Abstract
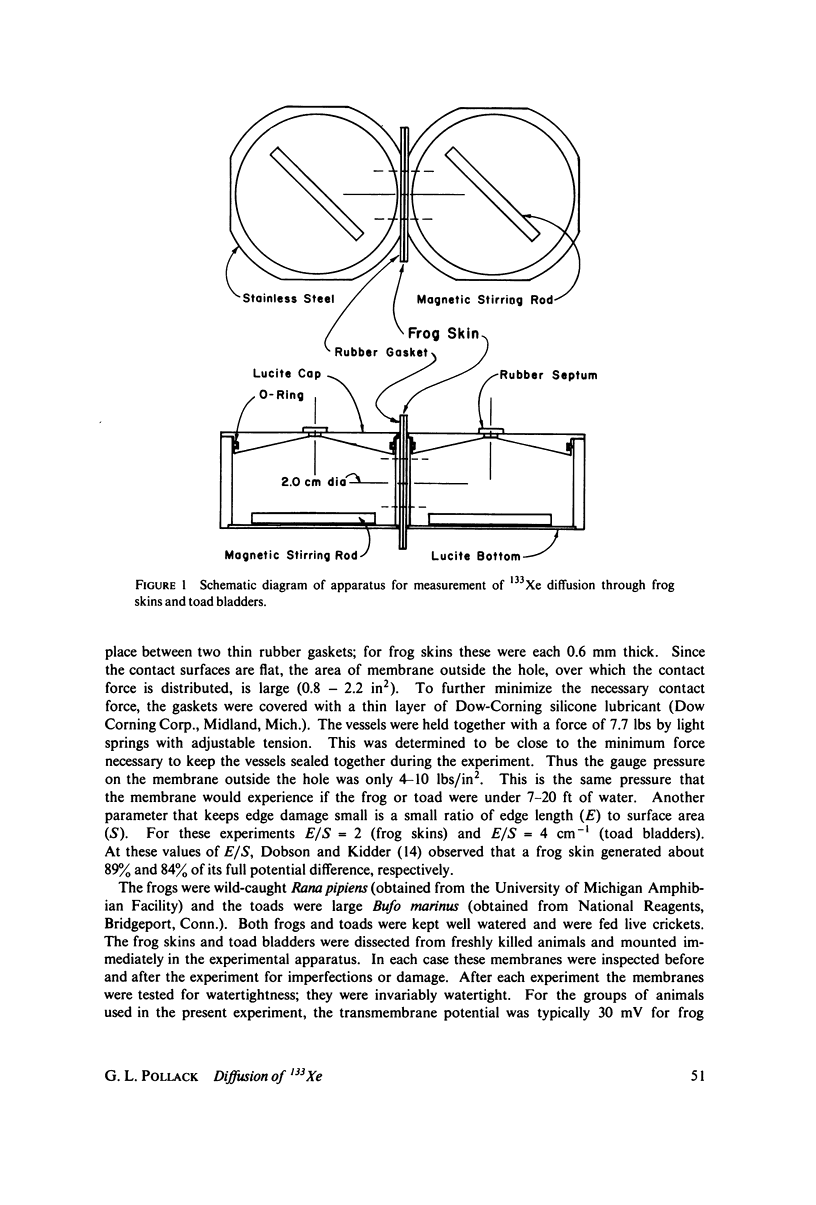
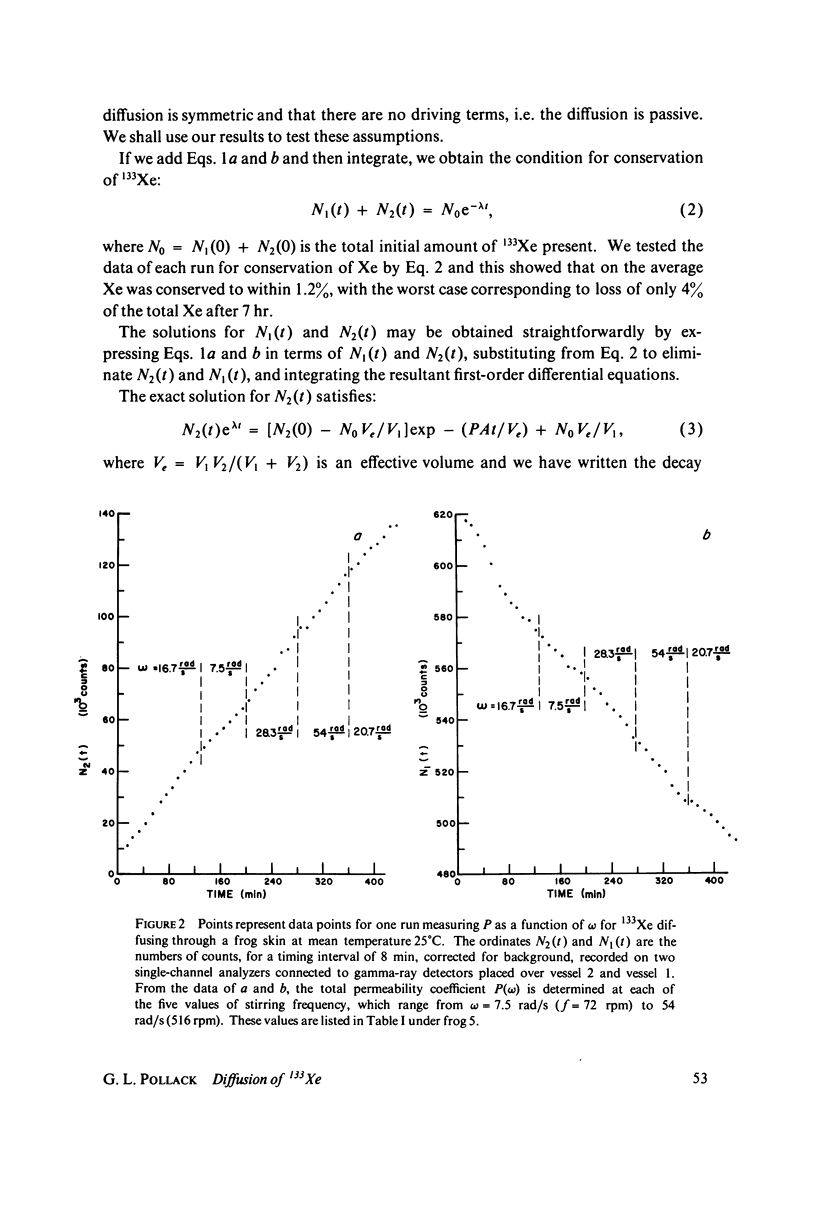
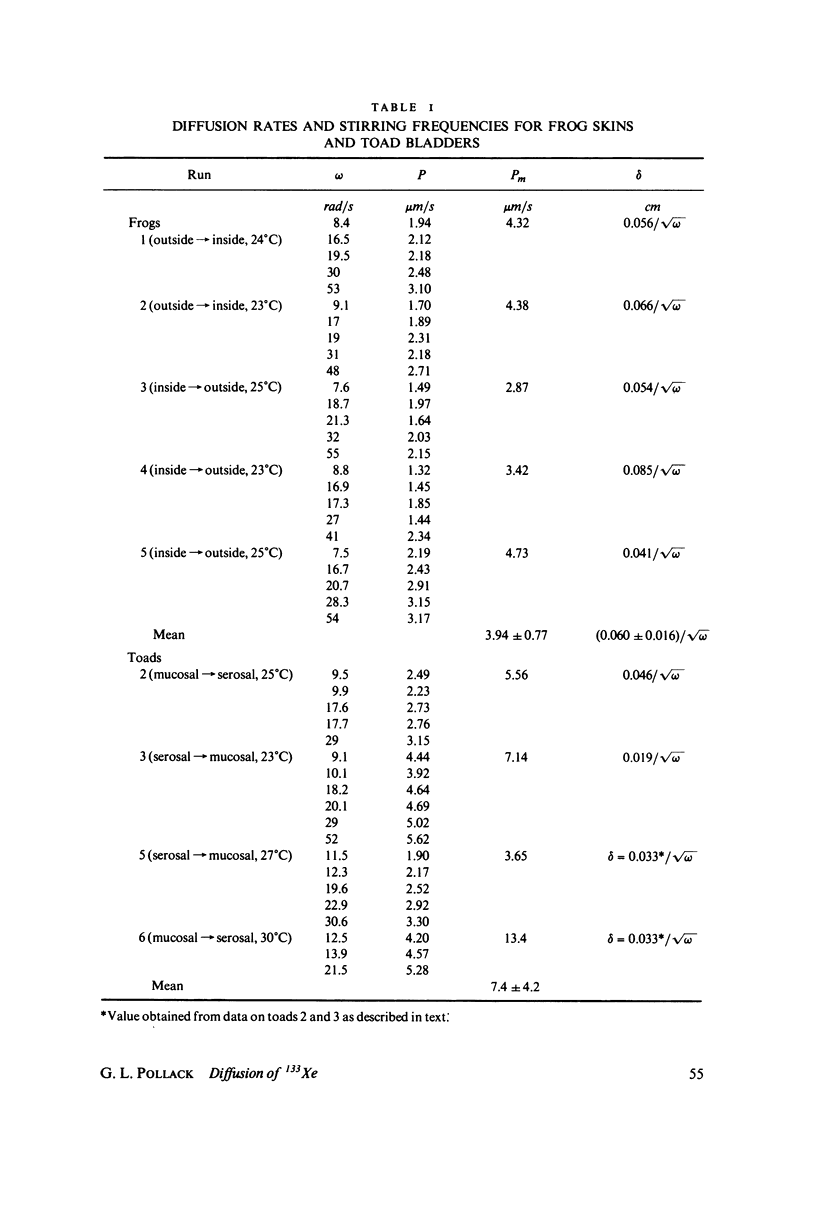
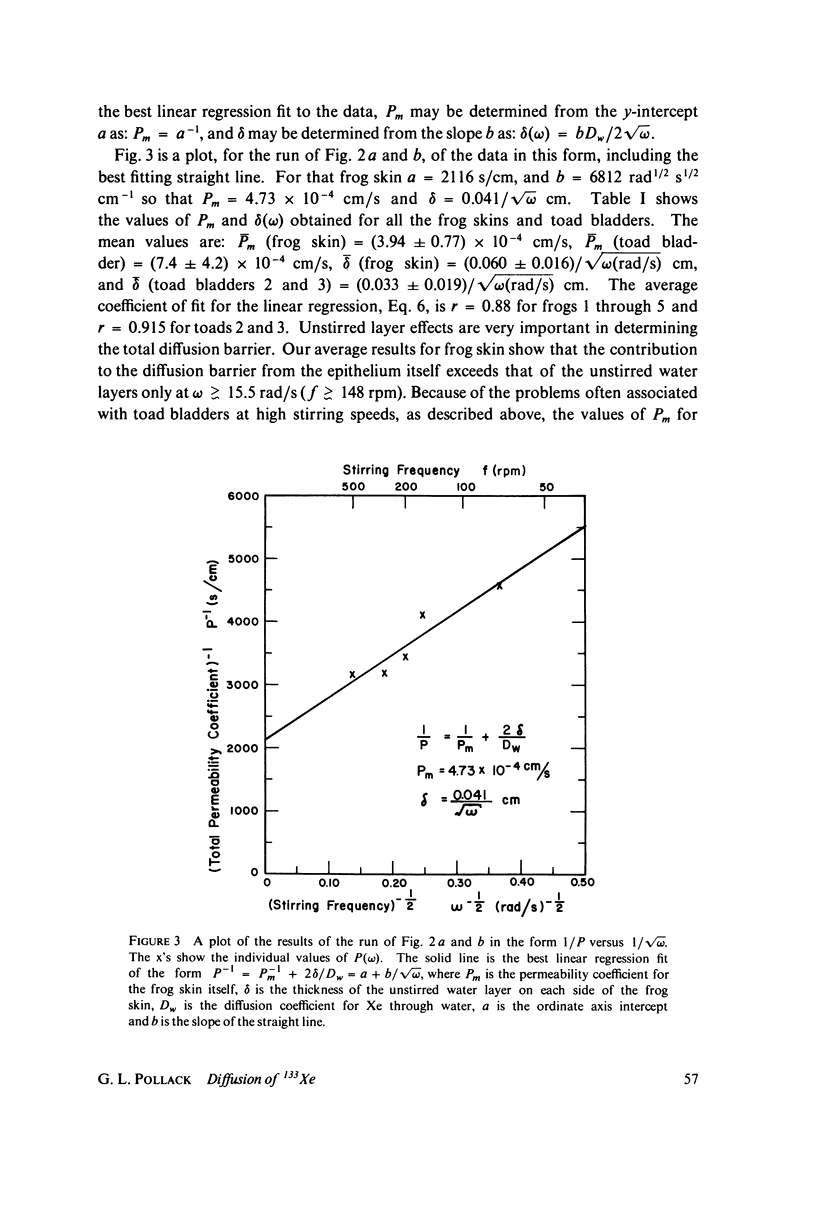
We have measured the total permeability coefficients P as a function of stirring frequency omega for 133Xe through frog skins and toad bladders. The permeability coefficients for the frog skins and toad bladders proper are, respectively, Pm = (3.9 +/- 0.8) X 10(-4) cm/s and (7.4 +/- 4.2) X 10(-4) cm/s. "Unstirred" water layer thickness delta is determined concurrently, from the frequency dependence of P(omega); the result for frog skin is delta = (0.060 +/- 0.016) square root of omega(rad/s) cm. The stirring frequency range is from omega = 7.5 rad/s (72 rpm) to 55 rad/s (530 rpm). The results support the conclusions that the principal barrier to Xe diffusion in these epithelia is inter- and intracellular water, and that the diffusion is passive and rapid. The experimental method may be straightforwardly adapted to the measurement of diffusion or counterdiffusion of any gamma-radioactive soluble or partly soluble solute through any flat membrane or through a solvent. We estimate the amount of total body-absorbed radioactivity due to environmental 133Xe to be 50 fCi for an ambient concentration of 2.6 pCi/m3 of air.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreoli T. E., Schafer J. A. Mass transport across cell membranes: the effects of antidiuretic hormone on water and solute flows in epithelia. Annu Rev Physiol. 1976;38:451–500. doi: 10.1146/annurev.ph.38.030176.002315. [DOI] [PubMed] [Google Scholar]
- Boeck W. L. Meteorological consequences of atmospheric krypton-85. Science. 1976 Jul 16;193(4249):195–198. doi: 10.1126/science.193.4249.195. [DOI] [PubMed] [Google Scholar]
- CULLEN S. C., GROSS E. G. The anesthetic properties of xenon in animals and human beings, with additional observations on krypton. Science. 1951 May 18;113(2942):580–582. doi: 10.1126/science.113.2942.580. [DOI] [PubMed] [Google Scholar]
- Dainty J., House C. R. Unstirred layers in frog skin. J Physiol. 1966 Jan;182(1):66–78. doi: 10.1113/jphysiol.1966.sp007809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson J. G., Jr, Kidder G. W., 3rd Edge damage effect in in vitro frog skin preparations. Am J Physiol. 1968 Apr;214(4):719–724. doi: 10.1152/ajplegacy.1968.214.4.719. [DOI] [PubMed] [Google Scholar]
- Dragomir C. T., Stefãnescu D. T., Georgescu I., Stere G. N., Ciucă L., Tudor U. F., Chirvasie R. Xeonon accumulation in the red blood cell. A process latered by suppressors of the membrane active transport function. Biochim Biophys Acta. 1975 May 21;389(3):530–540. doi: 10.1016/0005-2736(75)90163-7. [DOI] [PubMed] [Google Scholar]
- Farquhar M. G., Palade G. E. Cell junctions in amphibian skin. J Cell Biol. 1965 Jul;26(1):263–291. doi: 10.1083/jcb.26.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYS R. M., LEAF A. Studies on the movement of water through the isolated toad bladder and its modification by vasopressin. J Gen Physiol. 1962 May;45:905–919. doi: 10.1085/jgp.45.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helman S. I., Miller D. A. Edge damage effect on electrical measurements of frog skin. Am J Physiol. 1973 Oct;225(4):972–977. doi: 10.1152/ajplegacy.1973.225.4.972. [DOI] [PubMed] [Google Scholar]
- Kunz C. O., Paperiello C. J. Xenon-133: ambient activity from nuclear power stations. Science. 1976 Jun 18;192(4245):1235–1237. doi: 10.1126/science.192.4245.1235. [DOI] [PubMed] [Google Scholar]
- LEAF A., HAYS R. M. Permeability of the isolated toad bladder to solutes and its modification by vasopressin. J Gen Physiol. 1962 May;45:921–932. doi: 10.1085/jgp.45.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S. A., Diamond J. M. Active sodium transport by mammalian urinary bladder. Nature. 1975 Feb 27;253(5494):747–748. doi: 10.1038/253747a0. [DOI] [PubMed] [Google Scholar]
- PEACHEY L. D., RASMUSSEN H. Structure of the toad's urinary bladder as related to its physiology. J Biophys Biochem Cytol. 1961 Aug;10:529–553. doi: 10.1083/jcb.10.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi M., Piccinni Z. F. The penetration of water into the epithelium of toad urinary bladder and its modification by oxytocin. J Membr Biol. 1973;12(3):227–246. doi: 10.1007/BF01870003. [DOI] [PubMed] [Google Scholar]
- Raymond L., Weiskopf R. B., Halsey M. J., Goldfien A., Eger E. I., 3rd, Severinghaus J. W. Possible mechanism for the antiarrhythmic effect of helium in anesthetized dogs. Science. 1972 Jun 16;176(4040):1250–1252. doi: 10.1126/science.176.4040.1250. [DOI] [PubMed] [Google Scholar]
- Schafer J. A., Andreoli T. E. Cellular constraints to diffusion. The effect of antidiuretic hormone on water flows in isolated mammalian collecting tubules. J Clin Invest. 1972 May;51(5):1264–1278. doi: 10.1172/JCI106921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- VOUTE C. L. AN ELECTRON MICROSCOPIC STUDY OF THE SKIN OF THE FROG (RANA PIPIENS). J Ultrastruct Res. 1963 Dec;52:497–510. doi: 10.1016/s0022-5320(63)80081-7. [DOI] [PubMed] [Google Scholar]
- Walser M. Role of edge damage in sodium permeability of toad bladder and a means of avoiding it. Am J Physiol. 1970 Jul;219(1):252–255. doi: 10.1152/ajplegacy.1970.219.1.252. [DOI] [PubMed] [Google Scholar]



