Abstract
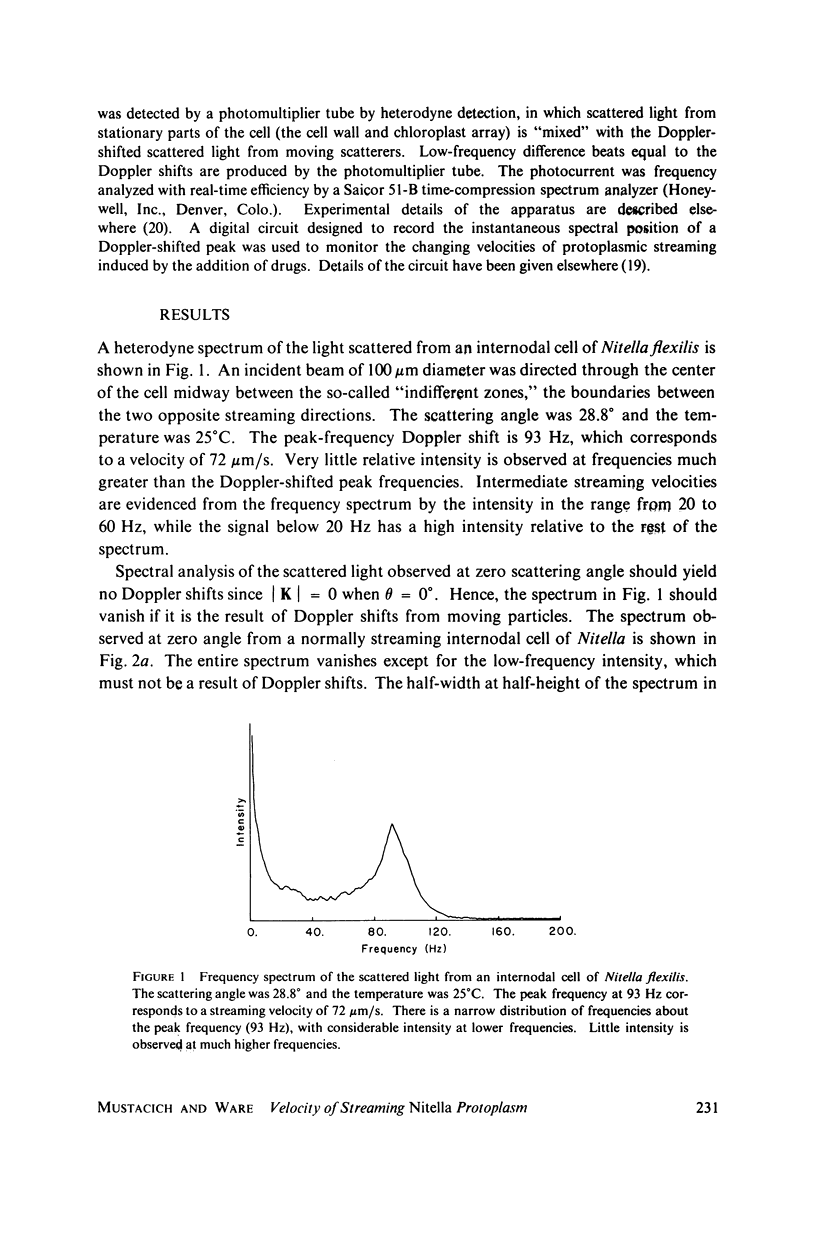
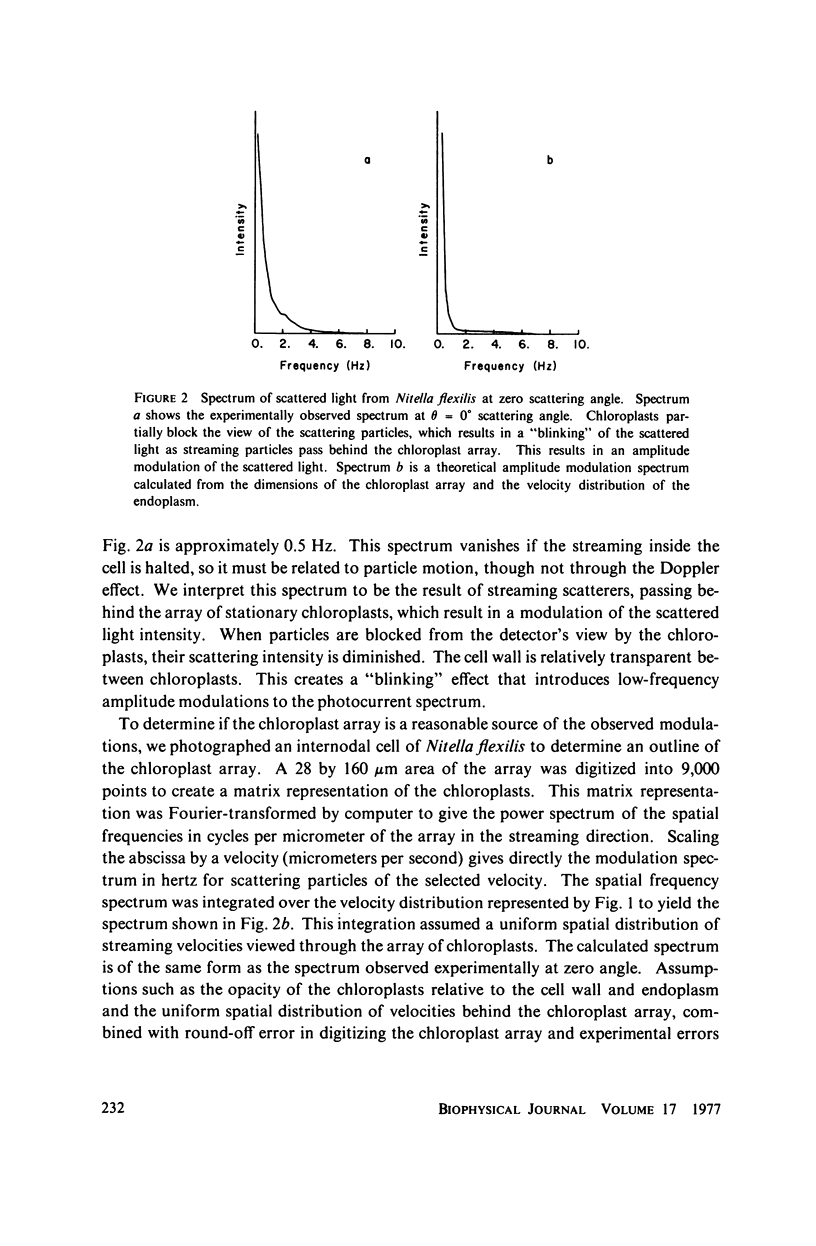
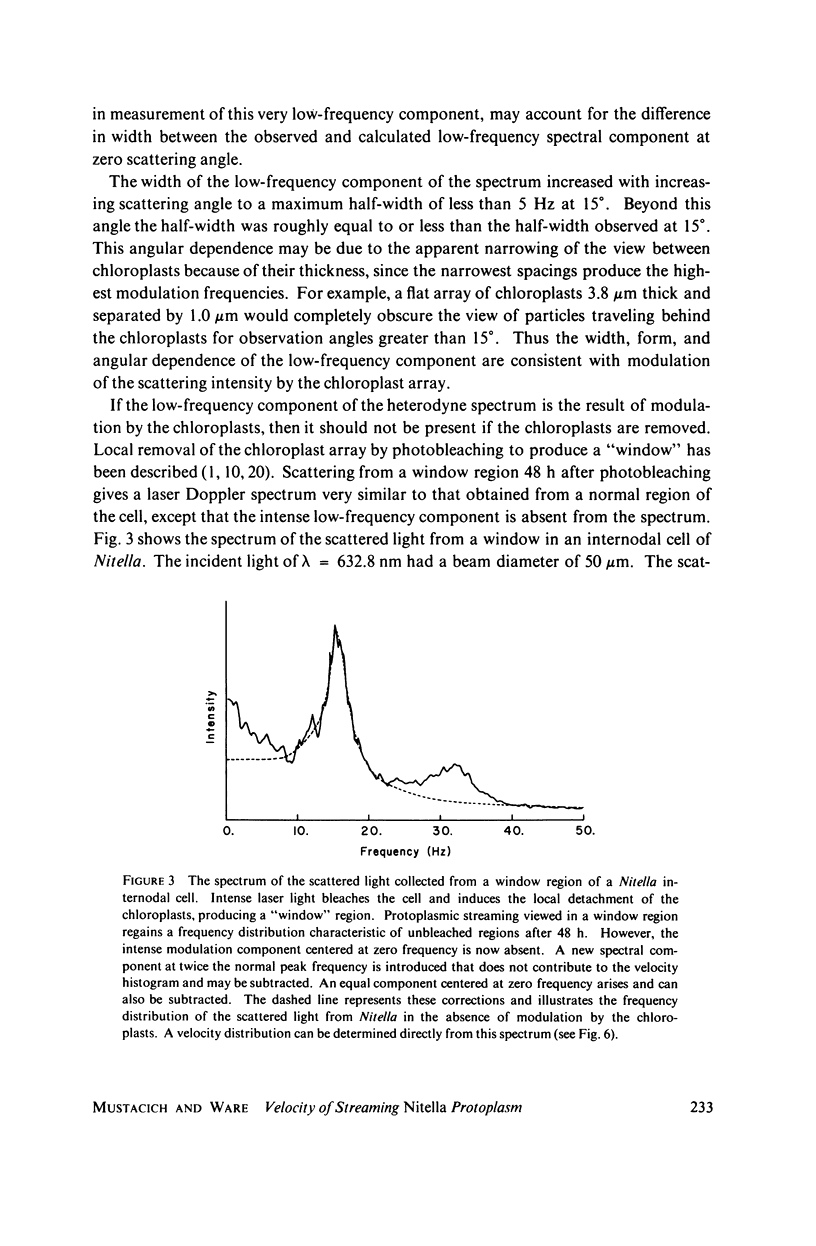
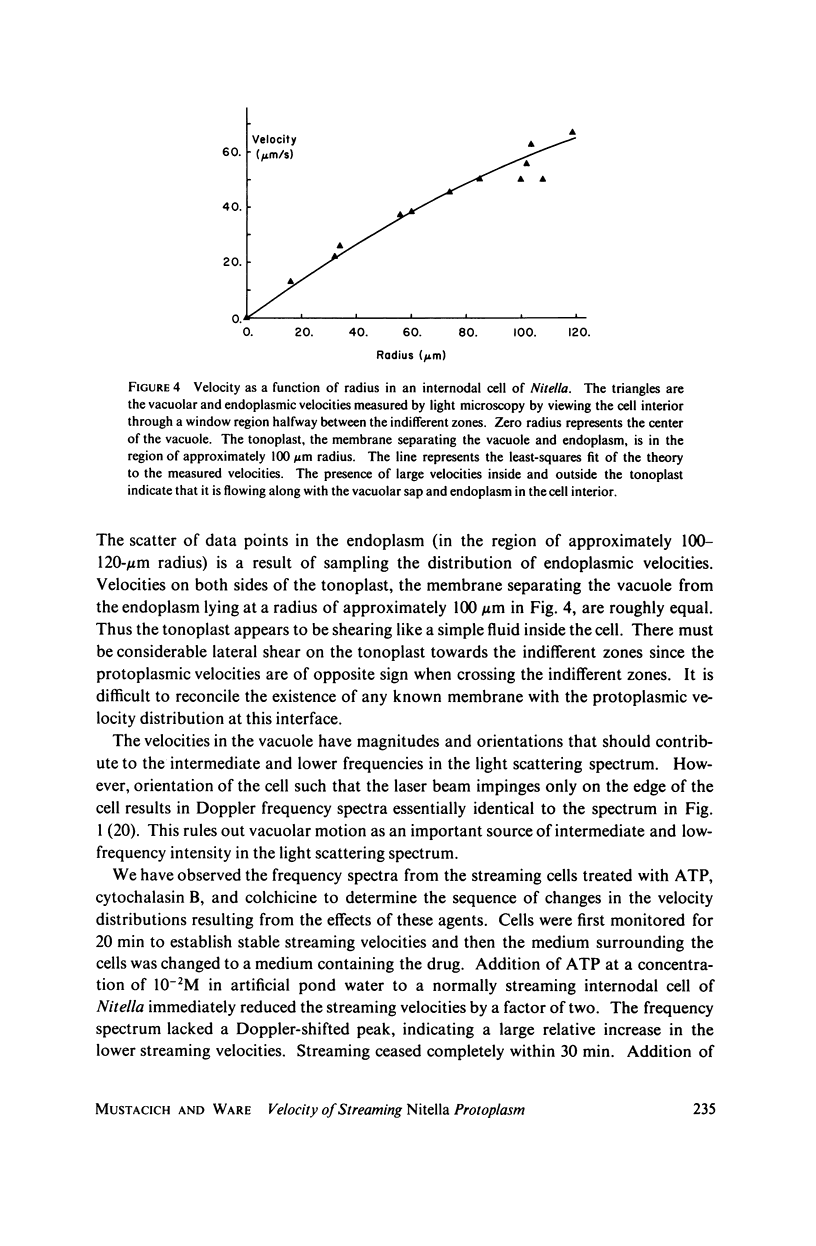
Laser light is Doppler-shifted in frequency by the streaming endoplasm of living cells of Nitella flexilis. The frequency spectrum of the scattered light can be interpreted as the histogram of velocities within the organism, with the exception of the intense low-frequency portion of the spectrum. We demonstrate that the lowest-frequency component is the result of amplitude modulation of the scattered light by the array of chloroplasts in the cell. Measurement of the streaming endoplasm in a photobleached "window" region allows correction of the frequency distribution for the modulation component. The complete velocity histogram for the streaming endoplasm is calculated directly from the corrected frequency distribution. Measurements of vacuolar and endoplasmic motions show that the tonoplast, the membrane separating the vacuole and the endoplasm, seems to be flowing along with the endoplasm and vacuolar sap. Placing the cell in medium containing ATP in concentrations greater than 10(-3) M greatly increases the contribution of low velocities to the velocity histogram. Cytochalasin B at high dosages (10-50 mug/ml) does not noticably change the shape of the velocity histogram, while at low dosages (1 mug/ml) there is an increase in the contribution of low velocities to the velocity histogram. Colchicine in high concentrations (1%) has no observable effect on the velocity histogram.
Full text
PDF


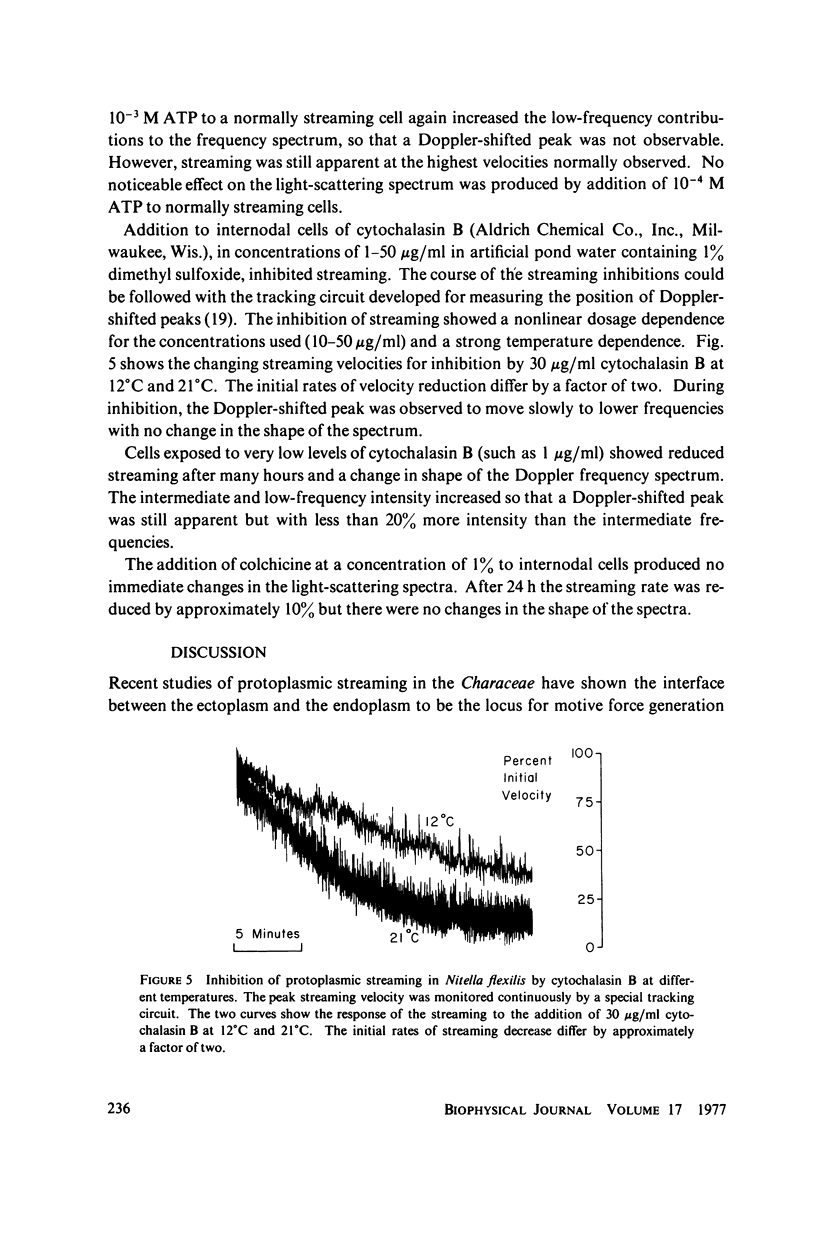
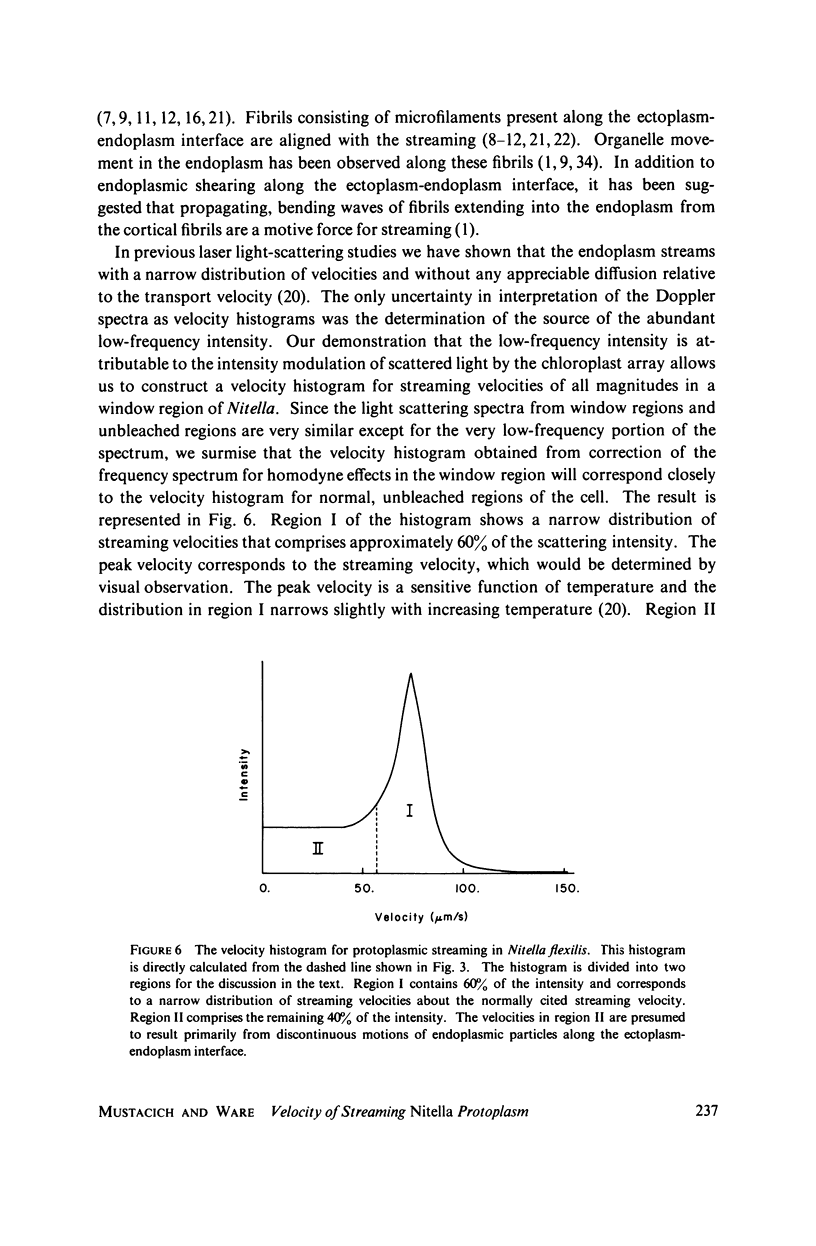




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen N. S. Endoplasmic filaments generate the motive force for rotational streaming in Nitella. J Cell Biol. 1974 Oct;63(1):270–287. doi: 10.1083/jcb.63.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisy G. G., Taylor E. W. The mechanism of action of colchicine. Binding of colchincine-3H to cellular protein. J Cell Biol. 1967 Aug;34(2):525–533. doi: 10.1083/jcb.34.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisy G. G., Taylor E. W. The mechanism of action of colchicine. Colchicine binding to sea urchin eggs and the mitotic apparatus. J Cell Biol. 1967 Aug;34(2):535–548. doi: 10.1083/jcb.34.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley M. O. Microfilaments and cytoplasmic streaming: inhibition of streaming with cytochalasin. J Cell Sci. 1973 Jan;12(1):327–343. doi: 10.1242/jcs.12.1.327. [DOI] [PubMed] [Google Scholar]
- Goldman R. D. The role of three cytoplasmic fibers in BHK-21 cell motility. I. Microtubules and the effects of colchicine. J Cell Biol. 1971 Dec;51(3):752–762. doi: 10.1083/jcb.51.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamitsubo E. A 'window technique' for detailed observation of characean cytoplasmic streaming. Exp Cell Res. 1972 Oct;74(2):613–616. doi: 10.1016/0014-4827(72)90430-2. [DOI] [PubMed] [Google Scholar]
- Kersey Y. M., Hepler P. K., Palevitz B. A., Wessells N. K. Polarity of actin filaments in Characean algae. Proc Natl Acad Sci U S A. 1976 Jan;73(1):165–167. doi: 10.1073/pnas.73.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersey Y. M., Wessells N. K. Localization of actin filaments in internodal cells of characean algae. A scanning and transmission electron microscope study. J Cell Biol. 1976 Feb;68(2):264–275. doi: 10.1083/jcb.68.2.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S., Santi D. V., Spudich J. A. Biochemical studies on the mode of action of cytochalasin B. Preparation of (3H)cytochalasin B and studies on its binding of cells. J Biol Chem. 1974 Apr 10;249(7):2268–2274. [PubMed] [Google Scholar]
- Mustacich R. V., Ware B. R. A study of protoplasmic streaming in Nitella by laser Doppler spectroscopy. Biophys J. 1976 May;16(5):373–388. doi: 10.1016/S0006-3495(76)85695-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai R., Rebhun L. I. Cytoplasmic microfilaments in streaming Nitella cells. J Ultrastruct Res. 1966 Mar;14(5):571–589. doi: 10.1016/s0022-5320(66)80083-7. [DOI] [PubMed] [Google Scholar]
- Palevitz B. A., Hepler P. K. Identification of actin in situ at the ectoplasm-endoplasm interface of Nitella. Microfilament-chloroplast association. J Cell Biol. 1975 Apr;65(1):29–38. doi: 10.1083/jcb.65.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puszkin E., Puszkin S., Lo L. W., Tanenbaum S. W. Binding of cytochalasin D to platelet and muscle myosin. J Biol Chem. 1973 Nov 25;248(22):7754–7761. [PubMed] [Google Scholar]
- ROBBINS E., GONATAS N. K. HISTOCHEMICAL AND ULTRASTRUCTURAL STUDIES ON HELA CELL CULTURES EXPOSED TO SPINDLE INHIBITORS WITH SPECIAL REFERENCE TO THE INTERPHASE CELL. J Histochem Cytochem. 1964 Sep;12:704–711. doi: 10.1177/12.9.704. [DOI] [PubMed] [Google Scholar]
- Takenaka T., Yoshioka T., Horie H. Physiological properties of protoplasmic drops of nitella. Adv Biophys. 1975;7:193–213. [PubMed] [Google Scholar]
- Tannenbaum J., Tanenbaum S. W., Lo L. W., Godman G. C., Miranda A. F. Binding and subcellular localization of tritiated cytochalasin D. Exp Cell Res. 1975 Mar 1;91(1):47–56. doi: 10.1016/0014-4827(75)90139-1. [DOI] [PubMed] [Google Scholar]
- Tilney L. G., Gibbins J. R. Microtubules in the formation and development of the primary mesenchyme in Arbacia punctulata. II. An experimental analysis of their role in development and maintenance of cell shape. J Cell Biol. 1969 Apr;41(1):227–250. doi: 10.1083/jcb.41.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L. G. Studies on the microtubules in heliozoa. IV. The effect of colchicine on the formation and maintenance of the axopodia and the redevelopment of pattern in Actinosphaerium nucleofilum (Barrett). J Cell Sci. 1968 Dec;3(4):549–562. doi: 10.1242/jcs.3.4.549. [DOI] [PubMed] [Google Scholar]
- Williamson R. E. A light-microscope study of the action of cytochalasin B on the cells and isolated cytoplasm of the characeae. J Cell Sci. 1972 May;10(3):811–819. doi: 10.1242/jcs.10.3.811. [DOI] [PubMed] [Google Scholar]
- Williamson R. E. Actin in the alga, Chara corallina. Nature. 1974 Apr 26;248(5451):801–802. doi: 10.1038/248801a0. [DOI] [PubMed] [Google Scholar]
- Williamson R. E. Cytoplasmic streaming in Chara: a cell model activated by ATP and inhibited by cytochalasin B. J Cell Sci. 1975 May;17(3):655–668. doi: 10.1242/jcs.17.3.655. [DOI] [PubMed] [Google Scholar]


