Abstract
The Age-Related Eye Disease Study (AREDS) was initially conceived as a long-term multicenter, prospective study of the clinical course of age-related macular degeneration (AMD) and age-related cataract. Data on progression rates and risk factors from the study will increase understanding of the clinical course of both conditions, generate hypotheses about etiology, and aid in the design of clinical trials of potential interventions. In addition to collecting natural history data, AREDS includes a clinical trial of high-dose vitamin and mineral supplements for AMD and a clinical trial of high-dose vitamin supplements for cataract. The clinical trials were initiated largely because of the widespread public use in the United States of commercially available pharmacologic doses of vitamins and minerals to treat these two eye conditions and the absence of definitive studies on the safety and efficacy of their use. Important design issues for the clinical trials include: defining cataract and AMD, estimating event rates, determining the type and dosage of vitamins and minerals to be tested for each condition, and identifying the parameters necessary for monitoring safety and efficacy. This paper describes the AREDS design, including the study rationale and operational structure, and the approach adopted to combine, for two diseases, clinical trials with a natural history study.
Keywords: Age-related macular degeneration, cataract, clinical trial, clinical course, antioxidants, zinc, vitamin C, vitamin E, beta-carotene
The common causes of blindness in the United States are associated with increasing age. Because older people are the fastest growing part of the U.S. population, age-related eye disorders will become increasingly prevalent in the coming decades. AREDS investigates two of these disorders: age-related macular degeneration and cataract. The study will provide descriptive data on the clinical course of the conditions, attempt to identify factors that influence their development and progression, and evaluate the potential efficacy of high-dose vitamins and minerals in arresting or retarding their progression.
AGE-RELATED MACULAR DEGENERATION
Age-related macular degeneration (AMD) a collection of clinically recognizable ocular findings, is a leading cause of registered blindness in both England [1] and the United States [2, 3]. Clinical findings associated with AMD include drusen, retinal pigment epithelial (RPE) abnormalities, geographic atrophy, RPE detachment, choroidal neovascularization and its consequences (e.g., serous sensory retinal detachment, often accompanied by hard exudates and subretinal hemorrhages), and disciform scar. The prevalence of ophthalmoscopically or photographically identifiable drusen increases with age, especially after the sixth decade. Eyes without drusen are generally not considered to have AMD. Larger and more extensive drusen seem to be associated with an increased risk of central visual acuity loss. This loss can come from the development of choroidal neovascularization, the neovascular form of the disease that causes most of the severe vision loss from AMD, or from the other non-neovascular lesions of AMD listed above, the most important of which is geographic atrophy.
CATARACT
Cataract is the term used for opacities in the normally transparent lens. These opacities can interfere with the formation of a sharp image on the retina. Age-related cataract, by far the most common type, is often categorized as nuclear, cortical, or posterior subcapsular in location. Nuclear and posterior subcapsular cataracts, which commonly affect the central visual axis, are the types most frequently associated with a need for cataract surgery. Cataract is a major public health problem throughout the world and a leading cause of visual impairment and blindness in the United States [4]. In developing countries, cataracts account for about half of all blindness because resources are often not adequate to provide surgical treatment [5].
TREATMENT OF CATARACT AND AGE-RELATED MACULAR DEGENERATION
There is no known effective preventive treatment for cataract or AMD. Surgical treatment for cataract is highly effective, although it entails some risk and is costly. There is no effective treatment for most cases of AMD. Laser photocoagulation has been documented to be beneficial in a small proportion of patients with well-defined choroidal neovascularization (CNV) [6–8], but recurrence of CNV is common and often results in further vision loss [9, 10]. There is no proven treatment for persons with the non-neovascular form of the disease.
AGE-RELATED EYE DISEASE STUDY
The Age-Related Eye Disease Study, a long-term multicenter, prospective study of 4757 persons age 55 to 80 years is designed to assess the clinical course, prognosis, and risk factors of both AMD and cataract. The National Eye Institute (NEI) of the National Institutes of Health (NIH) provides primary support for the study through contract intramural research funds, with additional support from Storz Ophthalmic Pharmaceuticals, currently owned by Bausch and Lomb Pharmaceuticals. Staff of the NEI first proposed the concept for AREDS in 1986, and the protocol was developed between 1990 and 1992. The first participant was enrolled in November 1992 and recruitment of non-African Americans was essentially complete in 3 years with the final non-African American participant enrolled in July 1996. Recruitment of African-American participants continued through January 1998. Participants are now being followed at 6-month intervals for at least 7 years.
STUDY OF CLINICAL COURSE AND PROGNOSIS
The study is designed to assess the clinical course of, and risk factors for, the development and progression of cataract and AMD by collecting data on possible risk factors, measuring changes in visual acuity, photographically documenting changes in macula or lens status, and assessing self-reported visual function. Studies prior to the initiation of AREDS suggest that smoking status, nutritional status, gender, race, medical factors, iris color, and genetic markers may be associated with both conditions [11–16]. AREDS provides an opportunity to test such hypotheses further. To describe disease incidence and progression, the study will refine and apply photographic classification scales for both AMD lesions and lens opacities (cortical, nuclear and posterior subcapsular).
A grading system has been developed for each of the lesions of AMD [17]. The major AMD outcomes in this study are the development of the neovascular form of the disease or the development of geographic atrophy that involves the center of the macula. Eyes developing either of these conditions are considered to have progressed to advanced AMD. Other lesions of AMD, particularly drusen (size, type, and extent) and RPE abnormalities (detachment, atrophy, and pigment disturbances) are individually graded for each study eye. There is evidence that the severity of these lesions or combinations of these lesions may be associated with the risk of developing vision loss from AMD [18, 19]. Attempts will be made to develop a severity scale for AMD using techniques similar to those used to develop the modified Airlie House severity scale for diabetic retinopathy [20].
The basis for the lens opacity grading scales has been previously described [21]. Nuclear opacities are graded by assigning slit-lamp photographs a severity grade on a scale defined by seven standard slit-lamp photographs of lenses with increasingly severe nuclear opacities. Decimal grades assigned by graders range from 0.9 (no opacity) to 7.1 (completely opaque). A densitometric evaluation of the standard photographs suggested reassignment of standard 2 to a grade of 1.5, and making subsequent adjustments provided more uniform incremental increases in opacity along the scale. The revised scale adopted by AREDS ranges from 0.9 to 6.1. Neitz retro-illumination photographs are used to grade cortical and posterior supcapsular (PSC) opacities, by estimating the percentage of pupillary area occupied by an opacity, with the central 5-mm circle representing the region of primary interest.
Analyses will be performed to explore the relationship between progression along these disease severity scales and subsequent decreases in visual acuity. A strong correlation might suggest that photographic progression along a severity scale can serve as a surrogate measure for subsequent visual acuity loss. When evaluating the efficacy of newly proposed therapeutic strategies, those scales may also be useful in identifying high-risk patients for future clinical trials.
CLINICAL TRIALS
Before the start of AREDS, several epidemiologic studies published data suggesting a possible role for antioxidants in reducing the risk of cancer, cardiovascular disease, and eye disease [22–24]. In addition, results from a small, randomized clinical trial suggested that pharmacologic doses of zinc might provide some protection against vision loss from AMD [25]. With limited treatment options for AMD and no preventive measures for either AMD or cataract, the findings from these studies, and extensive advertising, led to the widespread use of vitamin and mineral formulations containing antioxidants and zinc. This increased usage occurred despite an admonition by the authors of the zinc trial that “it is definitely premature to recommend widespread use of zinc” [25]. Extensive marketing of high-dose, commercially available preparations of antioxidant vitamins and zinc increased the visibility and availability of these products and contributed to their widespread use. An objective assessment of the efficacy and safety of nutritional intervention for preventing the development and progression of these conditions was judged to be needed. Because both AMD and cataract progress slowly, it appeared that a large and prolonged trial would be necessary to test the effect of nutritional supplementation. However, a specific sample size determination for such a clinical trial was difficult because of the limited data on the disease progression rates and the expected effect of nutritional supplementation. Because of these considerations and the need for additional natural history data on the two conditions, AREDS was designed to assess simultaneously the clinical course of AMD and cataract, and the potential safety and efficacy of pharmacologic doses of antioxidant vitamins and zinc in reducing the incidence or slowing the progression of AMD and/or cataract.
When AREDS planning began, pharmaceutical companies were marketing several formulations of the vitamins and minerals of interest. The AREDS formulation for the clinical trials was chosen based on recommendations from expert nutritionists, ophthalmologists, and biochemists who reviewed basic science, clinical trial, and epidemiologic data at a series of meetings sponsored by the NEI. Two carotenoids, lutein and zeaxanthin, which are known to be present in the central retina, were strong candidates for inclusion in the AREDS formulation, but they were not commercially available when AREDS started. Beta-carotene, a carotenoid with systemic antioxidant properties, was chosen because the manufacturers of ophthalmic nutritional supplements were promoting its effectiveness, clinical trials of heart disease and cancer were studying it, and it was commercially available. It was decided to include pharmacologic doses of the antioxidant vitamins C and E. This vitamin/antioxidant formulation was expected to affect possibly the progression of both cataract and AMD. Zinc had been reported to be beneficial for AMD, but there was no evidence of an effect on lens opacities. It was decided to assess whether zinc, alone or in combination with the vitamin/antioxidant formulation, could slow the progression of AMD. Formulations that included zinc also had copper added to offset potential zinc-induced copper-deficiency anemia. Study participants thought to be at risk of vision loss from AMD, based on the presence of drusen or RPE changes, were part of a factorial design evaluating both the zinc formulation and the vitamin/antioxidant formulation. Study participants without these AMD lesions were not assigned to zinc because of possible toxicity and no evidence of potential benefit. They were assigned to either the vitamin/antioxidant formulation or placebo. The exact doses of the ingredients in these formulations are currently proprietary.
The clinical trial component of AREDS consists of two clinical trials generally sharing one pool of participants. The majority of AREDS participants will be included in the primary analysis of both the clinical trial of AMD and the clinical trial of cataract. Some participants will be included in the primary analysis of only one trial. For example, bilateral aphakic or pseudophakic participants cannot be assessed for lens opacities but were enrolled in AREDS if they had drusen or RPE changes and could participate in the AMD clinical trial. Participants with no drusen or RPE changes were not assigned to the zinc formulation and will contribute only to the primary analysis of the cataract trial. The clinical trials are double masked in that neither the AREDS participant nor the study investigators know the participant’s treatment assignment. Figure 1 diagrams the randomization design and the number of participants enrolled in each of the clinical trials by AMD status. A total of 4757 participants were enrolled, 157 more than planned for by design. Bilaterally aphakic or pseudophakic participants are excluded from the cataract trial resulting in 4629 participants enrolled in this component for the primary comparison of antioxidants versus no antioxidant (129 more than planned). Likewise 1117 participants with little or no drusen are excluded from the AMD trial resulting in 3640 participants enrolled in this 2 × 2 factorial design of antioxidants and zinc (40 more than planned).
Figure 1.
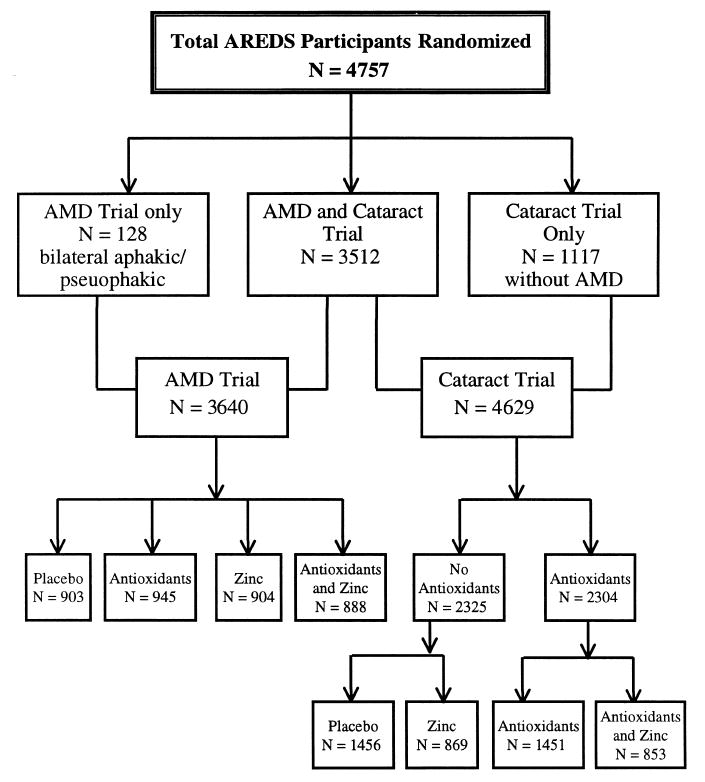
AREDS Randomization schema.
STUDY POPULATION
Eligible participants were age 55 to 80 years old at enrollment and had to be free of any illness or condition that would make long-term follow-up or compliance with study medications unlikely or difficult. On the basis of fundus photographs graded by a central reading center, best corrected visual acuity, and ophthalmologic evaluations, participants were enrolled in one of several AMD categories as shown in Table 1. Category 1 was established to provide a comparison group of approximately 1000 participants who were free of age-related macular changes. These participants were the group randomized only to placebo or antioxidants. Category 2 was projected to include approximately 1000 participants with mild or borderline age-related macular changes (multiple small drusen or intermediate drusen and/or pigment abnormalities) in one or both eyes. To qualify for either of these categories, both eyes had to have visual acuity of 20/32 or better (75 or more letters read correctly) measured by a standard protocol, media clear enough for good quality fundus photographs, and absence of any ocular disorder that might interfere with assessment of either AMD or lens opacities. Category 3 was established for participants who had at least one eye with visual acuity of 20/32 or better and large drusen (≥125 μm), extensive intermediate drusen (≥63 μm), and/or geographic atrophy that did not involve the center of the macula, and who did not have advanced AMD in either eye. The second eye of participants in this category either met the eligibility requirements of categories 1 through 3 (these patients are classified as category 3a), or had visual acuity worse than 20/32, not due to AMD, or had an otherwise disqualifying ocular disorder that was unilateral and unlikely to interfere with the assessment of AMD and lens opacity in the first eye (these patients are classified as category 3b and only the first eye will be included in analyses).
Table 1.
AMD Eligibility Categories
| First Eyea |
||||
|---|---|---|---|---|
| AMD Category | Drusen Sizeb | Drusen Areab | Pigment Abnormalities (PA)c | Second Eye |
| Category 1 | None or small (<63μm) | <125μm diameter circle (≈5–15 small drusen) | None | Same as first eye |
| Category 2 | Small (<63 μm)
or |
≥125 μm diameter circle | Absent or present, but geographic atrophy (GA) absent | Same as first eye or category 1 |
| Intermediate (≥63, <125 μm)
None required, if pigment abnormalities present |
At least one druse | |||
| Category 3a | Intermediate (≥63 μm, <125 μm) | ≥360 μm diameter circle, if soft indistinct drusen are present (≈20 intermediate drusen) | Absent or present but central GAb absent | Same as first eye or category 1 or 2 |
| or | ≥656 μm diameter circle(about 1/5 disc area), if soft indistinct drusen are absent (≈65 intermediate drusen) | |||
| Large (≥125 μm)
None required, if non-central GAb present |
At least one druse | |||
| Category 3b | First eye same as category 3a | VA <20/32 not due to AMD; or uniocular disqualifying disorder is present | ||
| Category 4a | First eye same as category 1, 2, or 3a | Advanced AMDd | ||
| Category 4b | First eye same as category 1, 2, or 3a | VA <20/32 due to AMD, but advanced AMDd not present | ||
Must have visual acuity ≥20/32, no advanced AMD, and no disqualifying lesions.
Drusen and geographic atrophy are assessed within 2 disc diameters of the center of the macula.
Pigment abnormalities (increased pigmentation or depigmentation) within 1 disc diameter of the center of the macula.
Geographic atrophy (GA) involving center of macula or signs of choroidal neovascularization (presence beneath the retinal pigment epithelium or sensory retina of fluid, blood, or fibrovascular or fibrous tissue).
Category 4 was established for participants who had advanced AMD or decreased vision (<20/32) in one eye and good vision in the second eye. These participants had visual acuity 20/32 or better, no advanced AMD, and no disqualifying lesion in their unaffected eye and had lesions of advanced AMD in the fellow eye (category 4a) or did not yet have the lesions of advanced AMD in the fellow eye, but had visual acuity worse than 20/32 due to AMD (category 4b). Photographic quality for all participants had to be sufficient to allow detailed retinal assessment (except for the eyes with advanced AMD, in which the quality needed only to be sufficient to allow its recognition). The accrual goal was 2600 participants evenly divided between AMD categories 3 and 4. Participants in categories 2, 3, and 4 were randomized in a factorial design to one of the four treatment arms.
Unlike AMD, there were no specific eligibility criteria regarding lens opacities, except that the media had to be sufficiently clear to allow for adequate fundus photographs, and visual acuity had to meet the requirements described above. Prior cataract extraction in one or both eyes did not disqualify a participant, except from category 1, and then only if aphakia/pseudophakia was bilateral. It was not necessary to prespecify recruitment goals in specific categories of lens opacity severity to assure adequate power for the clinical trial of cataract because lens opacities are expected to develop and progress with sufficient frequency within the AREDS eligible age range.
An important design feature of AREDS is the inclusion of participants who, at the time of screening, were current users of dietary supplements containing nutrients that are also contained in the study medication. Fifty-five percent of enrolled AREDS participants were supplementing with a multivitamin or at least one AREDS ingredient at the time of screening, and about half of these supplementing participants were taking recommended daily allowance (RDA) dosages rather than the pharmacologic dosages of the study medication. To accommodate such persons and standardize the use of nonstudy supplements, a daily dose of a widely available multivitamin tablet (Centrum®) is provided to each participant who wishes to take or continue taking a multivitamin. Approximately 66% of AREDS participants chose to take Centrum. Persons assigned to placebo who take Centrum have a dietary intake of vitamins C, E, beta-carotene, and zinc, plus an additional RDA amount that is contained in Centrum. Similarly, persons in the “active” treatment groups also will be increasing their intake by an RDA amount of each of the study ingredients if they take the Centrum. The statistical power of the study to test its primary hypothesis about pharmacologic doses of these nutrients may be reduced to the extent that prior use or the continued use of RDA type dosages of these nutrients affect the outcomes of interest. However, by including such persons in the study population, the AREDS population more closely resembles the supplementation habits of the general population in this age group.
STUDY OUTCOMES
The main study outcome variables are change in visual acuity and change in ocular status (AMD or lens opacities). Centrally graded fundus and lens photographs are used to assess the progression of retinal or lens disease. Five comparisons of the effect of treatment on primary outcomes will include:
Progression to advanced AMD (see below) comparing antioxidants and no antioxidants groups.
Progression to advanced AMD comparing zinc and no zinc groups.
15-letter decrease in visual acuity score comparing antioxidants and no antioxidants groups.
15-letter decrease in visual acuity score comparing zinc and no zinc groups.
Progression of lens opacity (see below) of any type or cataract surgery comparing antioxidants and no antioxidants groups.
If there is evidence of a statistically significant interaction between zinc and antioxidants then analyses will be restricted to outcomes within each of the four treatment cells.
An eye is defined as having progressed to advanced AMD when it has developed vision-threatening lesions. These lesions include signs of geographic atrophy involving the center of the macula or signs of choroidal neovascularization (defined as presence beneath the RPE or sensory retina of fluid, blood, or fibrovascular or fibrous tissue). Progression of lens opacity (nuclear, cortical, and/or PSC) is defined as follows:
Nuclear: 1.5 unit increase from baseline on a scale ranging from 0.9 to 6.1
Cortical: 10% absolute increase in area from baseline within the central 5-mm diameter circle of the lens
PSC: 5% absolute increase in area from baseline within the central 5-mm diameter circle of the lens
Other outcome variables that will be assessed as part of the clinical trials will include cardiovascular events, cancer, morbidity, and mortality from all causes and, possibly, from specific causes.
Sample Size and Power Considerations for Study of Clinical Course and Prognosis
Because the clinical trials are embedded in the study of clinical course and prognosis, expected 95% CI widths for incidence and progression rates were considered separately for placebo-only and for all enrolled participants. For the study of cataract, bilaterally and unilaterally phakic AREDS participants will compose the study cohort. Confidence intervals were calculated for various incidences for participants without a baseline opacity and progression rates for participants with a baseline opacity. CI widths are expected to range from 0.01 to 0.09. For the study of AMD, CIs for incidences of development of advanced AMD were calculated within AMD category for placebo only and all participants. CI widths are expected to range from 0.02 to 0.10.
For the study of risk factors of AMD and cataract, all enrolled participants (with the exception of bilateral aphakes or pseudophakes in the cataract study) will be analyzed and risk factors will be assessed in both univariate and multivariate models including treatment as a covariate. Incidence and progression rates from 0.10 to 0.30 were assumed and risk factors defined with prevalence of the factor at baseline ranging from 0.10 to 0.60. Relative risks from 1.2 to 3.0 are detectable with at least 90% power with the AREDS design.
Sample Size and Power Considerations for Clinical Trials
The design of an efficient clinical trial requires reliable estimates of disease progression overall and within selected subgroups. Data for progression rate estimates are sparse for cataract and AMD. Further, it is not known whether the RDA level of supplements in Centrum will affect the progression rates of either disease. Available resources at the time of study development permitted a total sample size of about 4600 participants (4757 were actually randomized). Calculations of therapeutic effect rates that could be detected with at least 80% power were performed assuming this fixed sample size and the conservatively estimated 5-year progression rates of participants assigned to placebo of 17% and 50% for AMD and cataract, respectively. A 5-year rate was assumed instead of a 7-year rate to maximize the number of patients with complete follow-up at the primary analysis endpoint. Clinical trials typically experience enrollment delays, and conservatively estimating available follow-up at the end of the trial during the planning phase protects the power of the trial from delays in recruitment. Preliminary analyses, based on changes in lens and AMD status of potential participants who had been evaluated with photographs for eligibility 2 years prior to randomization and at randomization, suggested that the assumed event rates were appropriate.
The power estimates included assumptions about expected adherence to study medication. Studies of nutritional supplements that are available over the counter are at higher than usual risk of having “drop-ins,” i.e., persons who begin to supplement with the active ingredients. Drop-ins are a special problem among subjects who think their condition may be worsening and who easily can obtain the study formulations commercially. Dropouts, i.e., persons who discontinue masked treatment, are a special problem among healthy subjects who may be less compliant with interventions than subjects who are ill. As the clinical trial progresses, the proportions of drop-ins and dropouts are likely to increase. The proportions may vary with the duration of the study and the extent to which information about possible health effects of the supplements becomes available from other studies during the course of AREDS. Such new information may not be sufficient to mandate a change in study design but can cause participants to begin or stop supplementing. The occurrence of drop-ins and dropouts dilutes the treatment effect, and in a factorial design dilution may be compounded across the factors.
In the cataract trial, potential drop-in and dropout rates were projected for each year of follow-up, assuming that after 7 years 15% of the participants would be lost to follow-up prior to experiencing an event, 30% would have begun to take a nonstudy supplement, and 20% would have stopped taking the study medication. With these assumptions, the percent reduction in the incidence and progression of cataract detectable with at least 90% power for event rates of 0.20, 0.30, and 0.50 was calculated by the methods of Wu et al. [26] for two-arm comparisons (Table 2) assuming 4500 participants would be enrolled in the cataract trial (4629 actually enrolled). For the factorial design of the AMD trial, treatment effect rates expected at the end of follow-up were adjusted by assuming that 10% of participants assigned to each treatment would drop out and assume the placebo event rate and 10% of participants would drop in and assume the full treatment (antioxidants and zinc) event rate. The total expected sample size of 3600 participants with AMD (3640 actually enrolled) was adjusted assuming 15% of the participants would be lost to follow-up. Approximate power for α = 0.05 and α = 0.01 are provided in Table 3 for varying effect sizes. The projected sample size will provide at least 80% power to detect treatment effects of 25% to 50%, depending on possible interactions between zinc and antioxidants. This range provides some assurance of detecting benefit from pharmacologic doses of the vitamins and minerals, whether Centrum provides independent benefit. If Centrum provides independent benefit and pharmacologic doses of vitamins and minerals are effective, observed treatment effects would be smaller than if Centrum provided no benefit.
Table 2.
Approximate Treatment Effects (Percent Reduction) Detectable with at Least 90 Percent Power by Placebo Event Rate for Cataract Triala
| Placebo Event Rate
|
||||
|---|---|---|---|---|
| Analysis group | Sample Size Available | 0.50 (Antioxidant Event Rate (15% reduction)) | 0.30 (Antioxidant Event Rate (25% reduction)) | 0.20 (Antioxidant Event Rate (30% reduction)) |
| Cataract | 3825b | 0.42 | 0.23 | 0.14 |
Table assumes: No interaction; two-arm comparisons; one-sided α = .025; 1-year treatment effect lag; drop-in rate 30%: 2% each of years 1 and 2, 4% each of years 3 and 4, 6% each of years 5, 6, and 7; dropout rate 20%: 2% year 1, 1% year 2, 2% year 3, 3% year 4, 4% each of years 5, 6, and 7.
Sample size of 3825 assumes 100 of the 4600 enrolled participants are bilaterally aphake or pseudophake and 15% of remaining participants are lost to follow-up or die prior to event.
Table 3.
Power Projections for AMD Clinical Trial (Assumed placebo event rate = 0.17; n = 3060.)a
| Rates of Progression to Advanced AMDb |
Approximate Power
|
|||||
|---|---|---|---|---|---|---|
| Situation (see below) | Placebo(n = 765) | Antioxidants (n = 765) | Zinc (n = 765) | Antioxidants + zinc (n = 765) | αc = 0.05 | αc = 0.01 |
| 1 | 0.162 | 0.128 | 0.128 | 0.094 | 0.87 | 0.68 |
| 2 | 0.163 | 0.109 | 0.109 | 0.109 | 0.84 | 0.65 |
| 3 | 0.170 | 0.170 | 0.116 | 0.170 | 0.84 | 0.65 |
| 4 | 0.164 | 0.164 | 0.117 | 0.117 | 0.86 | 0.67 |
| Situation | Percent reduction | Due to |
|---|---|---|
| 1 (Example: Main effects comparison: Antioxidants versus no antioxidants = 0.111 versus 0.145) | 25 | Antioxidants |
| 25 | Zinc | |
| 50 | Antioxidants and zinc (no interaction) | |
| 2 | 40 | Antioxidants |
| 40 | Zinc | |
| 40 | Antioxidants and zinc (negative interaction) | |
| 3 | 0 | Antioxidants |
| 40 | Zinc | |
| 0 | Antioxidants and zinc (negative interaction) | |
| 4 | 0 | Antioxidants |
| 35 | Zinc | |
| 35 | Antioxidants and zinc (no interaction) |
Calculated for 3600 participants with age-related macular changes; adjusted to 3060 for loss to followup and deaths prior to event.
Placebo rate of progression to advanced AMD of 0.17 is adjusted assuming 10% of participants would drop out and assume the placebo event rate and 10% of participants would drop in and assume the full treatment (antioxidants and zinc) event rate.
Two-sided α.
Analysis Plan
Unit of Analysis
Although AREDS will study both eyes of all participants, the primary unit of data analysis and the unit of randomization in the clinical trial will be the participant. In the study of clinical course and prognosis, the unit of analysis may be either the participant or the eye, depending on the analysis. When paired eyes are included in an analysis involving statistical inference, it is desirable to take into account the likely positive correlation between the eyes with respect to the variable studied [27, 28]. Single-eye analyses will consider either “better” or “worse” eyes based on the eye’s baseline status. The information from paired eyes will also be combined by classifying participants according to whether both eyes, one eye, or no eyes sustained a specified event (e.g., visual function loss).
Analytic Methods
AREDS is a longitudinal study with multiple outcome variables. When only one follow-up time is being considered, the simple t test and its nonparametric analogue, the Wilcoxon-Mann-Whitney test, will be used. Using analysis of covariance, these simple two-sample tests will be generalized to incorporate baseline values, covariates, and potential treatment interactions both with covariates (e.g., smoking) and between treatments (e.g., zinc and antioxidants). The stochastic ordering test described by Wei and Lachin [29] which allows for randomly missing data, is a natural multivariate analogue of the two-sample Wilcoxon-Mann-Whitney test and will be used to evaluate the repeated measures that will be taken at multiple time points.
Visual acuity outcomes (e.g., loss of 15 or more letters, occurrence of decrease in total visual acuity score to ≤35 letters) and morphologic endpoints (e.g., progression to advanced AMD, progression of lens opacity) will be analyzed in terms of one or more “events” using multivariate logistic regression. Time-dependent event rates will be analyzed by the life-table method [30–33] and will be portrayed graphically. Estimates of event rates will include confidence intervals, and treatment effects will be tested with the log-rank method. Standard contingency table methods will also be used to evaluate disproportionate event rates on treatment arms following a prespecified follow-up interval. Consideration will be given to estimating missing visual acuity values by interpolation, as was done in the Diabetic Retinopathy Study and the Early Treatment Diabetic Retinopathy Study [34]. Adjustments for missing observations by the methods of Choi and Stablein will also be considered [35]. Analyses will consider Centrum or other multivitamin use as a covariate.
The GEE method proposed by Liang and Zeger [36] will be used to analyze the longitudinal visual acuity data obtained from one eye per subject (left, right, or the average number of letters lost) using the SAS procedure GENMOD. When data from both eyes are to be used simultaneously in the analysis, the method based on second-order generalized estimating equations (GEE2) is most appropriate [37]. This method is an extension of GEE to handle correlation between multiple classes within a cluster. At present, the software developed for implementation of GEE2 is limited to binary outcome variables.
For analyses of data from both eyes, Cox’s proportional hazard model for multivariate time to event data developed by Wei, Lin, and Weissfeld [38] and Lee, Wei, and Amato [39] will be used. Separate analyses will be performed for each of the three primary endpoints, that is, for visual acuity events (loss of 15 or more letters) and the two morphologic events, progression to advanced AMD and progression of lens opacity. This approach is similar to the generalized estimating equation’s methodology of Liang and Zeger for analysis of longitudinal data [36]. The marginal distribution of time to event for each eye is formulated with the Cox proportional hazards model. It is natural to assume common baseline hazard functions for the two events from the same person. As in the GEE approach, modeling of the correlation between the paired eyes is not of interest and will be treated as a nuisance parameter. This approach yields consistent estimates of the regression parameters and robust variance–covariance matrix, leading to efficient estimation of treatment effect. Implementation of this approach will be carried out using one of the recently developed software packages such as COXPH (a function in S-PLUS 3.3 for Windows), MULCOX2 (Fortran), and SAS procedure PHREG. Simultaneous analysis of the three primary endpoints including data from both eyes will also be performed using this approach.
The goal of these different approaches to analysis is to assure consistency of results within the trial. Whether simple one-eye, two-measure models or more complex paired-eye, repeated-measure models are applied, the size and direction of observed effects should be consistent. GEE models will evaluate repeated outcomes measurements and estimate treatment effects in terms of the slope of change over time. Time-to-event analyses considering the participant as the unit of analysis will consider treatment differences in median time to event. If treatment is effective, both approaches should yield similar and significant results.
Disease-Specific Visual Outcome and Confounding
Morphologic and visual acuity changes are both important outcomes in the studies of clinical course and the clinical trials of cataract and AMD. However, it may often be difficult to determine the degree to which cataract, AMD, or other abnormalities are contributing to any observed visual decrease. Although the exclusion of persons with sight-threatening diseases makes the occurrence of visual impairment from causes other than cataract and macular degeneration unlikely during early follow-up, such impairments will become more frequent as follow-up progresses. Morphologic changes have the advantage of being disease-specific and photographically verifiable in a masked examination. A major disadvantage is their uncertain association with functional disability. The impact of these morphologic changes on visual function will be evaluated by examining the association with a doubling of the visual angle (15-letter decrease in visual acuity compared to randomization visit score).
Monitoring Guidelines
The multiplicity of endpoints in AREDS has led to the development of a complex sequential monitoring plan. For the purpose of sequential monitoring of ophthalmic endpoints, the plan assumes that there is no interaction between antioxidants and zinc and only main effects are analyzed. AREDS has extended the Lan and Lachin [40] group sequential approach to address multiple time-to-event outcome variables. Bonferroni adjustment distributes the type I alpha error among the five endpoints. The spending of the fraction of the overall type I error allocated to each outcome through interim analyses is then controlled by the fraction of the total “information” of the trial accumulated by the time of the interim analysis. A spending function that approximates the symmetric O’Brien-Fleming [41] boundary is used for AREDS because it is very conservative (requires a large treatment benefit or harm to signal stopping) during the early analyses and less conservative in the later analyses. Log-rank tests compare the response distributions of the two treatment groups, and the information fraction is estimated using patient exposure times, assuming the last follow-up visit for the clinical trial will occur on April 15, 2001.
A similar alpha-spending function approach is also being applied to sequential monitoring of mortality. To provide additional protection against the possibility that treatment increases mortality rates, we selected an overall alpha of 0.10 and a one-sided procedure in data monitoring that ignores any possible beneficial effects of active treatment on mortality. We selected a Pocock-type [42] spending function for sequential monitoring, which results in recommending early stopping under less extreme conditions than those of the O’Brien-Fleming boundaries.
Study Organization
An overview of the AREDS organizational structure is provided in Figure 2.
Figure 2.
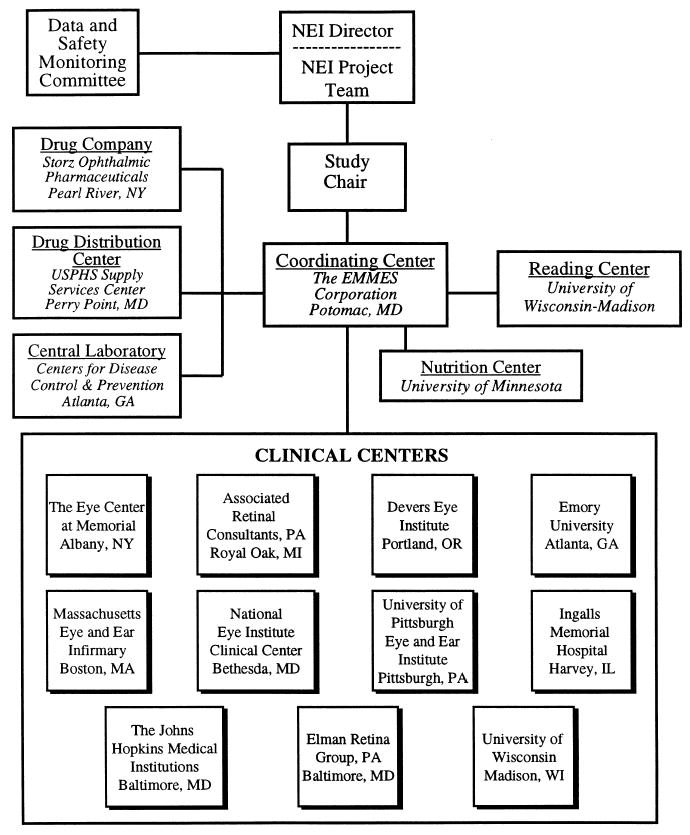
AREDS organization.
Public-Private Sector Collaboration
The past decade has seen growing interactive collaboration in research between the federal government and the private sector. To facilitate this collaboration, the NIH recently developed a standard Collaborative Research and Development Agreement (CRADA). Companies entering into a CRADA with the NIH participate both financially and scientifically in the conduct of a research program; each CRADA specifies the specific obligations of both the NIH and the corporation. The implementation of a CRADA follows models of collaboration that have long existed between laboratory scientists at the NIH and academically based collaborators. However, only recently have CRADAs been used to support NIH-sponsored field studies; AREDS represents one of the first multicenter clinical trials to use this arrangement.
The use of a CRADA to support a randomized trial like AREDS raises a number of issues, including the role of the private company in the overall supervision of the study, the responsibilities of the private company with regard to regulatory oversight of a trial with a new investigational drug, the access to study data by the private company to support its regulatory filings for commercial purposes, and the company’s need to minimize expenditures. The manner in which AREDS has addressed these issues, as described below, may provide guidance for future private/public sector collaborations.
Participating Centers
Eleven Clinical Centers are responsible for enrolling and following patients. A Coordinating Center is responsible for monitoring adherence to protocol procedures, assigning participants to study medication, monitoring data quality and timeliness, assigning ICD-9 codes to cause of death and hospitalizations, and providing statistical, analytic, and reporting support. Staff from the Coordinating Center train and certify clinical center staff in study procedures, perform annual site visits and data audits, and provide for the many administrative, logistic, and communications requirements of AREDS.
Fundus and lens photographs are assessed for quality and graded for disease severity at a central Photograph Reading Center. The Reading Center developed and implements both contemporaneous and serial monitoring of grading quality. The Photography Monitor at the Reading Center is responsible for training and certifying photographers at the Clinical Centers, and for monitoring photographic quality and equipment performance. Serum samples are shipped to the AREDS Central Laboratory for analysis and storage; the Central Laboratory also provides quality assurance testing of the study medications. The Nutrition Center provides the nutrient content of responses from a Food Frequency Questionnaire and 24-hour dietary recall interviews.
Drug Manufacturing and Distribution
Storz Ophthalmic Pharmaceuticals manufactures the AREDS study medications and run-in preparation, and Lederle/Whitehall Robbins provides Centrum (for participants who request a daily supplement vitamin). Supplies of the medication are shipped to the AREDS Drug Distribution Center where they are bottled and distributed to the Clinical Centers. Bottles are labeled with preassigned numbers to preserve masking. Ingredients in a sample of labeled bottles are assessed routinely by the Central Laboratory to monitor label accuracy.
Operations Committee
An Operations Committee consisting of the Study Chair, the NEI Project Officer, the NEI Contract Officer, the Principal Investigator of the Coordinating Center, the Director of the Reading Center, a Principal Investigator from one of the Clinical Centers, a Study Coordinator from a Clinical Center, and the Director of the Lens Project meets at least monthly. During these meetings, the Operations Committee reviews study status and progress, as well as data quality at the Clinical Centers and other participating centers.
Lens Project
The Lens Project is composed of members of the Reading Center and the Coordinating Center and is directed by an NEI representative. The purpose of the Lens Project is to define outcome measures and direct analyses of data related to the studies of cataract.
Morbidity and Mortality Committee
A Morbidity and Mortality Committee, consisting of two physicians and a medical records administrator, independently reviews samples of the ICD-9 coded cases of hospitalizations and all deaths to monitor the coding assignments made by the Coordinating Center staff.
Data and Safety Monitoring Committee
A Data and Safety Monitoring Committee (DSMC) monitors accumulating data for safety and efficacy. The committee consists of nine voting members: two ophthalmologists, one non-ophthalmologist physician, two biostatisticians, one bioethicist, one social scientist, one physician epidemiologist, and one nutritional epidemiologist. The ten nonvoting members include a representative from the FDA, a representative from Storz Ophthalmic Pharmaceuticals, the Director of the NEI, the AREDS Study Chair, the NEI Project Officer, the Director of the AREDS Lens Project, and representatives from the Coordinating Center, Reading Center, and the Central Laboratory.
The Study Chair and, ultimately, the Director of the NEI have overall responsibility for AREDS. The DSMC advises the Director of the NEI regarding the conduct of AREDS. As noted previously, Storz has ex officio membership on the DSMC. As such, Storz has access to study data provided to the DSMC for review. Storz also holds the Investigational New Drug (IND) application for AREDS and is required to report annually to the FDA on the study’s progress. The Coordinating Center prepares an annual report to the FDA and forwards it to Storz for final approval and submission to FDA. In all other respects, Storz functions as a member of the AREDS consortium and is therefore subject to the oversight mechanisms present in the study, e.g., site visits by members of the Operations Committee.
Study Implementation
In AREDS’s first phase, potential study participants were identified, study medication was formulated, study staff were trained in data collection procedures, and necessary equipment was purchased and modified as needed. In the second phase, participants were screened for eligibility, randomized and are now being followed regularly.
Participant Screening
Potentially eligible participants were screened during a qualification visit. Screening consisted of the signing of an informed consent statement, an interview, an eye examination, measurement of best-corrected visual acuity, and photography of the fundus and lens. Individuals who passed the screening evaluation and were interested in participating in AREDS were provided with a one-month “run-in” supply of placebo to assess potential for tolerance of the inactive ingredients and compliance with the treatment regimen. They were asked to take two tablets twice a day for 1 month. Participants taking fewer than 75% of the prescribed tablets were ineligible for enrollment. This requirement resulted in the elimination of 205 potential participants out of 6354 qualified (3.2%). Participants who had good compliance with the run-in medication, who had adequate pupillary dilation and no disqualifying lesions noted on photographs sent to the Reading Center for grading, and who signed a second consent form were stratified into AMD categories at the time of randomization, based on the Reading Center’s evaluation of fundus photographs from the Qualification Visit, and randomly assigned to one of four treatment groups. Randomized participants were seen at the AREDS Clinical Centers for follow-up every 6 months. Figure 3 illustrates the schedule of participant visits during screening and follow-up.
Figure 3.

Overview of study visits.
Data Collection
Baseline data and data on potential risk factors for the development and progression of both AMD and cataract were obtained by examination and interview at the time of randomization. These data, collected on all participants, included comorbidity (e.g., history of malignancy, cardiovascular disease), current and past medication and hormone use, and an assessment of nutrient intake using a modified Block Food Frequency Questionnaire (FFQ), which assessed dietary intake during the year prior to randomization. Twenty-four-hour dietary recall interviews were conducted by telephone by the Nutrition Center on a sample of 197 participants from the three Clinical Centers that collected serum samples (see below). An interview designed to calculate mean annual effective ocular sunlight exposure [43, 44] was implemented in 1996. Starting in 1998, study participants were asked to donate serum samples for DNA isolation and the creation of immortalized cell lines to be used in current and future studies of genetic markers for AMD and cataract.
The 25-item NEI Visual Function Questionnaire (NEI VFQ-25) with an appendix of 11 additional questions [45] was incorporated in 1997 to measure the effect of vision on daily living activities. The questionnaire is administered to all participants every 3 years as well as at the 5-year follow-up visit and at the participant’s last follow-up visit. Best-corrected visual acuity is measured at randomization and at yearly intervals using a standard protocol adapted from the Early Treatment of Diabetic Retinopathy Study (ETDRS). AREDS made minor modifications to the ETDRS protocol, including the use of a rear-lighted instead of a front-lighted box [46]. Standard lens and fundus photographs are taken at baseline, and follow-up photographs are taken yearly, beginning 2 years after enrollment. In addition, photos should be taken at unscheduled visits the first time the study participant’s visual acuity has dropped at least 10 letters from randomization.
Possible adverse experiences are monitored in all participants through interview data (e.g., fatigue, gastric complications, skin color change) every 6 months. Hematocrit levels are measured annually on all participants to assess possible copper-deficiency anemia, which may be associated with the administration of zinc. Serum samples were collected prerandomization and then are collected annually on all participants from the following Clinical Centers: Associated Retinal Consultants (prerandomization only), Devers Eye Institute, The Johns Hopkins Medical Institutions, and the NEI Clinical Center. Tests on the serum samples include measuring levels of vitamins E, C, and carotenoids, including lutein/zeaxanthin and alpha-carotene as well as zinc, copper, and cholesterol. Lutein/zeaxanthin and alpha-carotene are measured because pharmacologic doses of beta-carotene may affect serum levels of other carotenoids. HDL, total cholesterol, and triglycerides are also measured because of reports that zinc usage may affect serum lipid levels [47–50]. Hospitalization data, including discharge summaries, are obtained. Staff at the Coordinating Center use ICD-9 coding to assign the causes of hospitalization. Self-reported adverse experiences are recorded if the participant or the participant’s health care provider reports a possible relationship with the study medication. Deaths are reported to the Coordinating Center within 24 hours after the Clinical Center is notified. Clinical Center staff obtain death certificates and Coordinating Center staff assign causes of death according to ICD-9 codes.
Distributed Data Collection System
Each Clinical Center enters data into the AREDS Distributed Data Collection System developed by the Coordinating Center. Data are single-key entered by Clinical Center staff with interactive within and between form cross-checks and out-of-range checks. Data are then electronically transmitted by modem from the Clinical Centers to the Coordinating Center. The Coordinating Center performs additional cross-checks and distributes data quality and timeliness reports electronically to the centers monthly. The median error rate for the AREDS Clinical Centers calculated from annual data audits on 400,956 fields performed by the Coordinating Center over the last 3 years is 0.5 fields per 1000 fields audited. This represents a decrease in error rate from the first 2 years of the study in which 304,664 fields were audited with an error rate of 1.3 fields per 1000 fields audited. The reduction is due in large part to continuing education by the Coordinating Center during site visits and the importance the Clinic Coordinators place on data accuracy.
Eligibility verification and random treatment assignment were performed by the Coordinating Center using the on-site computers, with procedures to protect the integrity of randomization and to provide randomization backup in case of hardware malfunction. Multiple levels of data encryption ensure the integrity of the treatment assignment files. Each center has two treatment assignment databases: one for patients without AMD (category 1) containing approximately 100 records consisting of bottle numbers for placebo (five bottle numbers) or antioxidants (five bottle numbers), and one for patients with some AMD (categories 2, 3, or 4) containing approximately 420 records consisting of a different sequence of bottle numbers for placebo and antioxidants (ten bottle numbers each) as well as for zinc and antioxidant and zinc formulations (ten bottle numbers each). Each treatment assignment database residing on the hard drives at each Clinical Center is encrypted and includes check numbers to insure tamper-free operation and proper sequential treatment assignments. There were no cases of database corruption during randomization.
The Coordinating Center developed a backup randomization system that could be performed manually and exactly simulated the usual randomization process. When randomization was not possible using the computer at the clinic, usually because of temporary hardware failure, the clinic called the Coordinating Center and the randomization was performed manually and the assignment provided by telephone. A potential participant’s qualification visit data and Reading Center data were entered on a Coordinating Center computer and this computer was used to exactly simulate the process that would have occurred at the clinic computer to assign the randomization number. Whether performed at the clinic or centrally by the Coordinating Center, the computerized randomization system identified which of the two randomization tables (category 1 versus categories 2, 3, or 4) should be used for assigning a treatment, and the participant was randomly assigned a bottle number from the appropriate list. The manual system was used a total of seven times by four clinics.
Data Monitoring
One voting member of the DSMC, designated as the Board’s Medical Monitor, receives copies of death reports and cumulative death information each month. Because AREDS has an IND application on file with the FDA and held by the pharmaceutical company, all required safety reports are provided to the FDA and the pharmaceutical company.
AREDS is a double-masked study. The AREDS participants, investigators and Reading Center personnel are masked to study-wide outcome data and treatment assignments. The DSMC is charged with recommending termination of one or all of the therapies if benefit or harm is demonstrated using the monitoring guidelines established prior to evaluating study results.
Reports From Other Trials
It is difficult to predict the impact that results from other studies may have on the adherence of study participants to the protocol. In AREDS, the study medication contains compounds that are commercially available without prescription. Hence, the announcement of results from other trials can prompt participants to start or stop the supplements without guidance from AREDS. Because many trials of nutritional supplements are in progress and new information from these studies is often quickly available to the study participants, investigators must be prepared to advise participants when new information becomes available.
While participants were still being enrolled into AREDS, the results of three large, randomized clinical trials of beta-carotene were published. In 1994, the Alpha-Tocopherol Beta Carotene (ATBC) Lung Cancer Prevention Study of 29,000 male Finnish cigarette smokers reported a statistically significant increase in lung cancer incidence (about 18%) and mortality (about 9%) among men randomly assigned to 20 mg of beta-carotene per day [51]. In 1996, the Physician’s Health Study, which followed 22,071 male physicians in the United States who had been randomly assigned to take either 50 mg of beta-carotene or placebo every other day for 12 years, reported no effect of supplementation on the risk of cancer, cardiovascular disease, or death either in the total study group or in the 11% who smoked [52]. In the same issue of the New England Journal of Medicine, the Beta-Carotene and Retinol Efficacy Trial (CARET) reported that after an average of 4 years of follow-up the participants taking beta-carotene and vitamin A had 28% more new cases of lung cancer and 17% more deaths from all causes than the control group [53]. The cohort studied in CARET consisted of 14,254 men and women who were current or former heavy smokers and 4060 people with extensive asbestos exposure.
Following the report of the ATBC study, in accordance with a recommendation from the AREDS DSMC, all AREDS participants were informed of the ATBC findings by a special newsletter. Participants were told they were free to stop their study medication or continue without change. In 1996, following the publication of the CARET results and the Physician’s Health Study results, the DSMC further recommended that it would be prudent for current smokers to stop supplementing with study medication containing beta-carotene. At nine study sites participants were given a choice of continuing with assigned study medication, stopping all study medication, or being reassigned to a non-antioxidant containing formulation. At the other two clinics, however, the institutions’ Human Subject’s Committee determined that no persons who currently smoke should take study tablets containing beta-carotene. At these sites, smokers were offered the choice of stopping supplementation or being reassigned. The operational impact of this recommendation led to the bottling of additional placebo and zinc tablets with a new sequence of bottle numbers to preserve masking. No participant was informed of his or her original medication assignment. Approximately 8% (388) of AREDS participants were current cigarette smokers at enrollment. Of these participants, 28% (112) chose to stop supplementing following the 1996 publications and 32% (124) chose to be reassigned. The actual percent of participants who smoked and who stopped taking beta-carotene is about 30% (118) because approximately half of all participants were originally randomized to a non-antioxidant formulation, and these participants, although reassigned to a new bottle number, continued with their original assigned formulation. The impact on AREDS as a result of these studies was a 2.5 percentage point increase in the “dropout” rate over a 12-month period.
DISCUSSION
Collaboration with Industry
Collaboration between NIH-sponsored research and industry can facilitate answering important health questions in a cost-effective manner. The use of a CRADA in AREDS provided a framework for successful collaboration. A successful relationship must balance the needs and requirements of industry with the importance of maintaining the autonomy of NIH and the investigators in study decision processes. The composition and role of data and safety monitoring committees have been the subject of recent discussion in the literature [54]. In AREDS, the pharmaceutical company holds the IND with FDA and has safety reporting responsibilities. This responsibility requires a representative from the pharmaceutical company to be privy to the outcome data. The NEI believed that including industry as an ex officio member of the DSMC would provide the best forum for presentation and discussion of outcome data. Independence of the committee’s recommendations is maintained by restricting the industry representative to a nonvoting status. In addition the representative agreed to maintain the confidentiality of the data and not share it with other company members. During the design phase, the pharmaceutical company hoped that if the clinical trial proved one of the formulations is effective they would be able to submit a New Drug Application to the FDA and could then benefit from a limited time of exclusive marketing of the formulation with an approved indication. In order to maintain this exclusivity it was important to keep the exact doses of the formulation confidential. Therefore the dose of the formulation was described to prospective participants in terms of approximate multiples of the RDA doses of the components. The AREDS Research Group has no proprietary interest.
Expected Contribution of AREDS
The Age-Related Eye Disease Study will help us to better understand two important diseases that affect an aging population. The study is collecting data on the factors that influence the development and progression of lens opacities and AMD in persons supplementing and not supplementing with pharmacological doses of antioxidants and minerals. Because AREDS is not a population-based study, analyses and inference will consider the effect of the entrance criteria on the generalizability of the results. The analysis of potential risk factors for the two diseases will increase our understanding and perhaps generate new hypotheses. Safety and efficacy monitoring guidelines account for the multiplicity of outcomes, frequency of analyses, and accumulating data from other studies. If the disease classification scales developed and refined in AREDS identify early markers for populations at risk of future vision loss, these may be useful for more rapid evaluation of the efficacy of new interventions.
Nested within the natural history study are two randomized clinical trials generally sharing a common cohort. The design of the trials considers the potential effects of different interventions and the possibility that measurement of the progression of one eye disease will confound measurement of the progression of the other. Because the intervention consists of ingredients that are commercially available without prescription, accommodating the potential for a dilution of treatment effect is likely to be crucial to maintaining the designed power. Flexibility in multivitamin use while controlling for the type and or dosage of vitamin by providing Centrum to those who wish to supplement, and continuing education of the study population about new information as it becomes available, should help ensure the study’s power to detect treatment effects. The AREDS Research Group hopes that data from AREDS on progression rates and risk factors for AMD and cataract will further our understanding of the clinical course of both conditions, generate hypotheses about etiology, and aid in the design of clinical trials of potential interventions. The group’s approach to collaborating with industry on NIH-sponsored trials may provide a foundation for the design of future collaborative clinical trial efforts.
APPENDIX
AREDS RESEARCH GROUP
The Eye Center at Memorial
Aaron Kassoff, MD, Shalom Kieval, MD, Michel Mehu, JoAnne Buehler, Mary Eglow, RN, and Francine Kaufman.
Associated Retinal Consultants, PC
Raymond R. Margherio, MD, Morton S. Cox, MD, Bruce Garretson, MD, Tarek Hassan, MD, Alan Ruby, MD, Michael T. Trese, MD, Jane Camille Werner, MD, George A. Williams, MD, Virginia Regan, RN, Patricia Manatrey, RN, Kristi Cumming, RN, Mary Zajechowski, Rachel Falk, Patricia Streasick, and Lynette Szydlowski.
Devers Eye Institute
Richard F. Dreyer, MD, Colin Ma, MD, Carolyn Beardsley, and Harold Crider.
Emory University
Antonio Capone Jr, MD, Thomas M. Aaberg, MD, Daniel Martin, MD, David Saperstein, MD, Paul Sternberg, Jr, MD, Linda Curtis, Barbara Stribling, James Gilman, Bob Myles, and Ray Swords.
Ingalls Memorial Hospital
David H. Orth, MD, Timothy P. Flood, MD, Joseph Civantos, MD, Serge deBustros, MD, Kirk H. Packo, MD, Celeste MacLeod, Chris Morrison, Douglas A. Bryant, Don Doherty, and Sharon Sandoval.
Massachusetts Eye and Ear Infirmary
Johanna M. Seddon, MD, Michael K. Pinnolis, MD, Desiree A. Jones-Devonish, Valerie D. Crouse, MS, Kristin K. Snow, MS, Claudia Evans, OD, Nancy Davis, Charlene Callahan, David Walsh, Jennifer Dubois, and Ilene Burton, RN.
National Eye Institute Clinical Center
Frederick L. Ferris, III, MD, Emily Y. Chew, MD, Karl Csaky, MD, PhD, Sally A. McCarthy, RN, MSN, Katherine Hall Dabas, Linda Goodman, Young Ja Kim, RN, BSN, Patrick Lopez, Richard Mercer, Leanne M. Ayres, Toni LaRean, Anne Randall, Marilois Chicca, Patrick F. Ciatto, Ernest Kuehl, Iris Kivitz, and Dessie Koutsandreas.
University of Pittsburgh
Thomas R. Friberg, MD, Andrew Eller, MD, Michael B. Gorin, MD, PhD, Jane Alexander, and Barbara Mack.
The Johns Hopkins Medical Institutions
Susan B. Bressler, MD, Neil M. Bressler, MD, Gary Cassel, MD, Morton Goldberg, MD, Julia A. Haller, MD, Lois Ratner, MD, Andrew P. Schachat, MD, Steven H. Sherman, MD, Janet S. Sunness, MD, Sherrie Schenning, Catherine Sackett, CANP, Dennis Cain, David Emmert, Terry George, and Stacy Wheeler.
Elman Retina Group, PA
Michael J. Elman, MD, Rex Ballinger, OD, Arturo Betancourt, MD, David Glasser, MD, Joyce Lammlein, MD, Ronald Seff, MD, Margin Shuman, MD, JoAnn Starr, Anita Carrigan, Christine Ringrose, Terri Mathews, Peter Sotirakos, and Theresa Cain.
University of Wisconsin - Madison
Suresh R. Chandra, MD, Frank L. Myers, MD, T. Michael Nork, MD, Thomas Stevens, MD, Barbara Blodi, MD, Justin Gottlieb, MD, Tracy Perkins, MPH, Margo Blatz, Wendy Walker, Bob Harrison, Gene Knutson, Denise Krolnik, and Guy Somers.
University of Wisconsin - Reading Center
Matthew D. Davis, MD, Barbara E.K. Klein, MD, Ronald Klein, MD, Larry Hubbard, MA, Yvonne L. Magli, Judith Brickbauer, Sarah Ansay, Jane Armstrong, Michael Neider, Hugh Wabers, James Baliker, Linda Kastorff, Kristine Laher, Darlene Badal, Patricia L. Geithman, Kathleen D. Miner, William N. King, Kurt R. Osterly, Kristi L. Dohm, James A. Onofrey, Barbara Esser, Cynthia Hurtenback, Marian R. Fisher, Nancy L. Robinson, and James Reimers.
Centers for Disease Control and Prevention - Central Laboratory
Dayton Miller, PhD, Barbara Bowman, PhD, Elaine Gunter, and Anne Sowell, PhD.
Coordinating Center - The EMMES Corporation
Anne S. Lindblad, PhD, Fred Ederer, MA, FACE, Roy C. Milton, PhD, Gary Gensler, MS, Ravinder Anand, PhD, Gary Entler, Elaine Stine, Stuart H. Berlin, Phyllis R. Scholl, and Susan A. Mengers.
National Eye Institute Project Office
Frederick L. Ferris, III, MD, Emily Y. Chew, MD, Robert Sperduto, MD, and Natalie Kurinij, PhD.
ACKNOWLEDGMENTS
Data and Safety Monitoring Committee (DSMC)
Janet Wittes, PhD—Chair, Gladys Block, PhD, David DeMets, PhD, Stuart Fine, MD, Curt Furberg, MD, PhD, M Cristina Leske, MD, MPH, Professor Giovanni Maraini, Donald Patrick, PhD, MSPH, and Robert Veatch, PhD.
Data and Safety Monitoring Committee (DSMC) Ex-Officios
Anne Sowell, PhD, Wiley Chambers, MD, Ellen Strahlman, MD, Matthew D. Davis, MD, Fred Ederer, MA, FACE, Frederick L. Ferris, III, MD, Karen Gamble, Carl Kupfer, MD, Natalie Kurinij, PhD, Anne S. Lindblad, PhD, and Robert Sperduto, MD.
National Institutes of Health Division of Contracts and Grants
Karen Gamble.
Bausch and Lomb Pharmaceuticals
Ellen Strahlman, MD.
Footnotes
Supported by contracts from the National Eye Institute, National Institutes of Health, with additional support from Bausch and Lomb Pharmaceuticals.
References
- 1.Sorsby A. Reports on Public Health and Medical Subjects. No 114. London: Her Majesty’s Stationary Office, 1966.
- 2.Kahn HA, Moorhead HB. Statistics on Blindness in the Model Reporting Area. 1969–1970 Washington, D.C.: USDHEW, PHS Publication No. (NIH); 1973:73–427.
- 3.National Advisory Eye Council. Report of the Retinal and Choroidal Diseases Panel. Vision Research-A National Plan: 1983–1987 Bethesda, MD: U.S. Department of Health and Human Services; 1984.
- 4.National Advisory Eye Council. Report of the Cataract Panel. Vision Research—A National Plan: 1983–1987 Bethesda, MD: U.S. Department of Health and Human Services; 1984.
- 5.Thylefors B, Négrel AD, Pararajasegaram R, Dadzie KY. Global data on blindness. Bull WHO. 1995;73(1):115–121. [PMC free article] [PubMed] [Google Scholar]
- 6.Macular Photocoagulation Study Group. Argon laser photocoagulation for neovascular maculopathy. Five-year results from randomized clinical trials. Arch Ophthalmol. 1991;109:1109–1114. [PubMed] [Google Scholar]
- 7.Macular Photocoagulation Study Group. Krypton laser photocoagulation for neovascular lesions of age-related macular degeneration. Results of a randomized clinical trial. Arch Ophthalmol. 1990;108:816–824. doi: 10.1001/archopht.1990.01070080058036. [DOI] [PubMed] [Google Scholar]
- 8.Macular Photocoagulation Study Group. Laser photocoagulation of subfoveal neovascular lesions of age-related macular degeneration: updated findings from two clinical trials. Arch Ophthalmol. 1993;111:1200–1209. doi: 10.1001/archopht.1993.01090090052019. [DOI] [PubMed] [Google Scholar]
- 9.Macular Photocoagulation Study Group. Recurrent choroidal neovascularization after argon laser treatment for neovascular maculopathy. Arch Ophthalmol. 1986;104:503–512. doi: 10.1001/archopht.1986.01050160059012. [DOI] [PubMed] [Google Scholar]
- 10.Macular Photocoagulation Study Group. Persistent and recurrent neovascularization after krypton laser photocoagulation for neovascular lesions of age-related macular degeneration. Arch Ophthalmol. 1990;108:825–831. doi: 10.1001/archopht.1990.01070080067037. [DOI] [PubMed] [Google Scholar]
- 11.Hiller R, Sperduto RD, Ederer F. Epidemiologic associations with nuclear, cortical, and posterior subcapsular cataracts. Am J Epidemiol. 1986;124:926. doi: 10.1093/oxfordjournals.aje.a114481. [DOI] [PubMed] [Google Scholar]
- 12.Leske MC, Chylack LT, Wu SY, et al. The Lens Opacities Case-Control Study. Arch Ophthalmol. 1991;109:244. doi: 10.1001/archopht.1991.01080020090051. [DOI] [PubMed] [Google Scholar]
- 13.Kahn HA, Leibowitz HM, Ganley JP, et al. The Framingham Eye Study, II. Association of ophthalmic pathology with single variables previously measured in the Framingham Heart Study. Am J Epidemiol. 1977;106:33–41. doi: 10.1093/oxfordjournals.aje.a112429. [DOI] [PubMed] [Google Scholar]
- 14.Hyman LG, Lilienfeld AM, Ferris FL, 3d, et al. Senile macular degeneration: A case-control study. Am J Epidemiol. 1983;118:213–227. doi: 10.1093/oxfordjournals.aje.a113629. [DOI] [PubMed] [Google Scholar]
- 15.Delaney WV, Oates RP. Senile macular degeneration. A preliminary study. Ann Ophthalmol. 1982;14:21–24. [PubMed] [Google Scholar]
- 16.Maltzman BA, Mulvihill MN, Greenbaum A. Senile macular degeneration and risk factors: A case-control study. Ann Ophthalmol. 1979;11:1197–1201. [PubMed] [Google Scholar]
- 17.Klein R, Davis MD, Magli YL, et al. The Wisconsin age-related maculopathy grading system. Ophthalmol. 1991;98:1128–34. doi: 10.1016/s0161-6420(91)32186-9. [DOI] [PubMed] [Google Scholar]
- 18.Macular Photocoagulation Study Group. Risk factors for choroidal neovascularization in the second eye of patients with juxtafoveal or subfoveal choroidal neovascularization secondary to age-related macular degeneration. Arch Ophthalmol. 1997;115:741–747. doi: 10.1001/archopht.1997.01100150743009. [DOI] [PubMed] [Google Scholar]
- 19.Klein R, Klein BEK, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy. The Beaver Dam Study. Ophthalmology. 1997;104:7–21. doi: 10.1016/s0161-6420(97)30368-6. [DOI] [PubMed] [Google Scholar]
- 20.Early Treatment Diabetic Retinopathy Study Group. Fundus photographic risk factors for progression of diabetic retinopathy. Ophthalmol. 1991;98(Suppl):823–833. [PubMed] [Google Scholar]
- 21.Klein BEK, Magli YL, Neider MW, et al. Wisconsin system for classification of cataracts from photographs. NTIS Accession No. PB 90-138306.
- 22.Buring JF, Hennekens CH. In: Prasad K, ed. Nutrients in Cancer Prevention and Treatment Totowa, NJ: Humana; 1995;pp 223–234.
- 23.Stampfer MJ, Hennekens CH, Manson JE, et al. Vitamin E consumption and the risk of coronary disease in women. N Engl J Med. 1993;328:1444–1449. doi: 10.1056/NEJM199305203282003. [DOI] [PubMed] [Google Scholar]
- 24.Sperduto RD, Ferris FL, 3rd, Kurinij N. Do we have a nutritional treatment for age-related cataract or macular degeneration? Arch Ophthalmol. 1990;108:1403–1405. doi: 10.1001/archopht.1990.01070120051026. [DOI] [PubMed] [Google Scholar]
- 25.Newsome DA, Swartz M, Leone NC, et al. Oral zinc in macular degeneration. Arch Ophthalmol. 1988;106:192–198. doi: 10.1001/archopht.1988.01060130202026. [DOI] [PubMed] [Google Scholar]
- 26.Wu M, Fisher M, DeMets D. Sample sizes for long-term medical trials with time-dependent dropout and event rates. Control Clin Trials. 1980;1:109–121. doi: 10.1016/0197-2456(80)90014-8. [DOI] [PubMed] [Google Scholar]
- 27.Ederer F. Shall we count eyes or subjects? Arch Ophthalmol. 1973;89:1–2. doi: 10.1001/archopht.1973.01000040003001. [DOI] [PubMed] [Google Scholar]
- 28.Katz J. Two eyes or one? The data analyst’s dilemma. Ophthalmic Surg. 1988;19:585–589. [PubMed] [Google Scholar]
- 29.Wei LJ, Lachin JM. Two sample asymptotically distribution-free tests for incomplete multi-variate observations. J Am Stat Assoc. 1984;79:653–661. [Google Scholar]
- 30.Kaplan E, Meier P. Nonparametric estimates from incomplete observations. J Am Stat Assoc. 1958;53:457. [Google Scholar]
- 31.Turnbull BW. The empirical distribution function with arbitrarily grouped, censored and truncated data. J Roy Stat Soc Series B. 1976;38:290–295. [Google Scholar]
- 32.Finkelstein DM. A proportional hazards model for interval-censored failure time data. Biometrics. 1986;42:845–854. [PubMed] [Google Scholar]
- 33.Lindsey JC, Ryan LM. Tutorial in biostatistics: Methods for interval-censored data. Stat Med. 1998;17:219–238. doi: 10.1002/(sici)1097-0258(19980130)17:2<219::aid-sim735>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 34.Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for macular edema. Arch Ophthalmol. 1985;103:1796–1806. [PubMed] [Google Scholar]
- 35.Choi SC, Stablein DM. Comparing proportions with incomplete data under non-random mechanisms. Stat Med. 1988;7:929–939. doi: 10.1002/sim.4780070904. [DOI] [PubMed] [Google Scholar]
- 36.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;80:55–61. [Google Scholar]
- 37.Qaqish BF, Liang K-Y. Marginal models for correlated binary responses with multiple classes and multiple levels of nesting. Biometrics. 1992;48:939–950. [PubMed] [Google Scholar]
- 38.Wei LJ, Lin DY, Weissfeld L. Regression analysis of multivariate incomplete failure time data by modeling marginal distributions. J Am Stat Assoc. 1989;84:1065–1073. [Google Scholar]
- 39.Lee EW, Wei LJ, Amato DA. Cox-type regression analysis for large numbers of small groups of correlated failure time observations. In: JP Klein, PK Goel, eds. Survival Analysis: State of the Art Dordrecht: Kluwer Academic Publishers; 1992, pp 237–247.
- 40.Lan KKG, Lachin JM. Implementation of group sequential logrank tests in a maximum duration trial. Biometrics. 1990;46:759–770. [PubMed] [Google Scholar]
- 41.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–556. [PubMed] [Google Scholar]
- 42.Pocock SJ. Group sequential methods in the design and analysis of clinical trials. Biometrika. 1977;64:191–199. [Google Scholar]
- 43.Carson CPhD, Lee S, Livingston P, et al. Ocular exposure to UV-B in sunlight: The Melbourne visual impairment model. Bull WHO. 1996;74(4):353–360. [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenthal F, West S, Munoz B, et al. Ocular and facial skin exposure to ultraviolet radiation in sunlight: A personal exposure model with application to a worker population. Health Physics. 1991;61(1):77–86. doi: 10.1097/00004032-199107000-00008. [DOI] [PubMed] [Google Scholar]
- 45.The National Eye Institute 25-Item Visual Function Questionaire (VFQ-25). Santa Monica, Calif; RAND, 1996.
- 46.Ferris FL, 3rd, Sperduto RD. Standardized illumination for visual acuity testing in clinical research. Am J Ophthalmol. 1982;94:97–98. doi: 10.1016/0002-9394(82)90198-2. [DOI] [PubMed] [Google Scholar]
- 47.Hooper PL, Visconti L, Garry PJ, et al. Zinc lowers high-density lipoprotein-cholesterol levels. JAMA. 1980;244:1960–1961. [PubMed] [Google Scholar]
- 48.Freeland-Graves JH, Friedman BJ, Han WH, et al. Effect of zinc supplementation on plasma high-density lipoprotein cholesterol and zinc. Am J Clin Nutr. 1982;35:988–992. doi: 10.1093/ajcn/35.5.988. [DOI] [PubMed] [Google Scholar]
- 49.Goodwin JS, Hunt WC, Hooper P, et al. Relationship between zinc intake, physical activity, and blood levels of high-density lipoprotein cholesterol in a health elderly population. Metabolism. 1985;34:519–523. doi: 10.1016/0026-0495(85)90187-8. [DOI] [PubMed] [Google Scholar]
- 50.Black MR, Medeiros DM, Brunett E, et al. Zinc supplements and serum lipids in young adult white males. Am J Clin Nutr. 1988;47:970–975. doi: 10.1093/ajcn/47.6.970. [DOI] [PubMed] [Google Scholar]
- 51.The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 52.Hennekens CH, Buring JE, Manson JE, et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996;334:1145–1149. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- 53.Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 54.Meinert CL. Clinical Trials and treatment effects monitoring (and comments) Control Clin Trials. 1988;19:515–543. doi: 10.1016/s0197-2456(98)00027-0. [DOI] [PubMed] [Google Scholar]


