnormalities) and highly associated with the development of advanced AMD, especially when the status of both eyes was considered. The 5-year risk of advanced AMD using this scale increases in the approximate sequence of: 0 factors, 0.5%; 1 factor, 3%; 2 factors, 12%; 3 factors, 25%; and 4 factors, 50%. This scale may be useful clinically, either with ophthalmoscopy or slitlamp biomicroscopy, or in less optimal photographs using less complex grading procedures than those used in AREDS.
Footnotes
Financial Disclosure: None.
Funding/Support: This study was supported by contracts from the National Eye Institute, National Institutes of Health, Department of Health and Human Services, Bethesda, Md, with additional support from Bausch and Lomb Inc, Rochester, NY.
References
- 1.The Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch Ophthalmol. 2005;123:1484–1498. doi: 10.1001/archopht.123.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study system for classifying age-related macular degeneration from stereoscopic color fundus photographs: AREDS Report Number 6. Am J Ophthalmol. 2001;132:688–681. doi: 10.1016/s0002-9394(01)01218-1. [DOI] [PubMed] [Google Scholar]
- 3.Klein R, Davis MD, Magli YL, Segal P, Klein BE, Hubbard L. The Wisconsin Age-Related Maculopathy Grading System. Ophthalmology. 1991;98:1128–1134. doi: 10.1016/s0161-6420(91)32186-9. [DOI] [PubMed] [Google Scholar]
- 4.Bird AC, Bressler NM, Bressler SB, et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration: the International ARM Epidemiological Study Group. Surv Ophthalmol. 1995;39:367–374. doi: 10.1016/s0039-6257(05)80092-x. [DOI] [PubMed] [Google Scholar]
- 5.Early Treatment Diabetic Retinopathy Study Research Group. Fundus photographic risk factors for progression of diabetic retinopathy: ETDRS Report Number 12. Ophthalmology. 1991;98:823–833. [PubMed] [Google Scholar]
- 6.The Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS Report No 8. Arch Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Au Eong KG, Haller JA. Risk factors for age-related macular degeneration and choroidal neovascularization. In: Lim JI, ed. Age-Related Macular Degeneration. New York, NY: Marcel Dekker, Inc; 2002:389–395.
- 8.Klein R, Klein BEK, Jensen SC, Meuer SM. The live-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1997;104:7–21. doi: 10.1016/s0161-6420(97)30368-6. [DOI] [PubMed] [Google Scholar]
- 9.Klein R, Klein BEK, Tomany SC, Meuer SM, Huang G-H. Ten-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 2002;109:1767–1779. doi: 10.1016/s0161-6420(02)01146-6. [DOI] [PubMed] [Google Scholar]
- 10.Wang JJ, Foran S, Smith W, Mitchell P. Risk of age-related macular degeneration in eyes with macular drusen or hyperpigmentation: the Blue Mountains Eye Study cohort. Arch Ophthalmol. 2003;121:658–663. doi: 10.1001/archopht.121.5.658. [DOI] [PubMed] [Google Scholar]
- 11.van Leeuwen R, Klaver CCW, Vingerling JR, Hofman A, deJong PTVM. The risk and natural course of age-related maculopathy: follow-up at 6 1/2 years in the Rotterdam study. Arch Ophthalmol. 2003;121:519–526. doi: 10.1001/archopht.121.4.519. [DOI] [PubMed] [Google Scholar]
- 12.Macular Photocoagulation Study Group. Five-year follow-up of fellow eyes of patients with age-related macular degeneration and unilateral extrafoveal choroidal neovascularization. Arch Ophthalmol. 1993;111:1189–1199. doi: 10.1001/archopht.1993.01090090041018. [DOI] [PubMed] [Google Scholar]
- 13.Macular Photocoagulation Study Group. Risk factors for choroidal neovascularization in the second eye of patients with juxtafoveal or subfoveal choroidal neovascularization secondary to age-related macular degeneration. Arch Ophthalmol. 1997;115:741–747. doi: 10.1001/archopht.1997.01100150743009. [DOI] [PubMed] [Google Scholar]


