Abstract
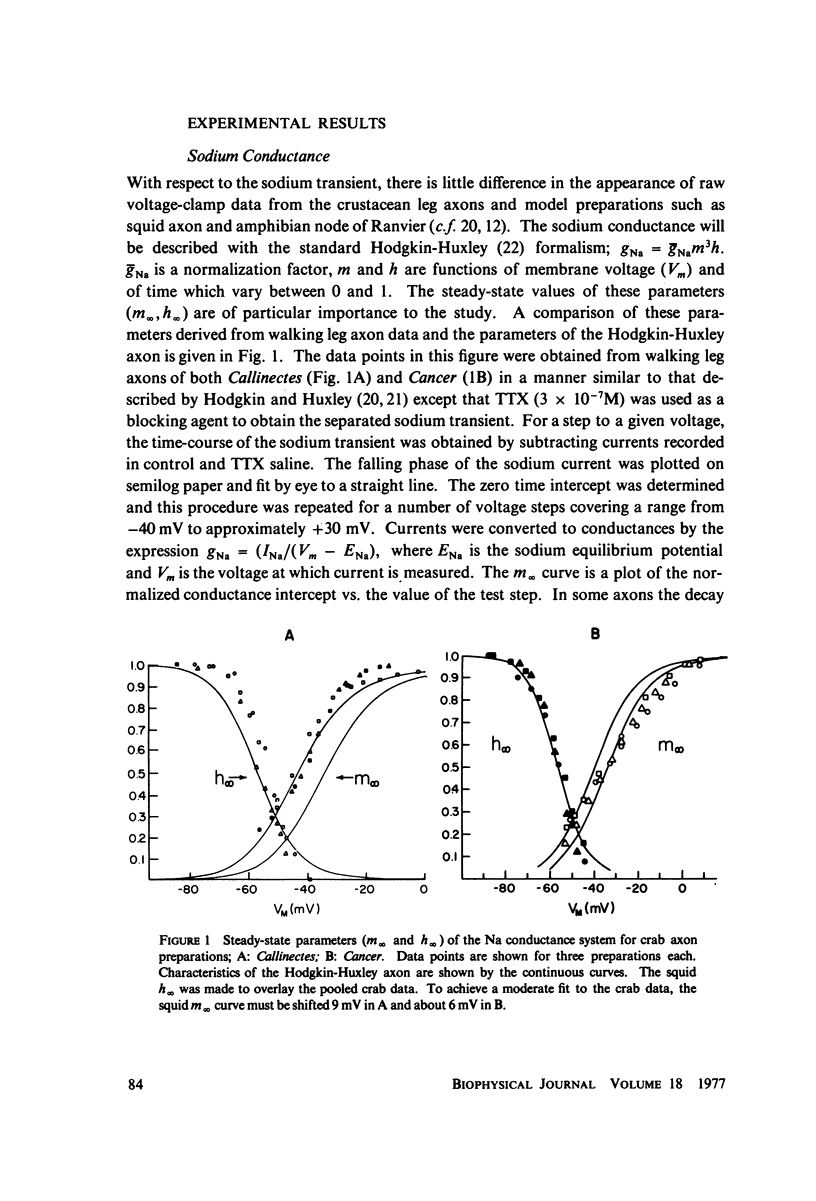
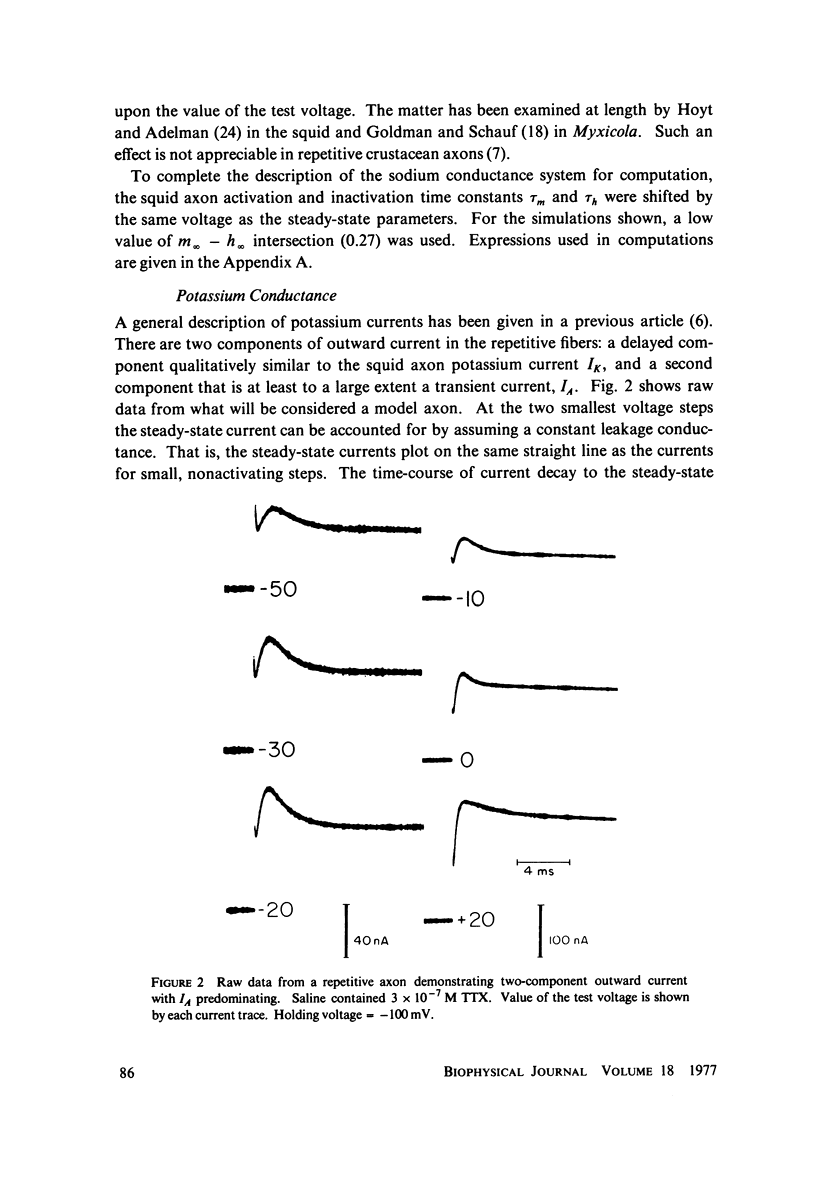
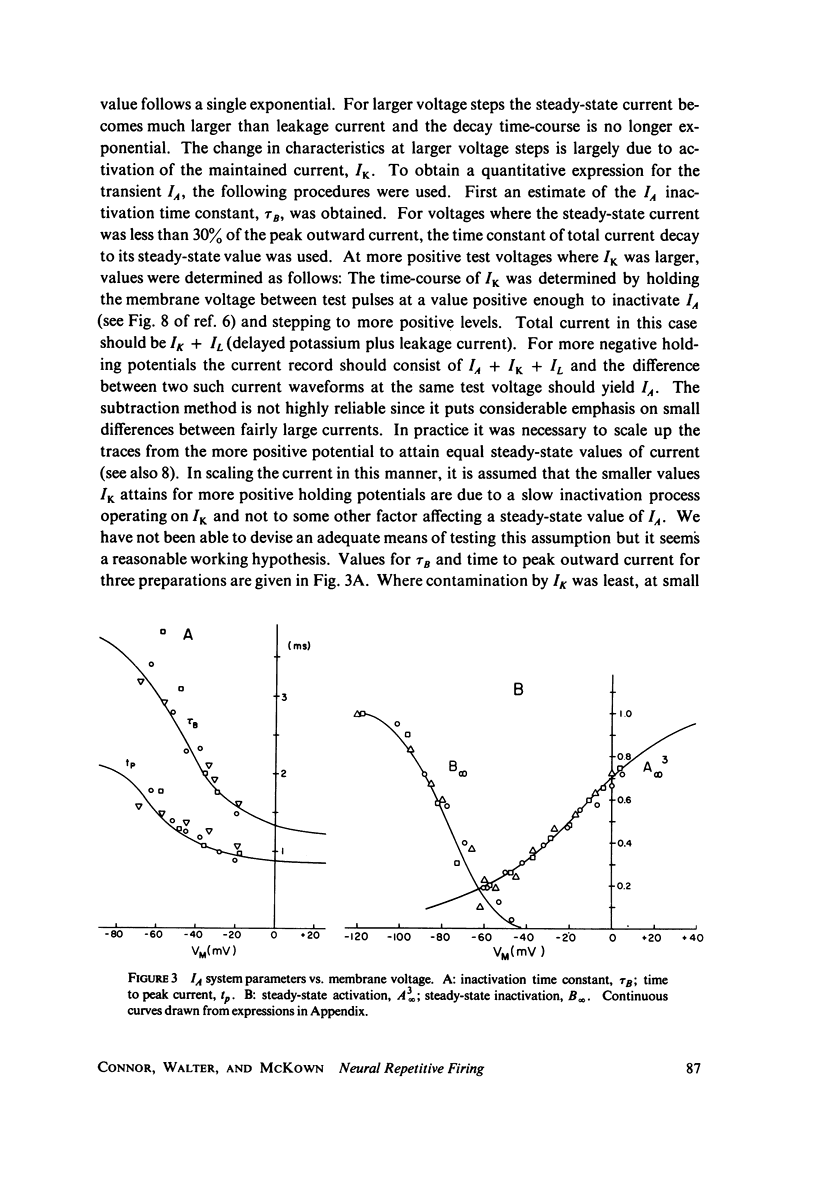
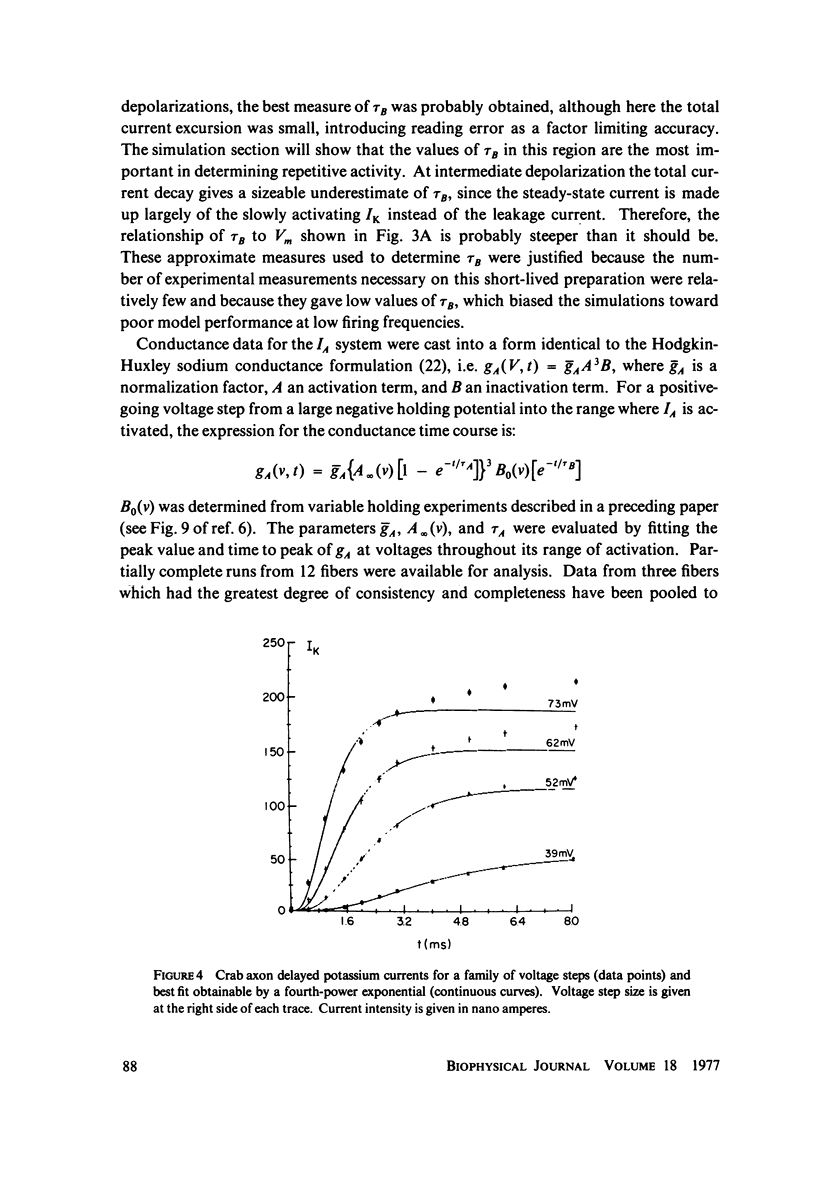
The Hodgkin-Huxley equations for space-clamped squid axon (18 degrees C) have been modified to approximate voltage clamp data from repetitive-firing crustacean walking leg axons and activity in response to constant current stimulation has been computed. The m infinity and h infinity parameters of the sodium conductance system were shifted along the voltage axis in opposite directions so that their relative overlap was increased approximately 7 mV. Time constants tau m and tau h, were moved in a similar manner. Voltage-dependent parameters of delayed potassium conductance, n infinity and tau n, were shifted 4.3 mV in the positive direction and tau n was uniformly increased by a factor of 2. Leakage conductance and capacitance were unchanged. Repetitive activity of this modified circuit was qualitatively similar to that of the standard model. A fifth branch was added to the circuit representing a transient potassium conductance system present in the repetitive walking leg axons and in other repetitive neurons. This model, with various parameter choices, fired repetitively down to approximately 2 spikes/s and up to 350/s. The frequency vs. stimulus current plot could be fit well by a straight line over a decade of the low frequency range and the general appearance of the spike trains was similar to that of other repetitive neurons. Stimulus intensities were of the same order as those which produce repetitive activity in the standard Hodgkin-Huxley axon. The repetitive firing rate and first spike latency (utilization time) were found to be most strongly influenced by the inactivation time constant of the transient potassium conductance (tau b), the delayed potassium conductance (tau n), and the value of leakage conductance (gL). The model presents a mechanism by which stable low frequency discharge can be generated by millisecond-order membrane conductance changes.
Full text
PDF



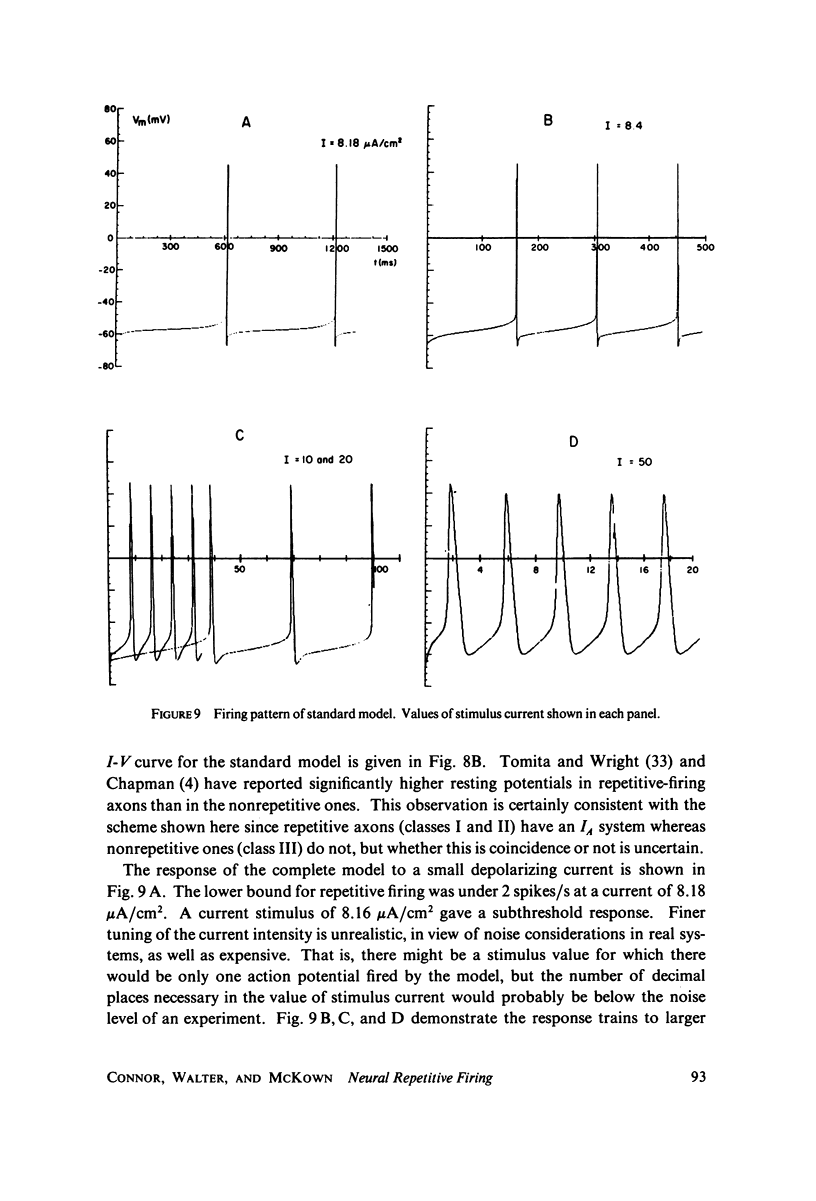
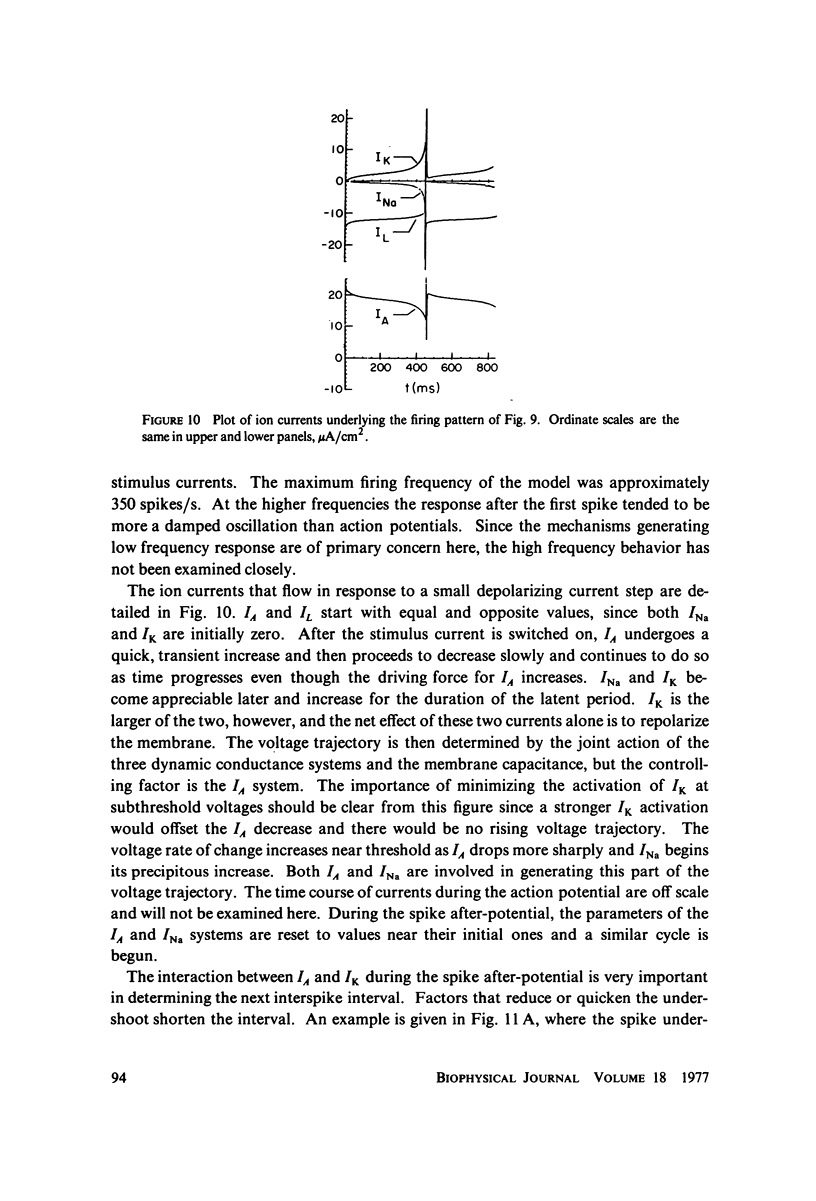
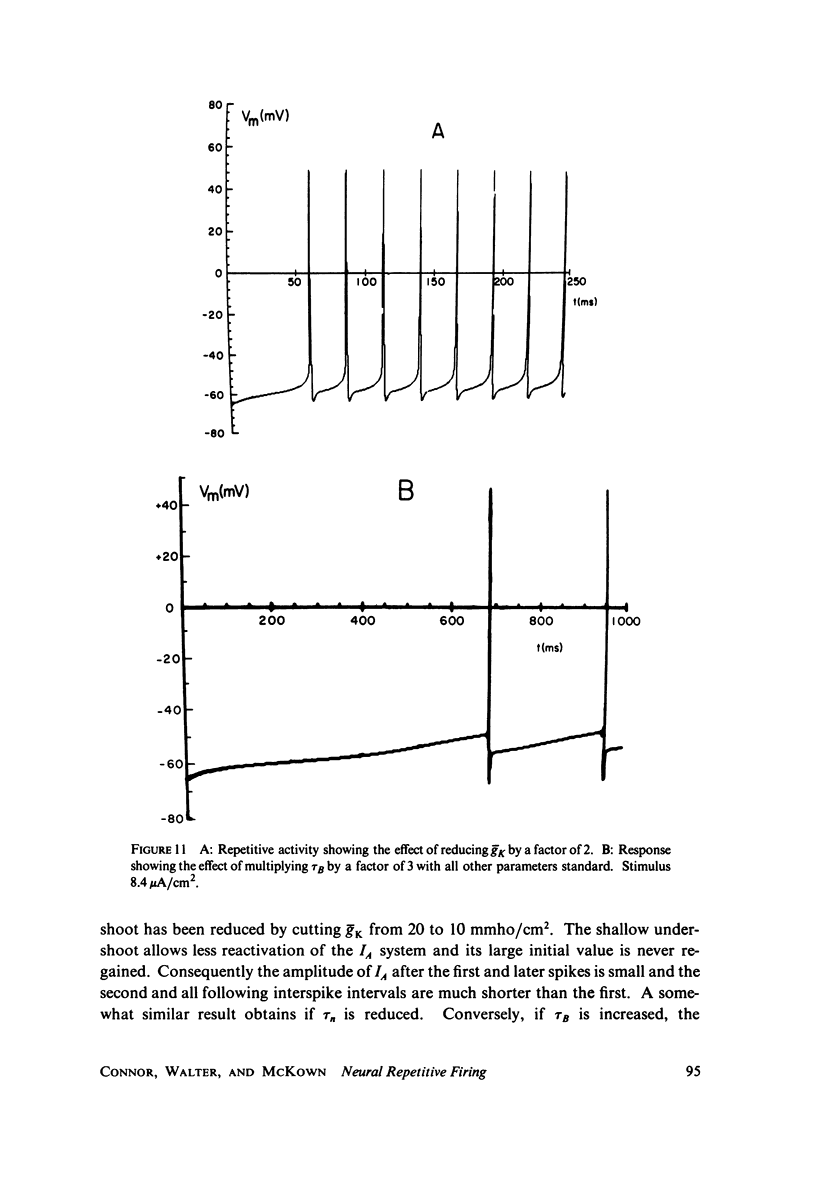
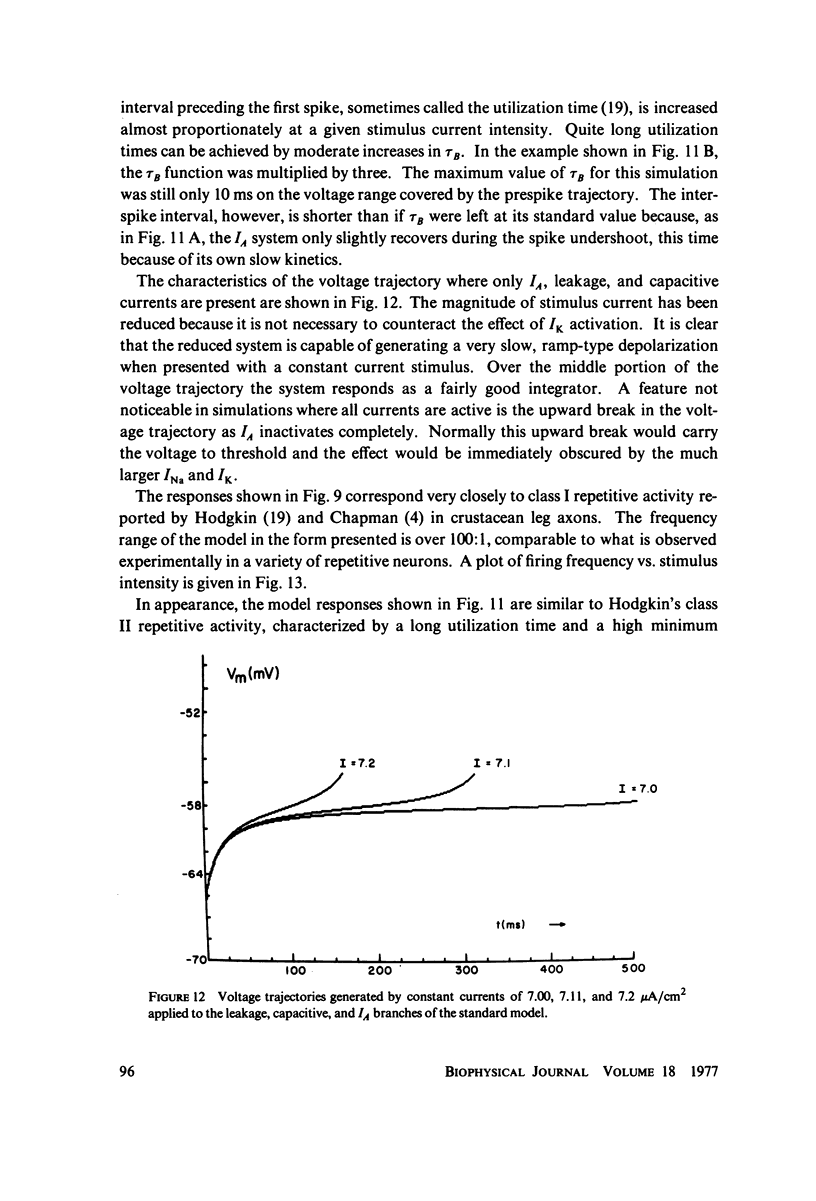





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AGIN D. HODGKIN-HUXLEY EQUATIONS: LOGARITHMIC RELATION BETWEEN MEMBRANE CURRENT AND FREQUENCY OF REPETITIVE ACTIVITY. Nature. 1964 Feb 8;201:625–626. doi: 10.1038/201625a0. [DOI] [PubMed] [Google Scholar]
- Bromm B., Frankenhaeuser B. Repetitive discharge of the excitable membrane computed on the basis of voltage clamp data for the node of Ranvier. Pflugers Arch. 1972;332(1):21–27. [PubMed] [Google Scholar]
- COLE K. S., MOORE J. W. Potassium ion current in the squid giant axon: dynamic characteristic. Biophys J. 1960 Sep;1:1–14. doi: 10.1016/s0006-3495(60)86871-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A. Neural repetitive firing: a comparative study of membrane properties of crustacean walking leg axons. J Neurophysiol. 1975 Jul;38(4):922–932. doi: 10.1152/jn.1975.38.4.922. [DOI] [PubMed] [Google Scholar]
- DALTON J. C. Effects of external ions on membrane potentials of a lobster giant axon. J Gen Physiol. 1958 Jan 20;41(3):529–542. doi: 10.1085/jgp.41.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE F. A., FRANKENHAEUSER B. Sodium currents in the myelinated nerve fibre of Xenopus laevis investigated with the voltage clamp technique. J Physiol. 1959 Oct;148:188–200. doi: 10.1113/jphysiol.1959.sp006281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr On the transduction of visual, mechanical, and chemical stimuli. Int J Neurosci. 1972 Jan;3(1):5–14. doi: 10.3109/00207457209147434. [DOI] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HUXLEY A. F. THE ACTION POTENTIAL IN THE MYELINATED NERVE FIBER OF XENOPUS LAEVIS AS COMPUTED ON THE BASIS OF VOLTAGE CLAMP DATA. J Physiol. 1964 Jun;171:302–315. doi: 10.1113/jphysiol.1964.sp007378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., VALLBO A. B. ACCOMMODATION IN MYELINATED NERVE FIBRES OF XENOPUS LAEVIS AS COMPUTED ON THE BASIS OF VOLTAGE CLAMP DATA. Acta Physiol Scand. 1965 Jan-Feb;63:1–20. doi: 10.1111/j.1748-1716.1965.tb04037.x. [DOI] [PubMed] [Google Scholar]
- FUORTES M. G., MANTEGAZZINI F. Interpretation of the repetitive firing of nerve cells. J Gen Physiol. 1962 Jul;45:1163–1179. doi: 10.1085/jgp.45.6.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman L., Schauf C. L. Inactivation of the sodium current in Myxicola giant axons. Evidence for coupling to the activation process. J Gen Physiol. 1972 Jun;59(6):659–675. doi: 10.1085/jgp.59.6.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F., KATZ B. Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):424–448. doi: 10.1113/jphysiol.1952.sp004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F. Ion movements during nerve activity. Ann N Y Acad Sci. 1959 Aug 28;81:221–246. doi: 10.1111/j.1749-6632.1959.tb49311.x. [DOI] [PubMed] [Google Scholar]
- Hoyt R. C., Adelman W. J., Jr Sodium inactivation. Experimental test of two models. Biophys J. 1970 Jul;10(7):610–617. doi: 10.1016/S0006-3495(70)86323-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernell D., Sjöholm H. Repetitive impulse firing: comparisons between neurone models based on 'voltage clamp equations' and spinal motoneurones. Acta Physiol Scand. 1973 Jan;87(1):40–56. doi: 10.1111/j.1748-1716.1973.tb05364.x. [DOI] [PubMed] [Google Scholar]
- Moore J. W., Cox E. B. A kinetic model for the sodium conductance system in squid axon. Biophys J. 1976 Feb;16(2 Pt 1):171–192. doi: 10.1016/s0006-3495(76)85673-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. W., Ramon F. On numerical integration of the Hodgkin and Huxley equations for a membrane action potential. J Theor Biol. 1974 May;45(1):249–273. doi: 10.1016/0022-5193(74)90054-x. [DOI] [PubMed] [Google Scholar]
- Partridge L. D., Stevens C. F. A mechanism for spike frequency adaptation. J Physiol. 1976 Apr;256(2):315–332. doi: 10.1113/jphysiol.1976.sp011327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro B. I., Lenherr F. K. Hodgkin-Huxley axon. Increased modulation and linearity of response to constant current stimulus. Biophys J. 1972 Sep;12(9):1145–1158. doi: 10.1016/S0006-3495(72)86151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein R. B. The frequency of nerve action potentials generated by applied currents. Proc R Soc Lond B Biol Sci. 1967 Jan 31;167(1006):64–86. doi: 10.1098/rspb.1967.0013. [DOI] [PubMed] [Google Scholar]
- Tomita T., Wright E. B. A study of the crustacean axon repetitive response. I. The effect of membrane potential and resistance. J Cell Physiol. 1965 Apr;65(2):195–209. doi: 10.1002/jcp.1030650207. [DOI] [PubMed] [Google Scholar]
- VALLBO A. B. ACCOMMODATION RELATED TO INACTIVATION OF THE SODIUM PERMEABILITY IN SINGLE MYELINATED NERVE FIBRES FROM XENOPUS LAEVIS. Acta Physiol Scand. 1964 Aug;61:429–444. [PubMed] [Google Scholar]
- Wright E. B., Tomita T. A study of the crustacean axon repetitive response. II. The effect of cations, sodium, calcium (magnesium), potassium and hydrogen (pH) in the external medium. J Cell Physiol. 1965 Apr;65(2):211–228. doi: 10.1002/jcp.1030650208. [DOI] [PubMed] [Google Scholar]


