Abstract
Objective
To assess the association of ocular disorders and high doses of antioxidants or zinc with mortality in the Age-Related Eye Disease Study (AREDS).
Methods
Baseline fundus and lens photographs were used to grade the macular and lens status of AREDS participants. Participants were randomly assigned to receive oral supplements of high-dose antioxidants, zinc, antioxidants plus zinc, or placebo. Risk of all-cause and cause-specific mortality was assessed using adjusted Cox proportional hazards models.
Results
During median follow-up of 6.5 years, 534 (11%) of 4753 AREDS participants died. In fully adjusted models, participants with advanced age-related macular degeneration (AMD) compared with participants with few, if any, drusen had increased mortality (relative risk [RR], 1.41; 95% confidence interval [CI], 1.08–1.86). Advanced AMD was associated with cardiovascular deaths. Compared with participants having good acuity in both eyes, those with visual acuity worse than 20/40 in 1 eye had increased mortality (RR, 1.36; 95% CI, 1.12–1.65). Nuclear opacity (RR, 1.40; 95% CI, 1.12–1.75) and cataract surgery (RR, 1.55; 95% CI, 1.18–2.05) were associated with increased all-cause mortality and with cancer deaths. Participants randomly assigned to receive zinc had lower mortality than those not taking zinc (RR, 0.73; 95% CI, 0.61–0.89).
Conclusions
The decreased survival of AREDS participants with AMD and cataract suggests that these conditions may reflect systemic rather than only local processes. The improved survival in individuals randomly assigned to receive zinc requires further study.
Various ocular disorders (eg, visual impairment and cataract and those in persons with diabetes mellitus, severe retinopathy, or visual impairment) have been reported1–17 to be significant predictors of a decreased life span, often even after extensive adjustment for potential confounders. Cataract surgery has been associated with decreased survival in many1–5 but not all6–8 studies. A common finding is the association between nuclear opacities, in particular, and decreased survival.6,7,9–11 Age-related macular degeneration (AMD), on the other hand, has not been found to be related to decreased survival in the few studies6,8,9 that have examined the relationship, although the ability to find an association is limited because few participants in these studies had advanced disease (neovascular AMD or geographic atrophy).
Why ocular factors should be associated with decreased survival is unclear. Inadequate adjustment for factors such as age or underlying disease related to the ocular conditions and mortality could explain the findings. The loss of vision could have a direct effect on mortality if it results in a susceptibility to accidents such as fatal falls or in depression, which has been reported to increase mortality.18,19 Also, ocular disorders, in particular cataract, may be markers of systemic processes that are associated with accelerated physiologic aging and earlier death.9,20 For example, generalized oxidative damage might play a role in the development of cataract and the aging process.
CME course available at www.archophthalmol.com
We undertook this study to determine whether visual impairment, type of lens opacity, cataract surgery, and advanced AMD are associated with overall or cause-specific mortality in participants in the Age-Related Eye Disease Study (AREDS), a long-term study of the clinical course of age-related cataract and AMD. Because AREDS included a randomized controlled trial of high-dose antioxidants and zinc used for a median of 6.5 years, we also examined the relationship between high-dose dietary supplements and mortality.
METHODS
Details of the AREDS design have been published previously.21 AREDS is an ongoing, multicenter study of the clinical course of cataract and AMD. The study included a randomized clinical trial that evaluated the effect of high daily doses of selected oral supplements (vitamin C, 500 mg; vitamin E, 400 IU; beta carotene, 15 mg; and zinc, 80 mg as zinc oxide with 2 mg of cupric oxide) on the incidence and progression of the 2 conditions. A total of 4757 persons aged 55 to 81 years at enrollment were entered into the study at 11 clinical centers between November 13, 1992, and January 15, 1998.
The ocular eligibility requirements were largely determined by the AMD component of AREDS. Stereoscopic color fundus photographs taken at baseline were used to assess macular status for this study. Photographs were assessed using the AREDS system for classifying AMD22 by trained and certified readers at a reading center, who classified each participant into 1 of 4 AMD categories: Category 1, few if any small drusen; Category 2, extensive small drusen or nonextensive intermediate-sized drusen; Category 3, extensive intermediate-sized drusen, at least 1 large druse, or noncentral geographic atrophy; and Category 4, unilateral advanced AMD or unilateral vision loss to worse than 20/32 attributable to AMD.
Except for the requirement that all participants have at least 1 eye with visual acuity of 20/32 or better and that the media be sufficiently clear for reasonable-quality fundus photography, lens opacity status was not otherwise considered in selecting participants. The large sample size requirements for the AMD component of the study and the expected high prevalence of lens opacities in the targeted age group made it likely that a diverse array of age-related lens opacities would be present in the cohort.
The AREDS system for classifying cataracts23 was used to assess the presence and severity of nuclear, cortical, and posterior subcapsular (PSC) lens opacities at baseline. Lens photographs were evaluated at a reading center by trained and certified examiners masked to the participants’ vision status. Nuclear opacities were graded on a decimal scale using cutoff points set by a series of standard slitlamp photographs with increasingly severe nuclear sclerosis. Nuclear opacity grades ranged from 0.9 (severity less than the lowest lens standard photograph) to 6.1 (severity exceeding the highest lens standard photograph). Each participant was classified into 1 of 2 nuclear opacity groups, irrespective of the presence of cortical or PSC opacities: less than grade 4 in both eyes or in the phakic eye if there is a history of unilateral cataract surgery vs grade 4 or higher in at least 1 eye.
The extent of cortical and PSC opacities was graded by estimating the area of lens involvement as seen on retroillumination photographs. Each participant was classified into 1 of 2 cortical opacity groups, irrespective of the presence of nuclear or PSC opacities: 5% or less of the central 5-mm area in both eyes or in the phakic eye if there is a history of unilateral cataract surgery vs greater than 5% of the central 5-mm area in at least 1 eye.
Similarly, each participant was classified into 1 of 2 PSC opacity groups, irrespective of the presence of nuclear or cortical opacities: 5% or less of the central 5-mm area in both eyes or in the phakic eye if there is a history of unilateral cataract surgery vs greater than 5% of the central 5-mm area in at least 1 eye.
Participants were also classified (yes or no) according to whether they had a history of cataract surgery.
Baseline best-corrected visual acuity was measured in each eye according to the ETDRS protocol as the number of letters read correctly, with scores ranging from 0 (<20/800) to 100 (20/10). Each participant was classified into 1 of 2 visual acuity groups: 20/40 or better in both eyes (equivalent to >69 letters) vs worse than 20/40 in 1 eye.
At enrollment, AREDS participants had to be free of any illness or condition that would, in the opinion of the enrolling physician, make long-term follow-up or compliance with use of the study interventions unlikely or difficult. Persons with a history of cancer with a poor prognosis for 7-year survival or a major cardiovascular or cerebrovascular event within the year before enrollment were ineligible for AREDS. Also excluded were persons with more than minimal diabetic retinopathy, previous ocular surgery (except cataract surgery and unilateral photocoagulation for AMD), and the presence of any other eye disease that could complicate assessment of the progression of lens opacities or AMD or that could affect visual acuity (eg, optic atrophy and acute uveitis).
Simple randomization, stratified by AREDS clinical center and AMD category, was used to enroll participants in the 2 × 2 factorial design of antioxidants and zinc. Participants in AMD categories 2 through 4 were assigned with a probability of 0.25 to receive placebo, antioxidants, zinc, or antioxidants and zinc. Participants in AMD Category 1 were assigned with a probability of 0.50 to receive placebo or antioxidants. The potential risks of high-dose zinc supplements24 if given to Category 1 participants were unlikely to be balanced by any possible benefits. This group was at low risk of developing vision loss due to AMD during the planned 5- to 8-year follow-up phase of the clinical trial, and there is no published evidence that taking zinc might reduce the risk of lens opacities.
Data on age, sex, race (white, other), education level (high school or less, more than high school), and smoking status (never, former, current) were available from an interviewer-administered baseline questionnaire. Data on diabetes mellitus, arthritis, angina, and cancer were based on solicited self-report. As part of the clinical trial component of AREDS, participants were given the option of taking a daily multivitamin supplement containing the recommended daily allowance of nutrients (Centrum; Wyeth Consumer Health Care International Inc, Madison, NJ). At the baseline examination, trained observers measured each participant’s height and weight, which were used to calculate body mass index. Hypertension was defined as systolic blood pressure greater than 160 mm Hg or diastolic blood pressure greater than 90 mm Hg or current use of antihypertensive medications. Data on the use of cholesterol-lowering medications, aspirin, or anti-inflammatory medications were also collected.
The primary outcome variable for this study is all-cause mortality. When mortality was reported, death was confirmed and the cause of death was abstracted from hospital records and from death certificates. Cause-specific mortality was based on International Classification of Diseases, Ninth Revision (ICD-9) codes, and analyses were performed for the major, broadly grouped causes of death. Codes were assigned by a certified ICD-9 diagnostic coder and reviewed by a physician medical monitor. A Morbidity and Mortality Committee consisting of 2 physicians and a diagnostic coder reviewed the assigned codes, and discrepancies were resolved by the medical monitor. Hospital discharge summaries were provided when available to assist in the review. The underlying cause of death was usually selected as the primary cause. In the absence of an underlying cause, immediate cause was selected as the primary cause. Cause of death was unknown for 37 deaths without a death certificate or an informative hospitalization history.
Age- and sex-adjusted Cox proportional hazards models predicting mortality were created with AMD category, visual acuity status, nuclear opacity status, cortical opacity status, PSC opacity status, history of cataract surgery, and assigned AREDS treatment at baseline as independent variables. We also constructed age- and sex-adjusted models predicting mortality with the following health status indicators: race, education, smoking status, and body mass index; use of Centrum, aspirm/anti-inflammatory drugs, or cholesterol-lowering medications; and the presence of angina, cancer, diabetes mellitus, hypertension, and arthritis. If a health status indicator adjusted for age and sex was not related to mortality, it was not considered further (P>.05). Significant health status indicators (P<.05) were added to models that examined the effect of each of the ocular characteristics on mortality. In addition, models that included statistically significant covariates and AMD and opacity characteristics simultaneously were assessed. Last, AMD and mortality analyses were conducted on the subset of AREDS participants who did not receive any zinc to evaluate whether the association between AMD and mortality is independent of an association between zinc supplements and mortality. Statistical significance was determined using P=.05, without adjustment for multiple comparisons. All analyses were carried out using SAS version 8.0 (SAS Institute Inc, Gary, NC).
RESULTS
At baseline, the median age of the 4757 AREDS participants was 69 years. Between November 13, 1992, and October 12, 2001, 534 (11%) of the 4753 participants with follow-up data for mortality died. Mortality rates, after adjustment for age and sex, for the median follow-up (6.5 years), are given in Table 1. Adjusted rates are higher for older persons, men, “other” races, those with less formal education, current and former smokers, participants with diabetes mellitus, participants with a lean body mass index, and those diagnosed as having comorbid conditions, such as cancer, hypertension, and angina.
Table 1.
All-Cause Mortality by Selected Nonocular Baseline Characteristics
| Characteristic | Participants, No. (%) | Deaths, No. (%) | Mortality, %* | RR (95% CI)† |
|---|---|---|---|---|
| Age, y | ||||
| 55–64 | 1012 | 44 (4.4) | 4.2 | 1.00 |
| 65–69 | 1586 | 145 (9.1) | 7.6 | 2.02 (1.44–2.83) |
| 70–74 | 1419 | 195 (13.7) | 12.5 | 3.16 (2.27–4.38) |
| 75–81 | 736 | 150 (20.4) | 19.0 | 5.14 (3.67–7.19) |
| Sex | ||||
| F | 2654 | 240 (9.0) | 7.3 | 1.00 |
| M | 2099 | 294 (14.0) | 11.7 | 1.61 (1.35–1.91) |
| Race | ||||
| White | 4546 | 509 (11.2) | 8.9 | 1.00 |
| Other | 207 | 25 (12.1) | 11.6 | 1.69 (1.13–2.52) |
| Education‡ | ||||
| High school or less | 1705 | 240(14.1) | 12.3 | 1.00 |
| More than high school | 3045 | 294 (9.7) | 7.3 | 0.64 (0.53–0.75) |
| Smoking status | ||||
| Never | 2105 | 166 (7.9) | 6.2 | 1.00 |
| Former | 2273 | 292 (12.9) | 10.1 | 1.53 (1.25–1.86) |
| Current | 375 | 76 (20.3) | 18.4 | 3.22 (2.45–4.23) |
| Body mass index§ | ||||
| Bottom 20% | 941 | 128 (13.6) | 11.0 | 1.32 (1.07–1.62) |
| Middle 60% | 2861 | 294 (10.3) | 8.4 | 1.00 |
| Top 20% | 948 | 111 (11.7) | 8.8 | 1.28 (1.03–1.59) |
| Diabetes mellitus | ||||
| No | 4466 | 467 (10.5) | 8.3 | 1,00 |
| Yes | 287 | 67 (23.3) | 20.3 | 2.21 (1.70–2.87) |
| Cancer | ||||
| No | 3910 | 412 (10.5) | 8.5 | 1.00 |
| Yes | 843 | 122 (14.5) | 11.3 | 1.32 (1.08–1.62) |
| Angina | ||||
| No | 4264 | 449 (10.5) | 8.4 | 1.00 |
| Yes | 489 | 85 (17.4) | 14.8 | 1.45 (1.15–1.83) |
| Hypertension | ||||
| No | 2869 | 251 (8.8) | 6.7 | 1.00 |
| Yes | 1884 | 283 (15.0) | 12.9 | 1.73 (1.46–2.05) |
| Arthritis | ||||
| No | 2578 | 285 (11.1) | 8.9 | 1.00 |
| Yes | 2175 | 249 (11.5) | 9.1 | 1.02 (0.86–1.21) |
| Centrum use|| | ||||
| No | 1613 | 193 (12.0) | 9.2 | 1.00 |
| Yes | 3140 | 341 (10.9) | 8.9 | 0.95 (0.80–1.14) |
| Aspirin or anti-inflammatory drug use | ||||
| No | 2932 | 316 (10.8) | 8.7 | 1.00 |
| Yes | 1821 | 218 (12.0) | 9.4 | 1.00 (0.84–1.19) |
| Cholesterol-lowering medication use | ||||
| No | 4337 | 487 (11.2) | 8.9 | 1.00 |
| Yes | 416 | 47 (11.3) | 10.4 | 1.06 (0.78–1.42) |
Abbreviations: CI, confidence interval; RR, relative risk.
Age- and sex-adjusted mortality for median follow-up (6.5 years).
Age- and sex-adjusted risk ratios.
Three participants refused to answer.
Not measured for 3 participants.
Wyeth Consumer Health Care International Inc, Madison, NJ.
All-cause mortality rates increased with increasing severity of macular disease (Table 2). After adjustment for age and sex, participants with advanced AMD in 1 eye (AMD Category 4) compared with participants with few, if any, drusen (AMD Category 1) had a significantly increased risk of mortality, and this association remained statistically significant after adjustment for demographic, lifestyle, and comorbid conditions (relative risk [RR], 1.41; 95% confidence interval [CI], 1.08–1.86).
Table 2.
Association of All-Cause Mortality With Baseline Ocular and Treatment Characteristics
| Characteristic | Participants, No. (%) | Deaths, No. (%) | Mortality, %* | RR (95% CI)† | RR(95%CI)‡ |
|---|---|---|---|---|---|
| AMD Category | |||||
| 1 | 1116 | 86 (7.7) | 6.2 | 1.00 | 1.00 |
| 2 | 1060 | 96(9.1) | 7.3 | 1.11 (0.83–1.49) | 1.09 (0.81–1.46) |
| 3 | 1620 | 176(10.9) | 9.3 | 1.22 (0.94–1.58) | 1.10 (0.85–1.43) |
| 4 | 957 | 176(18.4) | 14.7 | 1.80(1.38–2.34) | 1.41 (1.08–1.86) |
| Visual acuity | |||||
| ≥20/40 OU | 3940 | 376 (9.5) | 7.9 | 1.00 | 1,00 |
| <20/40 in 1 eye | 813 | 158(19.4) | 15.6 | 1.65 (1.36–2.00) | 1.36 (1.12–1.65) |
| Nuclear opacity§ | |||||
| Grade <4 in available eye(s) | 4001 | 403(10.1) | 8.0 | 1.00 | 1.00 |
| Grade ≥4 in at least 1 eye | 617 | 107(17.3) | 15.2 | 1.45 (1.16–1.81) | 1.40 (1.12–1.75) |
| Cortical opacity§ | |||||
| ≤5% in available eye(s) | 4090 | 431(10.5) | 8.3 | 1.00 | 1.00 |
| >5% in at least 1 eye | 524 | 78(14.9) | 13.6 | 1.26 (0.98–1.61) | 1.18 (0.92–1.51) |
| PSC opacity§ | |||||
| ≤5% in available eye(s) | 4503 | 490(10.9) | 8.6 | 1.00 | 1.00 |
| >5% in atleast 1 eye | 111 | 19(17.1) | 16.9 | 1.40 (0.86–2.27) | 1.33 (0.82–2.18) |
| Cataract surgery | |||||
| No | 4465 | 475(10.6) | 8.5 | 1.00 | 1.00 |
| Yes | 288 | 59(20.5) | 19.5 | 1.66 (1.26–2.19) | 1.55 (1.18–2.05) |
| AREDS treatment|| | |||||
| Antioxidant main effect | |||||
| No antioxidants | 1806 | 211(11.7) | 9.8 | 1.00 | 1.00 |
| Antioxidants | 1831 | 237(12.9) | 10.1 | 1.15 (0.96–1.39) | 1.17 (0.97–1.41) |
| Zinc main effect | |||||
| No zinc | 1847 | 257(13.9) | 10.9 | 1.00 | 1.00 |
| Zinc | 1790 | 191 (10.7) | 8.9 | 0.76 (0.63–0.92) | 0.73 (0.61–0.89) |
| Individual treatment groups | |||||
| Placebo | 903 | 117(13.0) | 10.9 | 1.00 | 1.00 |
| Antioxidants alone | 944 | 140(14.8) | 10.8 | 1.19 (0.93–1.52) | 1.15 (0.90–1.48) |
| Zinc alone | 903 | 94(10.4) | 8.5 | 0.79 (0.60–1.03) | 0.72 (0.55–0.95) |
| Antioxidants plus zinc | 887 | 97(10.9) | 9.2 | 0.88 (0.67–1.15) | 0.86 (0.65–1.12) |
Abbreviations: AMD, age-related macular degeneration; AREDS, Age-Related Eye Disease Study; CI, confidence interval; PSC, posterior subcapsular; RR, relative risk.
Age- and sex-adjusted mortality for median follow-up (6.5 years).
Age- and sex-adjusted risk ratios.
Adjusted for statistically significant covariates: age, sex, race, education, smoking status, body mass index, diabetes mellitus, angina, cancer, and hypertension.
There were 135 participants without slitlamp photographs and 139 participants without retroillumination photographs.
Includes 3637 AMD Category 2,3, and 4 participants only.
In analyses adjusted for age, sex, and statistically significant covariates, the presence of nuclear opacity in at least 1 eye was associated with a statistically significant increased risk in all-cause mortality (RR, 1.40; 95% CI, 1.12–1.75). A possible increased risk was also found for cortical (RR, 1.18; 95% CI, 0.92–1.51) and PSC (RR, 1.33; 95% CI, 0.82–2.18) opacities, but these increases were not statistically significant. Cataract surgery was statistically significantly associated with an increased risk of all-cause mortality (RR, 1.55; 95% CI, 1.18–2.05).
Among AREDS participants eligible to be randomly assigned to receive zinc (AMD Categories 2,3, and 4), age- and sex-adjusted all-cause mortality, as given in Table 2, was statistically significantly reduced in the main effects analyses for participants randomly assigned to receive any zinc (zinc alone group and antioxidants plus zinc group) compared with participants not randomly assigned to receive zinc (placebo group and antioxidants alone group) (RR, 0.76; 95% CI, 0.63–0.92). The relationship persisted after adjustment for statistically significant covariates (RR, 0.73; 95% CI, 0.61–0.89). No statistical association was found with randomization to receive high-dose antioxidants and mortality whether assessing the antioxidants main effect or the antioxidants alone effect. Covariate-adjusted survival curves for AREDS treatment and statistically significant ocular characteristics are shown in the Figure. The comparison of individuals randomly assigned to receive zinc alone vs those assigned to receive placebo is statistically significant only after adjustment for all covariates (RR, 0.72; 95% CI, 0.55–0.95).
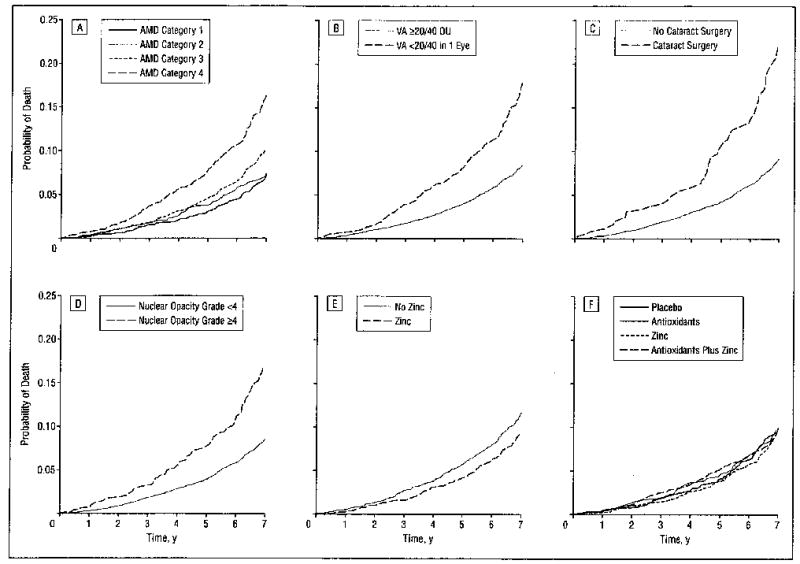
Covariate-adjusted estimates of the probability of all-cause mortality by age-related macular degeneration (AMD) Category (A), visual acuity (VA) (B), cataract surgery (C), nuclear opacity grade (D), zinc treatment (E), and Age-Related Eye Disease Study treatment (F).
The AREDS clinical trial results reported25 that individuals randomly assigned to receive antioxidants and zinc reduced their risk of progression to AMD and loss of visual acuity. To examine whether the association between AMD and mortality was independent of the association found for zinc and mortality, an analysis was conducted assessing AMD and mortality in the subgroup of AREDS participants in AMD Categories 2 through 4 not randomly assigned to receive any zinc-containing supplement along with participants in AMD Category 1. In this subgroup of participants, advanced AMD remained a statistically significant risk factor for mortality in a covariate-adjusted model (RR, 1.53; 95% CI, 1.13–2.07).
Compared with participants having better than 20/40 visual acuity in both eyes, those with visual acuity worse than 20/40 in 1 eye had a statistically significantly increased risk of all-cause mortality (RR, 1.36; 95% CI, 1.12–1.65). Ninety-one percent of participants who had reduced vision at baseline (visual acuity <20/40 in 1 eye) were in AMD Category 4. Thus, the association between visual impairment and mortality cannot be distinguished from the association between AMD and mortality.
When AMD category and “any” lens opacity (defined as nuclear, cortical, or PSC opacity or cataract surgery) are included in a covariate-adjusted model (model 1), AMD Category 4 and “any” opacity are statistically significantly associated with all-cause mortality (Table 3). Two additional models were developed to determine whether advanced AMD along with specific lens opacities are particularly associated with mortality. In model 2, nuclear opacity replaces any lens opacity from model 1 and, along with AMD Category 4, remains statistically significant. Finally, in model 3, cataract surgery replaces any lens opacity from model 1 and, along with AMD Category 4, remains statistically significantly associated with mortality in a multivariate model.
Table 3.
Multivariate Adjusted Models Assessing All Ocular Variables
| Characteristic | RR (95% CI)* | |
|---|---|---|
| Full model 1† | ||
| AMD Category 2 | 1.11 (0.83–1.49) | |
| AMD Category 3 | 1.09 (0.84–1.42) | |
| AMD Category 4 | 1.38 (1.05–1.82) | |
| Any opacity‡ | 1.34 (1.12–1.61) | |
| Full model 2§ | ||
| AMD Category 2 | 1.09 (0.82–1.47) | |
| AMD Category 3 | 1.11 (0.85–1.45) | |
| AMD Category 4 | 1.38 (1.04–1.81) | |
| Nuclear grade ≥4 in at least 1 eye | 1.39 (1.11–1.73) | |
| Full model 3|| | ||
| AMD Category 2 | 1.08 (0.81–1.45) | |
| AMD Category 3 | 1.07 (0.82–1.39) | |
| AMD Category 4 | 1.35 (1.03–1.78) | |
| Cataract surgery | 1.49 (1.12–1.97) | |
Abbreviations: AMD, age-related macular degeneration; CI, confidence interval; RR, relative risk.
Adjusted for statistically significant covariates: age, sex, race, education, smoking status, body mass index, diabetes mellitus, angina, cancer, and hypertension.
Covariate-adjusted Cox proportional hazards model including AMD category and any opacity.
Includes nuclear, cortical, and posterior subcapsular opacity types and cataract surgery.
Covariate-adjusted Cox proportional hazards model including AMD category and nuclear opacity.
Covariate-adjusted Cox proportional hazards model including AMD category and cataract surgery.
The ICD-9 codes for cause of death were available for 497 of the deaths (93%). Most of these deaths were from diseases of the circulatory system (40%) and neoplasms (34%). We grouped all other causes of death, except “unknown” (n=37), into an “other” category (27%). The specific causes of death for each of these broad categories are given in Table 4.
Table 4.
Cause-Specific Mortality
| Cause of Death | Deaths, No. (n = 534) |
|---|---|
| Circulatory system | |
| Ischemic heart disease | 77 |
| Other forms of heart disease | 48 |
| Cerebrovascular disease | 30 |
| Heart failure | 24 |
| Disease of arteries | 8 |
| Pulmonary circulation | 7 |
| Other circulatory diseases | 2 |
| Hypertensive disease | 1 |
| Subtotal | 197 |
| Neoplasms | |
| Lung | 43 |
| Colorectal | 17 |
| Pancreatic | 13 |
| Prostate | 11 |
| Leukemia | 11 |
| Lymphoma | 8 |
| Brain | 7 |
| Breast | 6 |
| Bladder | 5 |
| Kidney | 5 |
| Ovarian | 5 |
| Esophagus | 3 |
| Liver | 3 |
| Skin | 3 |
| Stomach | 3 |
| Other neoplasms | 24 |
| Subtotal | 167 |
| Other | |
| Respiratory system | 50 |
| Infectious and parasitic diseases | 16 |
| Injury | 15 |
| Genitourinary system | 13 |
| III-defined conditions | 12 |
| Digestive system | 11 |
| Nervous system | 10 |
| Endocrine diseases | 3 |
| Mental disorders | 2 |
| Blood and blood-forming organs | 1 |
| Subtotal | 133 |
| Unknown | 37 |
In covariate-adjusted analyses of cause-specific mortality, AMD Category 4 was statistically significantly associated with cardiovascular deaths (RR, 1.92; 95% CI, 1.18–3.12) (Table 5). Nuclear opacity (RR, 1.56; 95% CI, 1.05–2.31) and cataract surgery (RR, 2.29; 95% CI, 1.45–3.60) were statistically significantly associated with cancer deaths. Nuclear opacity was also statistically significantly associated with “other” deaths (RR, 1.64; 95% CI, 1.07–2.51).
Table 5.
Associations of Cause-Specific Mortality With Baseline Ocular and Treatment Characteristics
|
RR (95% Cl)* |
|||
|---|---|---|---|
| Characteristic | Circulatory System | Neoplasms | Other Causes |
| AMD category | |||
| 1 | 1.00 | 1.00 | 1.00 |
| 2 | 1.41 (0.83–2.39) | 1.09 (0.65–1.84) | 0.60 (0.34–1.07) |
| 3 | 1.45 (0.90–2.33) | 1.16 (0.73–1.85) | 0.75 (0.47–1.21) |
| 4 | 1.92 (1.18–3.12) | 1.50 (0.92–2.44) | 0.97 (0.59–1.60) |
| Nuclear opacity | |||
| Grade <4 in available eye(s) | 1.00 | 1.00 | 1.00 |
| Grade ≥4 in at least 1 eye | 1.19 (0.82–1.73) | 1.56 (1.05–2.31) | 1.64 (1.07–2.51) |
| Cortical opacity | |||
| ≤5% In available eye(s) | 1.00 | 1.00 | 1.00 |
| >5% In at least 1 eye | 1.26 (0.86–1.86) | 1.41 (0.92–2.17) | 0.82 (0.47–1.45) |
| PSC opacity | |||
| ≤5% In available eye(s) | 1.00 | 1.00 | 1.00 |
| >5% in at least 1 eye | 1.78 (0.91–3.50) | 0.91 (0.31–2.70) | 1.63 (0.66–4.05) |
| Cataract surgery | |||
| No | 1.00 | 1.00 | 1.00 |
| Yes | 1.21 (0.75–1.93) | 2.29 (1.45–3.60) | 1.18 (0.63–2.21) |
| AREDS treatment† | |||
| Antioxidant main effect | |||
| No antioxidants | 1.00 | 1.00 | 1.00 |
| Antioxidants | 1.35 (0.99–1.84) | 1.18 (0.84–1.66) | 0.83 (0.56–1.24) |
| Zinc main effect | |||
| No zinc | 1.00 | 1.00 | 1.00 |
| Zinc | 0.77 (0.57–1.05) | 0.78 (0.56–1.09) | 0.64 (0.43–0.96) |
Abbreviations: AMD, age-related macular degeneration; AREDS, Age-Related Eye Disease Study; CI, confidence interval; PSC, posterior subcapsular; RR, relative risk.
Adjusted for statistically significant covariates: age, sex, race, education, smoking status, body mass index, diabetes mellitus, angina, cancer, and hypertension.
Includes AMD Category 2, 3, and 4 participants only.
Given the suggestion of a mortality benefit for participants supplementing with zinc, analysis of cause-specific mortality in AMD Category 2 through 4 participants was performed. Zinc supplementation did not show a protective association with circulatory or neoplasm causes of death. A protective effect was found for “other” causes (RR, 0.64; 95% CI, 0.43–0.96). Further analysis of this group found a nonsignificant protective effect for respiratory causes, the largest subgroup of the other causes (RR, 0.78; 95% CI, 0.41–1.47). No cause-specific associations of antioxidants with mortality were found.
COMMENT
All-cause mortality was 11% during a median of 6.5 years of follow-up and was increased in AREDS participants in AMD Category 4 and in those with nuclear lens opacities or a history of cataract surgery when potentially confounding covariates were taken into account. No statistically significant associations with all-cause mortality were noted for persons with less severe age-related macular changes (AMD Categories 2 and 3), cortical lens opacities, or PSC lens opacities.
In an analysis of cause-specific mortality, AMD Category 4 was associated with an increased risk of death caused by diseases of the circulatory system. Persons with nuclear opacities or a history of cataract surgery had increased mortality from neoplasms and “other” causes compared with persons without these lens-related characteristics.
A statistically significant predictor of mortality in AREDS was AMD Category 4. Three population-based studies6,8,9 have reported no association between mortality and AMD, but each had few participants with advanced AMD. With eligibility criteria that permitted visual impairment only in 1 eye (all AREDS participants had at least 1 eye with 20/32 or better visual acuity at baseline), we noted an association between impaired vision and increased mortality. However, the AREDS mortality findings for visual acuity and AMD were not independent. Ninety-one percent of participants in AREDS who had visual acuity less than 20/40 at baseline in 1 eye were in AMD Category 4. This explains the similarity of findings for AMD and visual impairment and mortality.
Large population-based studies have reported associations between impaired vision and increased mortality. In 2 of these studies,6,9 vision impairment was defined as corrected acuity of 20/40 or worse in the better eye. In age- and sex-adjusted analyses, the Beaver Dam Eye Study9 noted a statistically significant 57% increase in mortality for persons with impaired vision, but the finding was not significant after adjustment for various systemic characteristics. The 70% increased mortality risk for persons with visual impairment in the Blue Mountains Eye Study6 was statistically significant, even after adjustment for potentially important covariates. Data from that study suggested that the visual impairment finding was independent of the presence of cataract. The Melbourne Visual Impairment Project8 noted a significant increase in mortality among persons with mild visual impairment, defined as best-corrected acuity less than 6/12. Data from the National Health Interview Survey,17 which uses self-reports of visual impairment and ocular disease, suggest that after adjustment for various covariates, including the presence of cataract, glaucoma, and retinopathy (but not AMD), women who reported bilateral severe visual impairment (bilateral “blindness”) and lesser degrees of visual impairment, compared with women who reported no impairment, had significantly higher all-cause and cardiovascular disease–related mortality. Since data on AMD were not collected in the National Health Interview Survey, it was not possible to examine whether the presence of AMD was related to the visual impairment findings.
We found that nuclear opacities and a history of cataract surgery were associated with decreased survival. Unlike the case for AMD, the cataract variables were less closely linked with visual impairment (visual acuity <20/40 in the worse eye) in the AREDS population. Only 20% of the visually impaired individuals had nuclear opacities, and 24% of those with nuclear opacities had visual impairment
Our findings for nuclear cataract are largely consistent with those from earlier studies. Five population-based studies6,7,9–11 have reported that nuclear opacities are important predictors of mortality independent of covariate adjustment. The fact that the association between nuclear cataract and mortality has been noted across studies of different racial groups in different geographical areas and using different cataract classification systems strengthens the validity of the observation that cataracts, in particular nuclear cataracts, are associated with decreased survival. Our finding that survival might be decreased for persons with a history of cataract surgery, however, is not consistently supported by earlier studies. Higher mortality has been reported1–5 for persons undergoing cataract surgery compared with those in the general population or those undergoing other elective surgical procedures. On the other hand, several population-based studies6–8 that reported relationships between cataract and decreased survival found no relationship between mortality and history of cataract surgery at baseline. The many selective factors, including the presence of co-morbid conditions, that determine whether a person undergoes an elective procedure such as cataract surgery make it difficult to interpret cataract surgery and mortality data.
As in several other studies7,10,26 that have examined cause-specific mortality, we found that persons with nuclear opacities were at higher risk of death from neoplasms. In the Salisbury Eye Evaluation Project,7 mixed cataracts that included a nuclear component were associated with a more than 2-fold increase in risk of death from cancer. Similarly, the Barbados Eye Studies10 reported that compared with persons without cataract, persons with mixed and “any” nuclear opacities tended to have a higher risk of death from neoplasms. In a follow-up study of persons who had participated in the Italian-American Case-Control Study of Age-related Cataract,26 persons with mixed cataract had a significantly increased RR of mortality from malignancies. None of these published studies, including ours, found a statistically significant association between lens opacities and death caused by circulatory diseases, although such an association has been suggested in a study5 of persons with cataract extraction.
It is not clear how ocular disorders might be related to decreased survival. One possibility is that adjustment for important comorbid conditions may have been incomplete. A hypothesis suggested by this study, and supported at least in part by other studies, is that nuclear cataract and AMD might each be associated with a major cause of death, that is, cancer and diseases of the circulatory system, respectively. This study and others7,10,26 suggest a link between nuclear cataract and cancer mortality. Even when history of cancer at the baseline examination was included as a covariate in the full model, nuclear cataract was significantly associated with increased mortality. However, adjustment for cancer was probably incomplete because limited life expectancy from diseases such as cancer was an exclusion criterion for the study, and, therefore, most life-threatening cancers probably became manifest subsequent to the baseline examination. It is possible that similarities in the developmental processes of the 2 conditions or the development of cataract as a by-product of cancer therapy (an explanation suggested by West et al7) could explain the link. The association we noted between AMD and all-cause mortality and death from circulatory diseases may be explained by an underlying vascular basis for AMD, a hypothesis suggested by some epidemiologic studies.27–30 Even with adjustment for some risk factors possibly associated with cardiovascular death and AMD (ie, age, smoking, and hypertension), the relationship persisted, but, as for cataract, adjustment may have been incomplete.
Other investigators6,7 have raised the possibility of a more direct effect of vision disorders on mortality, perhaps from the depression and dependency that can accompany vision loss. Depression has been associated with earlier death.18,19 The AREDS eligibility requirement that participants have at least 1 eye with 20/32 or better vision may have decreased the possibility of ocular-related depression. Also, an increased risk of falls and automobile accidents in the visually impaired might result in excess mortality. There were few deaths from accidents in our cohort, and our cause-specific analyses showed no evidence that the excess mortality we noted for persons with the ocular conditions was due to death from accidents.
Persons randomly assigned to receive zinc either alone or in combination with antioxidants compared with persons not randomly assigned to receive zinc (placebo or antioxidants alone) had significantly improved survival from all causes. The positive association of zinc with decreased all-cause mortality did not seem to be due to reduction in deaths involving cardiovascular/circulatory disease or cancer (Table 5). Other researchers31 have reported a beneficial effect of moderate doses of zinc and selenium in improving immunity and resistance to infections in an elderly institutionalized population. It is possible that the beneficial effects of zinc on mortality in this study may be related to an improved immune response, which is known to decrease with aging. A separate cause-specific analysis found that the zinc effect was in the protective direction for respiratory-specific mortality compared with no zinc, but this finding was not statistically significant (RR, 0.78; 95% CI, 0.41–1.47). We know that in the AREDS population, an intake of 80 mg of zinc oxide and 2 mg of cupric oxide, with or without antioxidant vitamins, for 5 years resulted in a median increase in serum zinc levels of 17% compared with 2% for participants not assigned to receive zinc. Whether zinc supplementation has an effect on mortality cannot be determined from this study alone. Additional studies are necessary before any conclusions can be made regarding the health benefits of zinc supplementation.
Strengths of this study include the large number of participants with the ocular disorders of interest (in particular advanced AMD), the use of standardized techniques for diagnosing the eye conditions, the relatively long follow-up, the near complete collection of mortality data, the ability to perform cause-specific analyses because of the large number of deaths, and the availability of data on potentially important covariates. A concern in interpreting the findings, as in all clinic-based studies, was the potential for bias, in particular selection bias. The control group for AMD (Category 1) and persons with intermediate-sized drusen or extensive small drusen (Category 2) were substantially more likely to have been volunteers from nonmedical sources. It has been shown that volunteers for prevention studies have more formal education, are more health conscious, and are more often employed in professional and skilled positions. Indeed, persons with no drusen to intermediate-sized drusen at enrollment had greater educational achievement and smoked less than persons in AMD Categories 3 and 4. There is less concern that selection bias might explain the cataract findings because the cataract cases were more evenly distributed across the AMD categories, making the distribution of volunteers more comparable among those with and without cataract. Inclusion of AMD category in the cataract analyses should also have compensated for residual imbalances in the distribution of volunteers among those with and without cataract.
This is the first large randomized trial to report a potential benefit of the use of high doses of zinc on survival. Other randomized studies of zinc supplementation and mortality are needed to confirm these findings. The ocular results of this study are consistent with those of other studies showing a potential link between various ocular disorders and survival. Cumulative evidence from clinic- and population-based studies suggests that cataract and AMD may reflect systemic rather than only local processes.
AREDS Research Group
The Eye Center at Memorial (Albany, NY)
Principal Investigator: Aaron Kassoff, MD; Co-Investigator: Jordan Kassoff, MD; Clinic Coordinators: JoAnne Buehler; Mary Eglow, RN; Susan Silverman; Photographer: Michel Mehu (Past Participating Personnel: Co-Investigator: Shalom Kieval, MD; Clinic Coordinator: Francine Kaufman; Examiner: Michael Mairs, MD; Photographers: Barbara Graig, RN; Andrea Quattrocchi; Technicians: Denise Jones; Joan Locatelli, RN)
Associated Retinal Consultants, PC (Royal Oak, Mich)
Principal Investigator: Alan Ruby, MD; Co-Investigators: Antonio Capone, Jr, MD; Bruce Garretson, MD; Tarek Hassan, MD; Michael T. Trese, MD; George A. Williams, MD; Clinic Coordinators: Virginia Regan, RN; Patricia Manatrey, RN; Photographers: Patricia Streasick; Lynette Szydlowski; Fran McIver; Craig Bridges; Technicians: Cheryl Stanley; Kristi Cumming, RN; Beth Mitchell, RN; Joanne Holloway, RN; Bobbie Lewis, RN; Mary Zajechowski (Past Participating Personnel: Principal Investigator: Raymond R. Margherio, MD [deceased]; Co-Investigators: Morton S. Cox, MD; Jane Camille Werner, MD; Photographers: Rachel Falk; Patricia Siedlak; Technician: Cheryl Neubert, RN)
Devers Eye Institute (Portland, Ore)
Principal Investigator: Michael L. Klein, MD; Co-Investigators: J. Timothy Stout, MD, PhD; Andreas K. Lauer, MD; Clinic Coordinator: Carolyn Beardsley; Photographers: Hiroko Anderson; Patrick Wallace; Technicians: Garland Smith; Shannon Howard (Past Participating Personnel: Principal Investigator: Richard F. Dreyer, MD; Co-Investigators: Colin Ma, MD; Richard G. Chenoweth, MD; John D. Zilis, MD; Adrian O’Malley, MD; Joseph E. Robertson, MD; David J. Wilson, MD; Photographers: Milton Johnson; Patrick Rice; Howard Daniel; Technicians: Harold Crider; Sheryl Parker; Kathryn Sherman)
Emory University (Atlanta, Ga)
Principal Investigator: Daniel F. Martin, MD; Co-Investigators: Thomas M. Aaberg Sr, MD; G. Baker Hubbard, MD; Enrique Garcia, MD; Clinic Coordinator: Linda T. Curtis; Photographers: Alex DeLeon; Bob Myles; Research Associate: Hannah Yi (Past Participating Personnel: Principal Investigators: Antonio Capone Jr, MD; Michael Lambert, MD; Travis Meredith, MD; Co-Investigators: Thomas M. Aaberg Jr, MD; Paul Sternberg, Jr, MD; David Saperstein, MD; Jennifer I. Lim, MD; Clinic Coordinators: Barbara Stribling; Bora Ju; Photographers: Denise Armiger; Jim Gilman; Debbie Jordan; Sandra Strittman; Ray Swords
Ingalls Memorial Hospital (Harvey, Ill)
Principal Investigator: David H. Orth, MD; Co-Investigators: Timothy P. Flood, MD; Joseph Civantos, MD; Serge deBustros, MD; Kirk H. Packo, MD; Pauline T. Merrill, MD; Jack A. Cohen, MD; David Chow, MD; Clinic Coordinators: Celeste Figliulo; Chris Morrison; Photographers: Douglas A. Bryant; Don Doherty; Marian McVicker (Past Participating Personnel: Technician: Tana Drefcinski)
Massachusetts Eye and Ear Infirmary (Boston, Mass)
Principal Investigator: Johanna M. Seddon, MD, ScM; Co-Investigator: Michael K. Pinnolis, MD; Clinic Coordinators: Mala Sachdeva; Tatyana Taytsel; Ilene Burton; Photographers: David Walsh; Charlene Callahan; Technicians: Claudia Evans, OD (Past Participating Personnel: Clinic Coordinators: Kristin K. Snow, MS; Desiree A. Jones-Devonish; Valerie D. Crouse, MS; N. Jennifer Rosenberg, RN, MPH; Nancy Davis; Photographer: Jennifer Dubois-Moran)
National Eye Institute Clinical Center (Bethesda, Md)
Principal Investigator: Emily Y. Chew, MD; Co-Investigators: Karl Csaky, MD, PhD; Frederick L. Ferris III, MD; Clinic Coordinators: Katherine Hall Shimel, RN; Merria A. Woods; Photographers: Denise Cunningham; Ernest M. Kuehl; Marilois Palmer; Technicians: Gloria Babilonia-Ayukawa, RN, MHCA; Guy E. Foster; Young Ja Kim, RN; Iris J. Kivitz; Dessie Koutsandreas; Antoinette LaReau; Richard F. Mercer; Roula Nashwinter; John Rowan; Greg Short (Past Participating Personnel: Clinic Coordinator: Sally A. McCarthy, RN, MSN; Photographer: Patrick F. Ciatto; Technicians: Leanne M. Ayres; Linda Goodman; Patrick Lopez; Cheryl Perry; Anne Randalls)
University of Pittsburgh (Pittsburgh, Pa)
Principal Investigator: Thomas R. Friberg, MD, MS; Co-Investigators: Andrew W. Eller, MD; Michael B. Gorin, MD, PhD; Clinic Coordinator: Barbara Mack; Photographers: Diane Y. Curtin; Phyllis P. Ostroska; Edward Fijewski (Past Participating Personnel: Clinic Coordinators: Jane Alexander; Shannon Nixon; Technicians: Melissa K. Paine; Patricia S. Corbin; Photographer: Joseph Warnicki)
Wilmer Eye Institute, Johns Hopkins University School of Medicine (Baltimore, Md)
Principal Investigator: Susan B. Bressler, MD; Co-Investigators: Neil M. Bressler, MD; Gary Cassel, MD; Daniel Finkelstein, MD; Morton Goldberg, MD; Julia A. Haller, MD; Lois Ratner, MD; Andrew P. Schachat, MD; Steven H. Sherman, MD; Janet S. Sunness, MD; Clinic Coordinators: Sherrie Schenning; Catherine Sackett, RN; Photographers: Dennis Cain; David Emmert; Mark Herring; Jacquelyn McDonald; Rachel Falk; Technician: Stacy Wheeler (Past Participating Personnel: Clinic Coordinator: Mary Mcmillan; Photographer: Terry George)
Elman Retina Group, PA (Baltimore, Md)
Principal Investigator. Michael J. Elman, MD; Co-Investigators: Rex Ballinger, OD; Arturo Betancourt, MD; Michael Herr, MD; Joyce Lammlein, MD; Robert Z. Raden, MD; Ronald Seff, MD; Martin Shuman, MD; Clinic Coordinators: JoAnn Starr; Dena Firestone; Michelle Sloan; Photographers: Peter Sotirakos; Theresa Cain; Technician: Terri Mathews (Past Participating Personnel: Co-Investigators: David Glasser, MD; Dahlia Hirsch, MD; Daniel Killingsworth, MD; Paul Kohlhepp, MD; Clinic Coordinators: Christine Ringrose; Anita Carrigan)
University of Wisconsin (Madison, Wis)
Co-Principal Investigators: Suresh R. Chandra, MD;Justin L. Gottlieb, MD; Co-Investigators: Michael S. Ip, MD; Ronald Klein, MD, MPH; T. Michael Nork, MD, MS; Thomas S. Stevens, MD; Barbara A. Blodi, MD; Michael Altaweel, MD; Barbara E. K. Klein, MD; Matthew D. Davis, MD; Clinic Coordinators: Michelle Olson; Alyson Skoldberg; Erika Christiansen; Barbara Soderling; Jennie R. Perry-Raymond; Kathryn Burke; Photographers: Gene Knutson; John Peterson; Denise Krolnik; Technicians: Robert Harrison; Guy Somers, RN (Past Participating Personnel: Principal Investigator: Frank L. Myers, MD; Co-Investigators: Ingolf Wallow, MD; Timothy W. Olsen, MD; George Bresnik, MD; G. De Venecia, MD; Clinic Coordinators: Tracy Perkins, MPH; Wendy Walker; Jennifer L. Miller; Margo Blatz; Photographers: Michael Neider; Hugh D. Wabers; Greg Weber; Technicians: Beth Amspaugh; Jennifer Buechner; Helen E. Lyngaas Myers)
University of Wisconsin-Reading Center (Madison, Wis)
Co-Principal Investigators: Matthew D. Davis, MD; Barbara E. K. Klein, MD, MPH; Ronald Klein, MD, MPH; Co-Investigators: Barbara Blodi, MD; Ronald Danis, MD; Larry Hubbard; Photography Protocol Monitors: Michael Neider; Pamela Vargo; Hugh D. Wabers; Senior Grader: Jane Armstrong; Graders: Wendy Benz; Kristi L. Dohm; Christina Fink; Trina Harding; Cynthia Hurtenbach; Kristine Lang; Susan Reed; Statisticians: Marian R. Fisher, PhD; Ronald Gangnon, PhD; Li-Yin Lee; Head of Computing and Statistician: Alistair Carr; Computing Staff: James Baliker; Data Manager: Linda Kastorff; Associate Director, Operations: Nancy Robinson; Research Project Manager. Kathleen E. Glander; Grants/Contracts Administrator: Jean Surfus (Past Participating Personnel: Senior Graders: Sarah Ansay; Yvonne L. Magli; Graders: Darlene Badal; Shirley Craanen; Julee Elledge; Barbara Esser; Patricia L. Geithman; Kathleen D. Miner; James Reimers; Mary Webster; Statisticians: Chunyang Gai; William King; Computing Staff: Kurt Osterby; Administration Program Specialist: James Onofrey; Coordination Staff: Judith Brickbauer)
Centers for Disease Control and Prevention-Central Laboratory (Atlanta, Ga)
Rosemary L. Schleicher, PhD; Dayton T. Miller, PhD; Anne L. Sowell, PhD; Elaine W. Gunter, MT (Past Participating Personnel: Barbara A. Bowman, PhD)
Coordinating Center-The EMMES Corporation (Rockville, Md)
Principal Investigators: Anne S. Lindblad, PhD; Roy C. Milton, PhD; Traci E. Clemons, PhD; Co-Investigators: Gary Gensler, MS; Molly Rankin, MS; Genetics Monitor: Alice Henning, MS; Ophthalmic Training & Certification: Gary Entler; Project Manager: Wendy McBee, MA; Database Administrators: Valerie Watson; Candice Davis; Elaine Stine; Computer Analysts: Stuart H. Berlin; Kumar Thotapally; Administration: Michelle Jackson (Past Participating Personnel: Administration: Kate Tomlin; Sophia Pallas; Phyllis R. Scholl; Susan A. Mengers; Co-Investigators: Fred Ederer, MA, FACE; Ravinder Anand, PhD; Protocol Monitor: Kiana Roberts)
National Eye Institute Project Office (Bethesda, Md)
Study Chairman and Principal Investigator: Frederick L. Ferris III, MD; Co-Investigators: Robert D. Sperduto, MD; Natalie Kurinij, PhD; Emily Y. Chew, MD; John Paul SanGiovanni, ScD
Footnotes
The Writing Team is from The EMMES Corp, Rockville, Md (Dr Clemons); the National Eye Institute, Bethesda, Md (Drs Kurinij and Sperduto); and the Johns Hopkins Medical Institutions, Baltimore (Dr Bressler).
This study was supported by contracts from the National Eye Institute, National Institutes of Health, Department of Health and Human Services, with additional support from Bausch & Lomb Inc, Rochester, NY.
References
- 1.Street DA, Javitt JC. National five-year mortality after inpatient cataract extraction. Am J Ophthalmol. 1992;113:263–268. doi: 10.1016/s0002-9394(14)71577-6. [DOI] [PubMed] [Google Scholar]
- 2.Benson WH, Farber ME, Caplan RJ. Increased mortality rates after cataract surgery: a statistical analysis. Ophthalmology. 1988;95:1288–1292. doi: 10.1016/s0161-6420(88)33019-8. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch RP, Schwartz B. Increased mortality among elderly patients undergoing cataract extraction. Arch Ophthalmol. 1983;101:1034–1037. doi: 10.1001/archopht.1983.01040020036004. [DOI] [PubMed] [Google Scholar]
- 4.Ninn-Pedersen K, Stenevi U. Cataract patients in a defined Swedish population 1986–90, VII: inpatient and outpatient standardized mortality ratios. Br J Ophthalmol. 1995;79:1115–1119. doi: 10.1136/bjo.79.12.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu FB, Hankinson SE, Stampfer MJ, et al. Prospective study of cataract extraction and risk of coronary heart disease in women. Am J Epidemiol. 2001;153:875–881. doi: 10.1093/aje/153.9.875. [DOI] [PubMed] [Google Scholar]
- 6.Wang JJ, Mitchell P, Simpson JM, Gumming RG, Smith W. Visual impairment, age-related cataract, and mortality. Arch Ophthalmol. 2001;119:1186–1190. doi: 10.1001/archopht.119.8.1186. [DOI] [PubMed] [Google Scholar]
- 7.West SK, Munoz B, Istre J, et al. Mixed lens opacities and subsequent mortality. Arch Ophthalmol. 2000;118:393–397. doi: 10.1001/archopht.118.3.393. [DOI] [PubMed] [Google Scholar]
- 8.Taylor HR, McCarty CA, Nanjan MB. Vision impairment predicts five-year mortality. Trans Am Ophthalmol Soc. 2000;98:91–99. [PMC free article] [PubMed] [Google Scholar]
- 9.Klein R, Klein BEK, Moss SE. Age-related eye disease and survival: the Beaver Dam Eye Study. Arch Ophthalmol. 1995;113:333–339. doi: 10.1001/archopht.1995.01100030089026. [DOI] [PubMed] [Google Scholar]
- 10.Hennis A, Wu SY, Li X, Nemesure B, Leske MC the Barbados Eye Studies Group. Lens opacities and mortality: the Barbados Eye Studies. Ophthalmology. 2001;108:498–504. doi: 10.1016/s0161-6420(00)00542-x. [DOI] [PubMed] [Google Scholar]
- 11.Thompson JR, Sparrow JM, Gibson JM, Rosenthal AR. Cataract and survival in an elderly nondiabetic population. Arch Ophthalmol. 1993;111:675–679. doi: 10.1001/archopht.1993.01090050109039. [DOI] [PubMed] [Google Scholar]
- 12.Minassian D, Mehra V, Johnson G. Mortality and cataract: findings from a population-based longitudinal study. Bull World Health Organ. 1992;70:219–223. [PMC free article] [PubMed] [Google Scholar]
- 13.Podgor MJ, Cassel GH, Kannel WB. Lens changes and survival in a population-based study. N Engl J Med. 1985;313:1438–1444. doi: 10.1056/NEJM198512053132303. [DOI] [PubMed] [Google Scholar]
- 14.Cohen DL, Neil HA, Sparrow J, Thorogood M, Mann JI. Lens opacity and mortality in diabetes. Diabet Med. 1990;7:615–617. doi: 10.1111/j.1464-5491.1990.tb01459.x. [DOI] [PubMed] [Google Scholar]
- 15.Klein R, Moss S, Klein B, DeMets D. Relation of ocular and systemic factors to survival in diabetes. Arch Intern Med. 1989;149:266–272. [PubMed] [Google Scholar]
- 16.Klein R, Klein BEK, Moss SE, Cruickshanks K. Associations of ocular disease and mortality in a diabetic population. Arch Ophthalmol. 1999;117:1487–1495. doi: 10.1001/archopht.117.11.1487. [DOI] [PubMed] [Google Scholar]
- 17.Lee DJ, Gómez-Marin O, Lam BL, Zheng DD. Visual acuity impairment and mortality in US adults. Arch Ophthalmol. 2002;120:1544–1550. doi: 10.1001/archopht.120.11.1544. [DOI] [PubMed] [Google Scholar]
- 18.Whooley MA, Browner WS. Association between depressive symptoms and mortality in older women: Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1998;158:2129–2135. doi: 10.1001/archinte.158.19.2129. [DOI] [PubMed] [Google Scholar]
- 19.Ariyo AA, Haan T, Tangen CM, et al. Depressive symptoms and risks of coronary heart disease and mortality in elderly Americans: Cardiovascular Health Study Collaborative Research Group. Circulation. 2000;102:1773–1779. doi: 10.1161/01.cir.102.15.1773. [DOI] [PubMed] [Google Scholar]
- 20.Varma SD. Scientific basis for the medical therapy of cataracts by antioxidants. Am J Clin Nutr. 1991;53:335S–345S. doi: 10.1093/ajcn/53.1.335S. [DOI] [PubMed] [Google Scholar]
- 21.The Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study (AREDS): design implications: AREDS Report No. 1. Control Clin Trials. 1999;20:573–600. doi: 10.1016/s0197-2456(99)00031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study (AREDS) system for classifying age-related macular degeneration from stereoscopic color fundus photographs: AREDS Report No. 6. Am J Ophthalmol. 2001;132:668–681. doi: 10.1016/s0002-9394(01)01218-1. [DOI] [PubMed] [Google Scholar]
- 23.The Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study (AREDS) system for classifying cataracts from photographs: AREDS Report No. 4. Am J Ophthalmol. 2001;131:167–175. doi: 10.1016/s0002-9394(00)00732-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Food and Nutrition Board, National Academy of Sciences. Zinc. In: Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, DC: National Academy Press; 2002.
- 25.Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related macular degeneration and vision loss: AREDS Report No. 8. Arch Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams SL, Ferrigno L, Mora P, Rosmini F, Maraini G. Baseline cataract type and 10-year mortality in the Italian-American Case-Control Study of Age-related Cataract. Am J Epidemiol. 2002;156:127–131. doi: 10.1093/aje/kwf012. [DOI] [PubMed] [Google Scholar]
- 27.Vingerling JR, Dielemans I, Bots ML, Hofman A, Grobbee DE, de Jong PTVM. Age-related macular degeneration is associated with atherosclerosis: the Rotterdam Study. Am J Epidemiol. 1995;142:404–409. doi: 10.1093/oxfordjournals.aje.a117648. [DOI] [PubMed] [Google Scholar]
- 28.Hyman L, Schachat AP, He Q, Leske MC. Hypertension, cardiovascular disease, and age-related macular degeneration. Arch Ophthalmol. 2000;118:351–358. doi: 10.1001/archopht.118.3.351. [DOI] [PubMed] [Google Scholar]
- 29.Sperduto RD, Miller R. Systemic hypertension and age-related maculopathy in the Framingham Study. Arch Ophthalmol. 1986;104:216–219. doi: 10.1001/archopht.1986.01050140070022. [DOI] [PubMed] [Google Scholar]
- 30.Snow KK, Seddon JM. Do age-related macular degeneration and cardiovascular disease share common antecedents? Ophthalmic Epidemiol. 1999;6:125–143. doi: 10.1076/opep.6.2.125.1558. [DOI] [PubMed] [Google Scholar]
- 31.Girodon F, Galan P, Monget AL, et al. the MIN.VIT.AOX geriatric network Impact of trace elements and vitamin supplementation on immunity and infections in institutionalized elderly patients: a randomized controlled trial. Arch In-tern Med. 1999;159:748–754. doi: 10.1001/archinte.159.7.748. [DOI] [PubMed] [Google Scholar]


