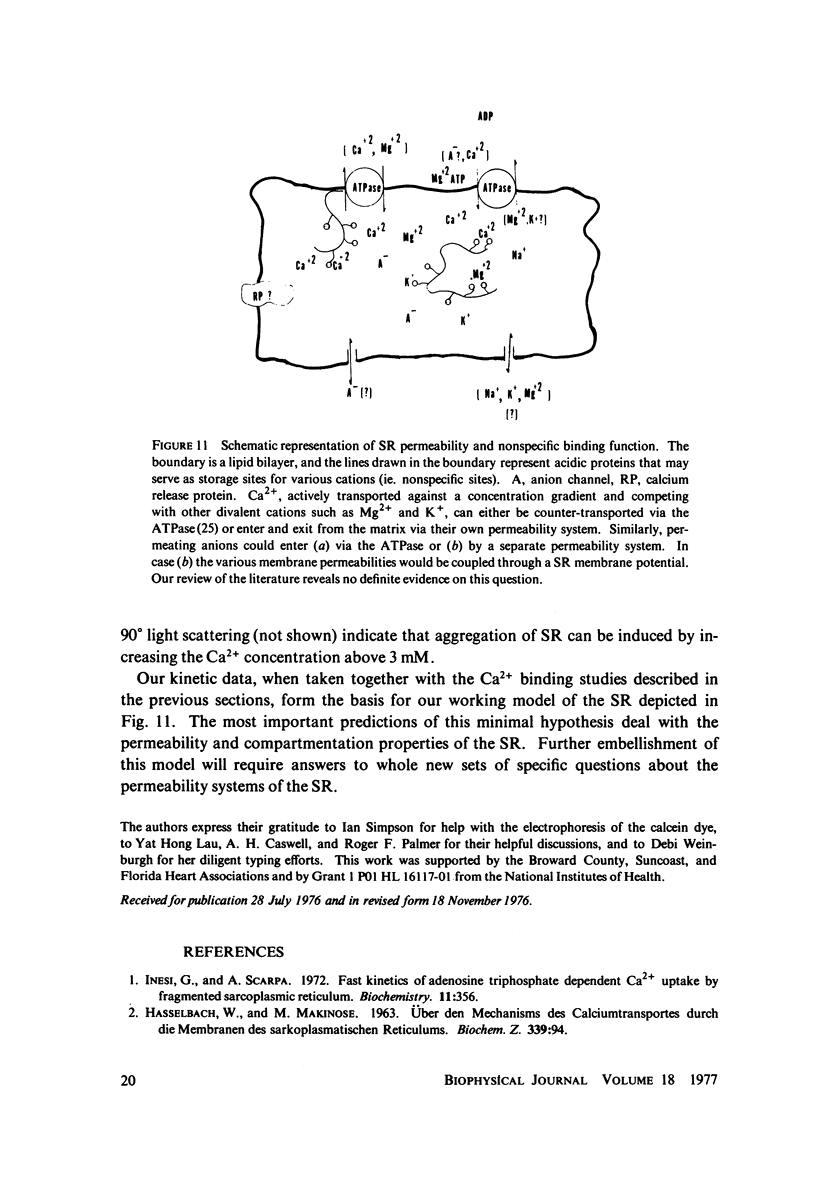
Abstract
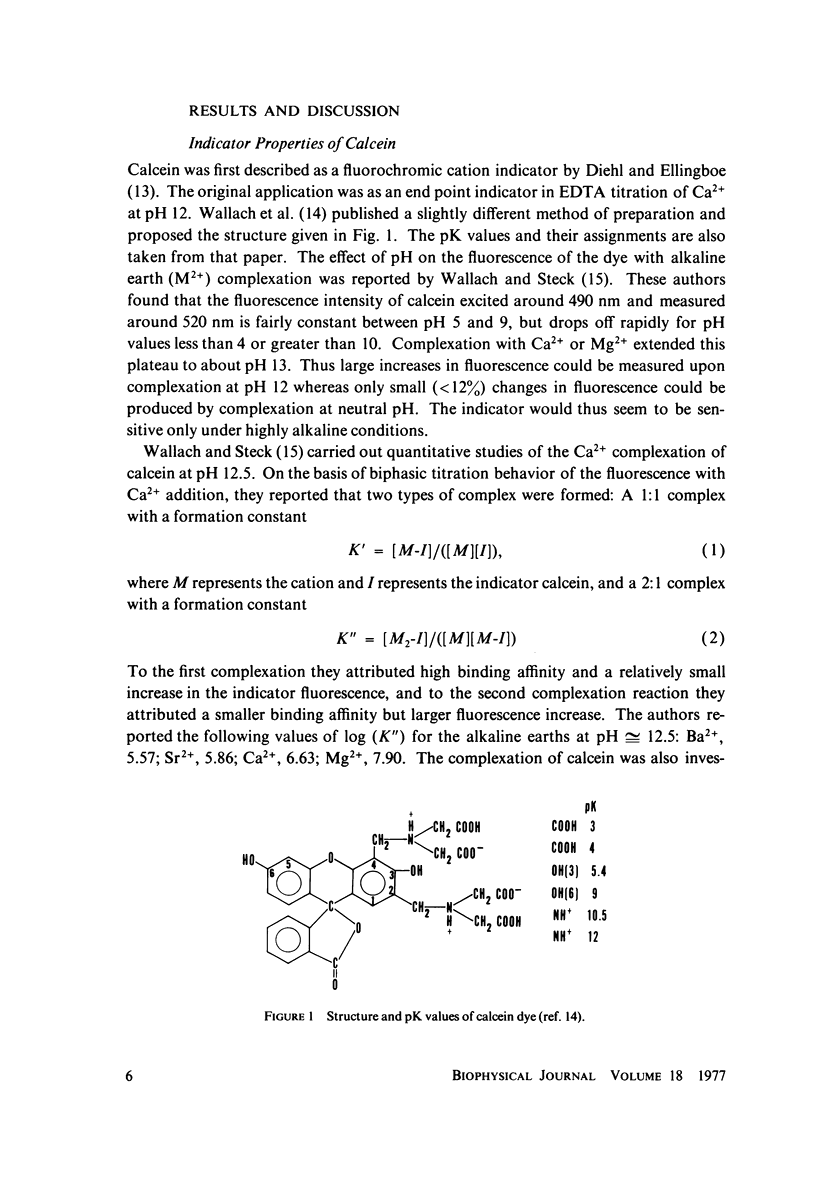
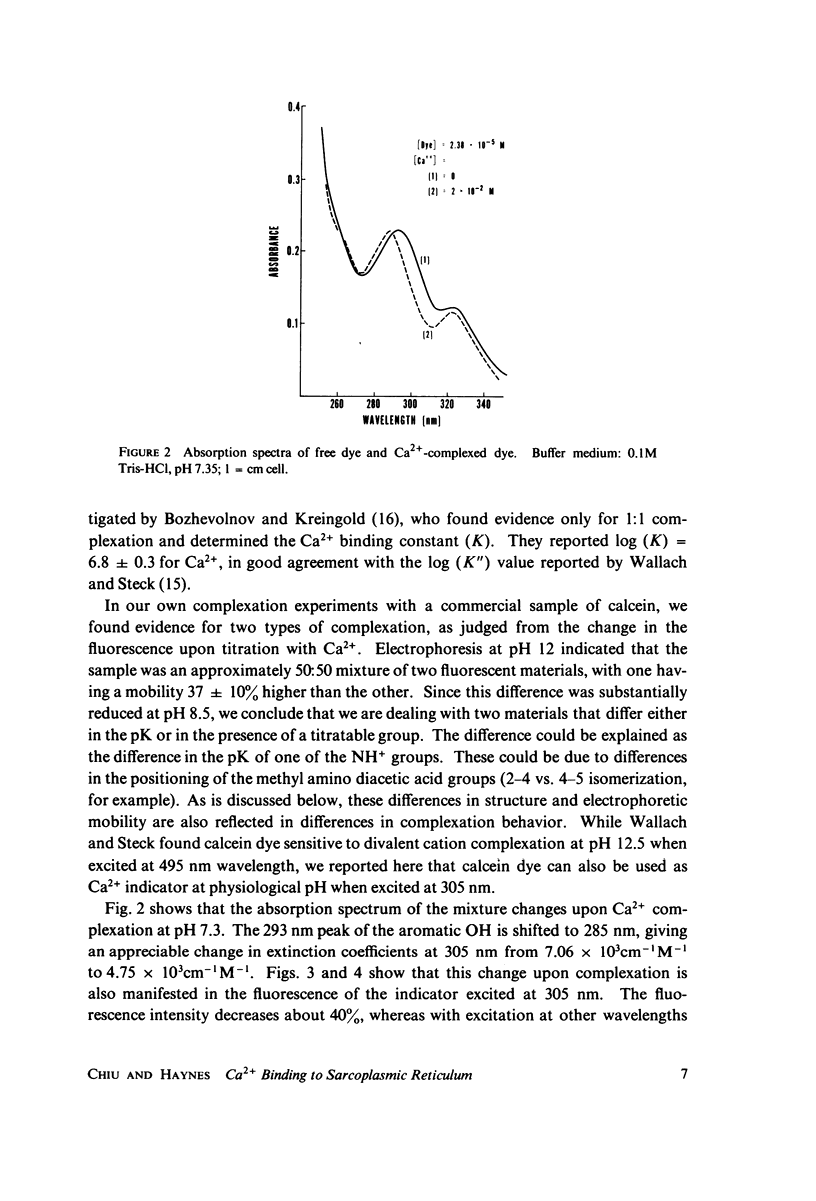
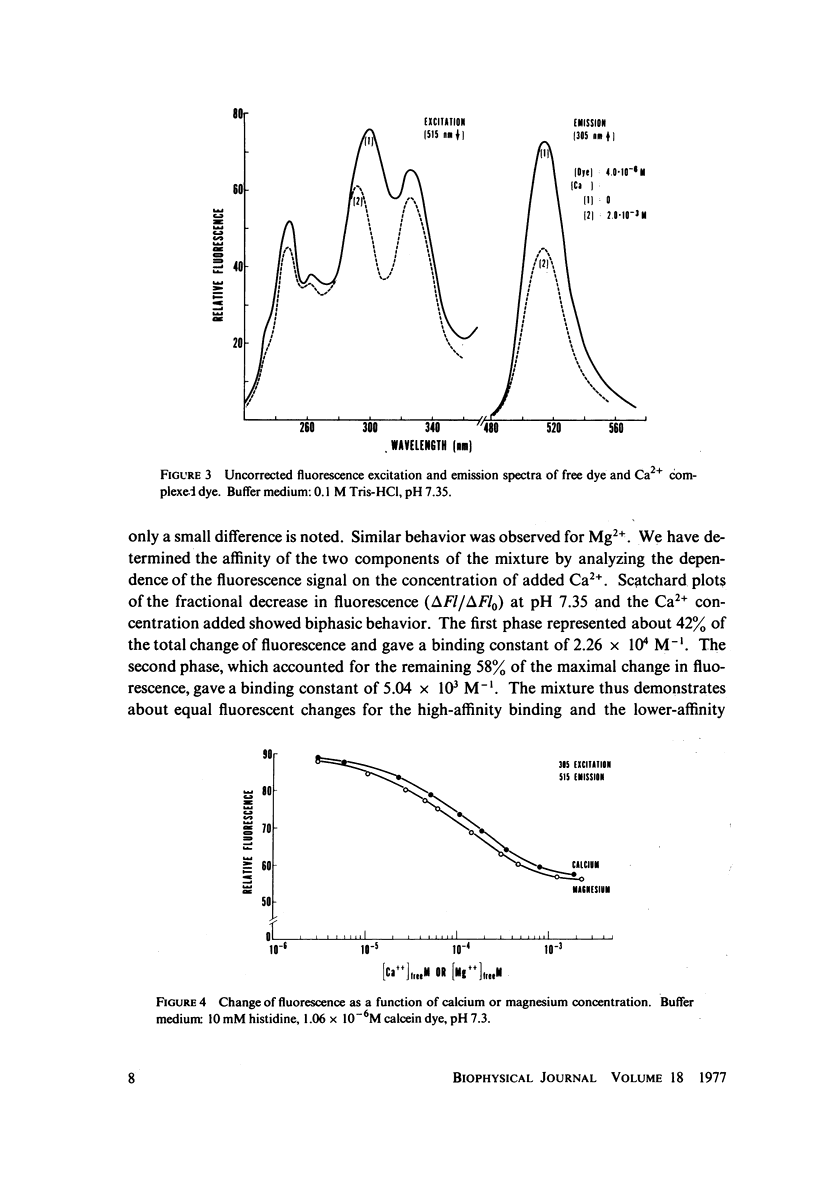
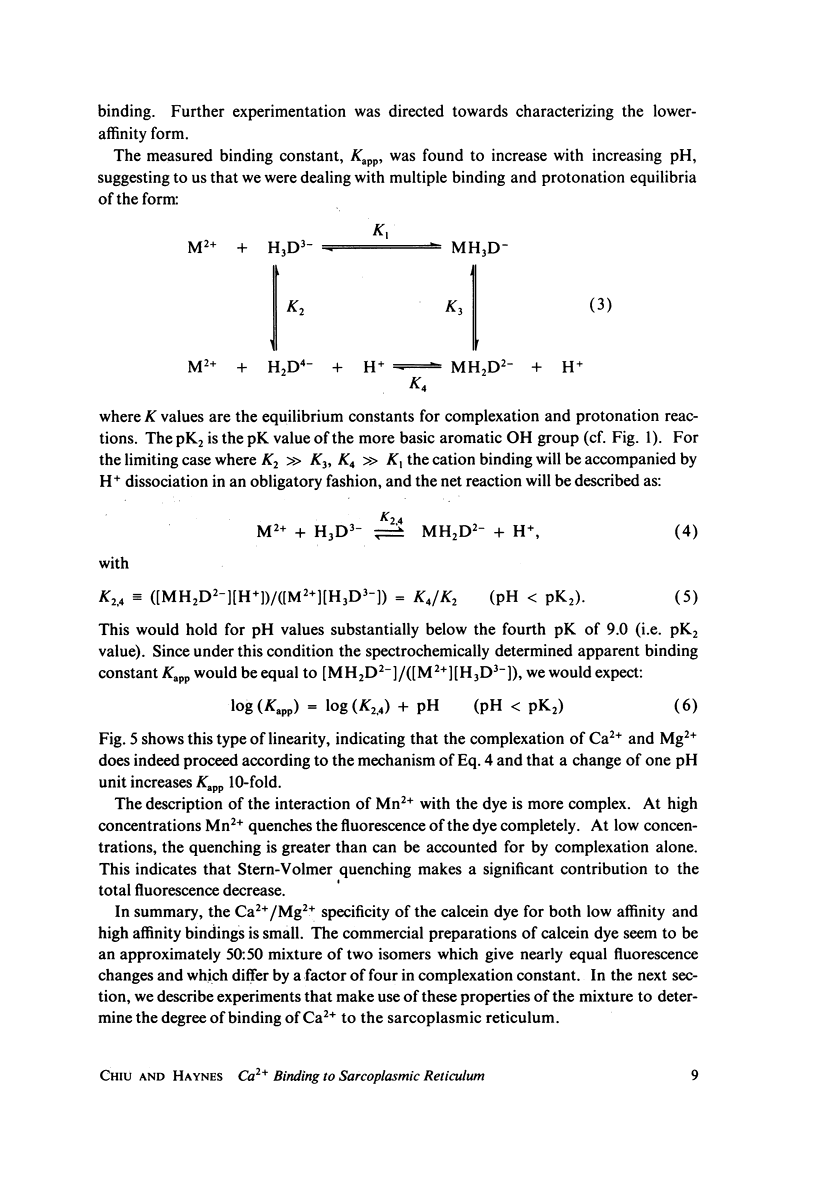
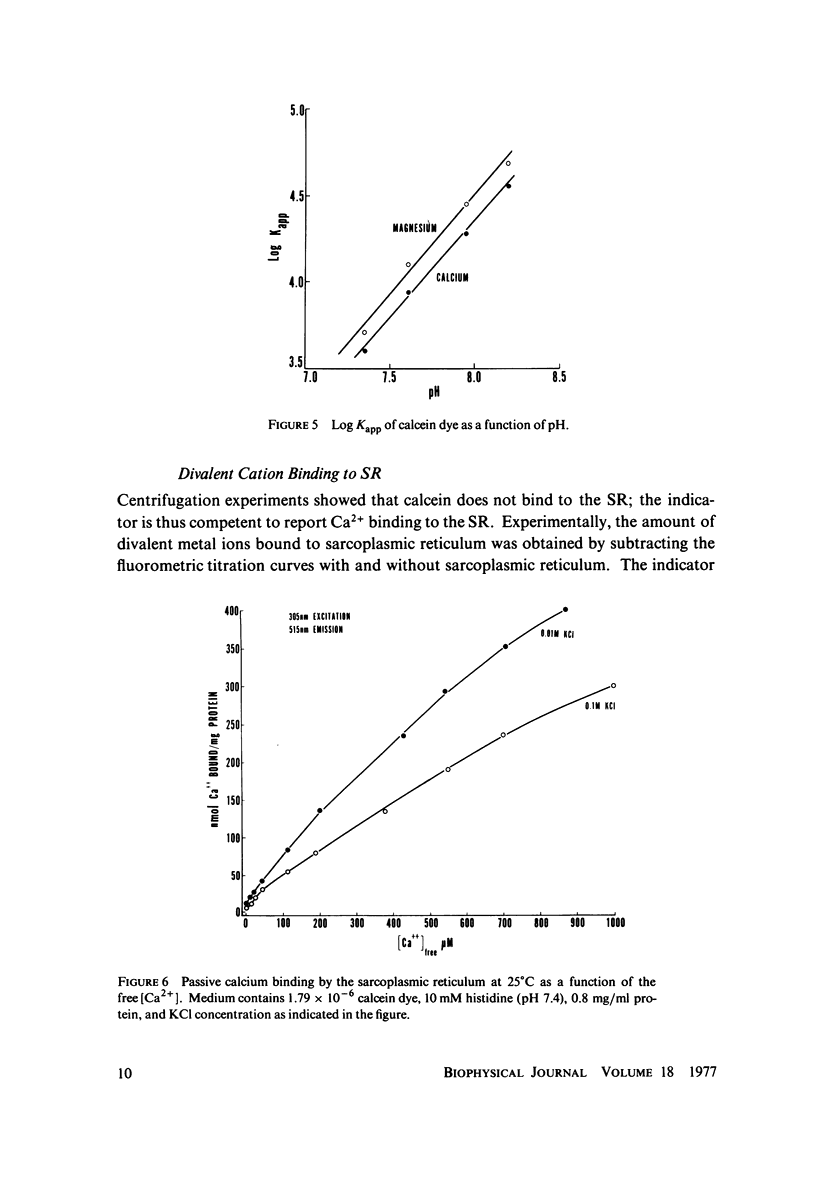
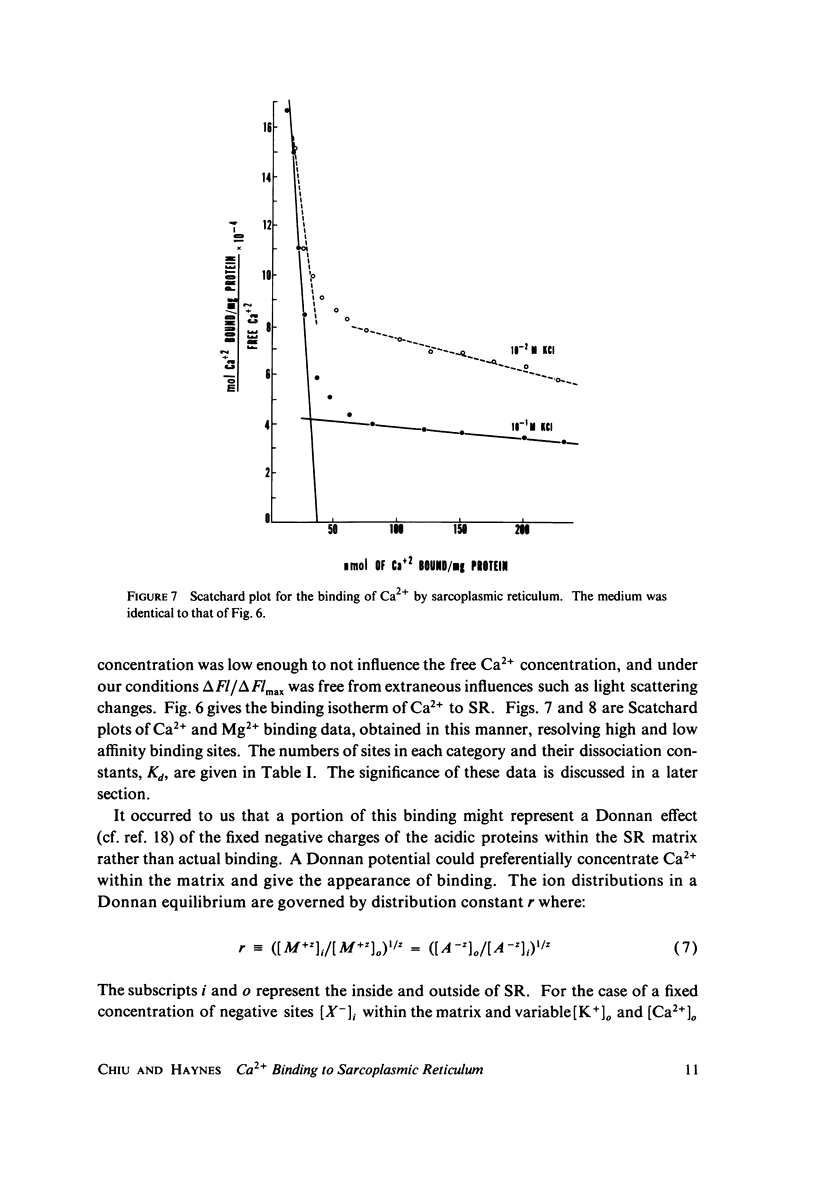
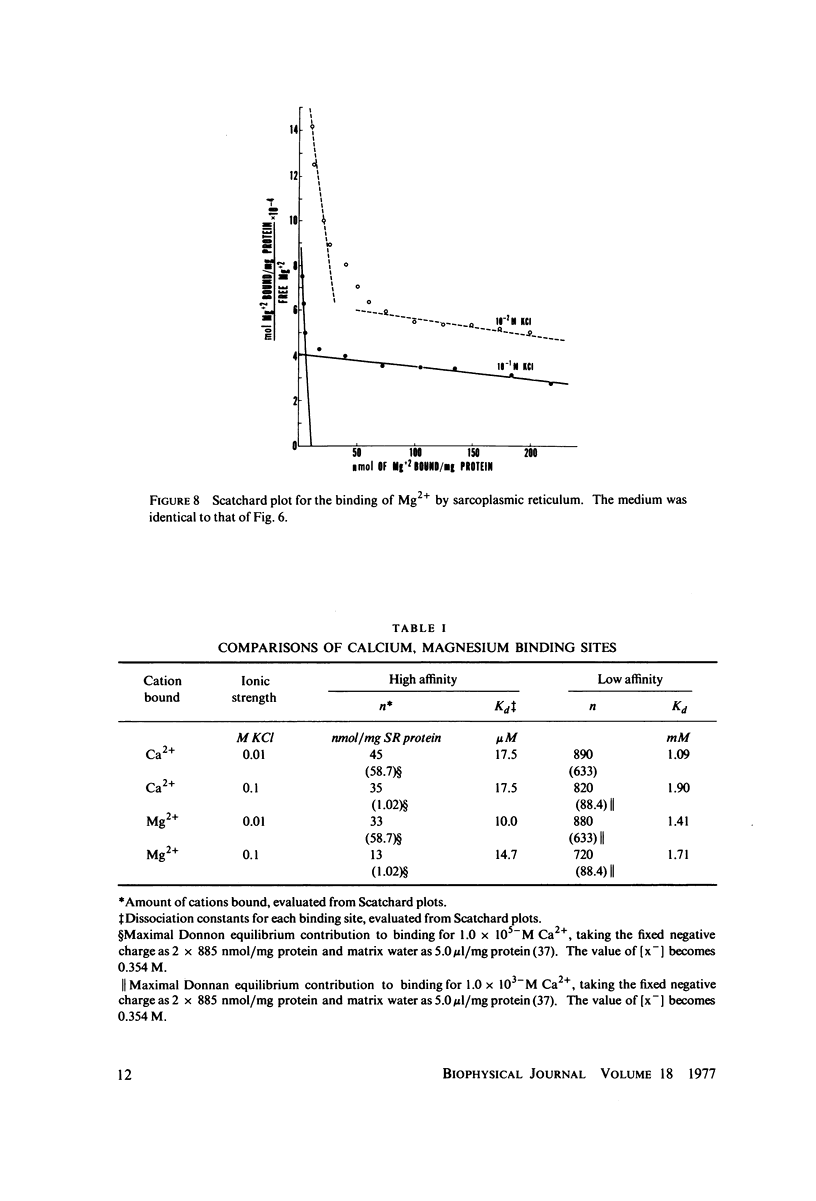
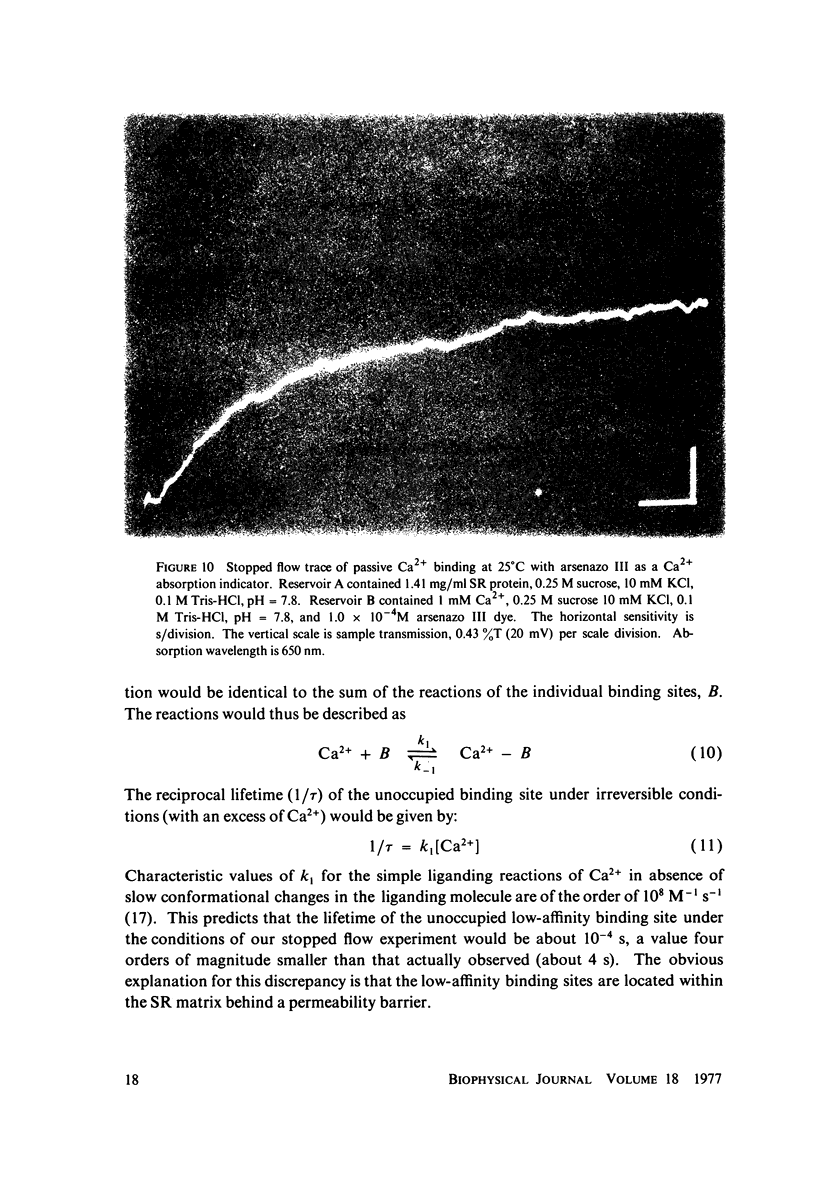
The fluorescent calcium indicator, calcein, has been used as a high-affinity indicator of Ca2+ in the aqueous phase at physiological pH in the study of high-affinity calcium binding to sarcoplasmic reticulum (SR). The binding constant of the indicator at physiological pH is 10(3)-10(4) M-1 and increases with increasing pH. The binding mechanism of the indicator with Ca2+ and Mg2+ is described. Application of calcein as an aqueous indicator of Ca2+ binding to the SR at room temperature has revealed two classes of binding sites: one with high capacity and low affinity (ca. 820 nmol/mg protein, Kd = 1.9 mM), and another with low capacity and higher affinity (ca. 35 nmol/mg protein, Kd = 17.5 micronM). The divalent cation specificity of the low-affinity site is low and Ca2+/Mg2+ specificity of the high-affinity site is high. Quantitative studies of the bindings indicate that the high-affinity site residues in the Ca2+ ATPase (carrier) protein and represents complexation in the active site of the carrier and that the low-affinity site residues in the nonspecific acidic binding proteins. The contribution of Donnan equilibrium effects to the measured binding is shown to be insignificant. Stopped flow kinetic studies of Ca2+ passive binding with calcein and arsenazo III dyes have demonstrated that the binding to high-affinity site is very fast and that the overall binding reaction with the low-affinity site is slow, with a time course of about 4 s. Our analysis has shown that at least part of the low-affinity acidic proteins are within the SR matrix and that Ca2+ can reach them only by transversing the membrane via the Ca2+ carrier (Ca2+ ATPase). A model of the SR is proposed that accounts for several functional properties of the organelle in terms of its known protein composition and topological organization.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carvalho A. P. Binding of cations by microsomes from rabbit skeletal muscle. J Cell Physiol. 1966 Feb;67(1):73–83. doi: 10.1002/jcp.1040670109. [DOI] [PubMed] [Google Scholar]
- Carvalho A. P., Leo B. Effects of ATP on the interaction of Ca++, Mg++, and K+ with fragmented sarcoplasmic reticulum isolated from rabbit skeletal muscle. J Gen Physiol. 1967 May;50(5):1327–1352. doi: 10.1085/jgp.50.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell A. H., Pressman B. C. Kinetics of transport of divalent cations across sarcoplasmic reticulum vesicles induced by ionophores. Biochem Biophys Res Commun. 1972 Oct 6;49(1):292–298. doi: 10.1016/0006-291x(72)90043-5. [DOI] [PubMed] [Google Scholar]
- Chevallier J., Butow R. A. Calcium binding to the sarcoplasmic reticulum of rabbit skeletal muscle. Biochemistry. 1971 Jul 6;10(14):2733–2737. doi: 10.1021/bi00790a012. [DOI] [PubMed] [Google Scholar]
- Duggan P. F., Martonosi A. Sarcoplasmic reticulum. IX. The permeability of sarcoplasmic reticulum membranes. J Gen Physiol. 1970 Aug;56(2):147–167. doi: 10.1085/jgp.56.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiehn W., Migala A. Calcium binding to sarcoplasmic membranes. Eur J Biochem. 1971 May 28;20(2):245–248. doi: 10.1111/j.1432-1033.1971.tb01387.x. [DOI] [PubMed] [Google Scholar]
- Haynes D. H. 1-Anilino-8-naphthalenesulfonate: a fluorescent indicator of ion binding electrostatic potential on the membrane surface. J Membr Biol. 1974 Jul 12;17(3):341–366. doi: 10.1007/BF01870191. [DOI] [PubMed] [Google Scholar]
- Ikemoto N., Bhatnager G. M., Gergely J. Fractionation of solubilized sarcoplasmic reticulum. Biochem Biophys Res Commun. 1971 Sep 17;44(6):1510–1517. doi: 10.1016/s0006-291x(71)80257-7. [DOI] [PubMed] [Google Scholar]
- Ikemoto N. The calcium binding sites involved in the regulation of the purified adenosine triphosphatase of the sarcoplasmic reticulum. J Biol Chem. 1974 Jan 25;249(2):649–651. [PubMed] [Google Scholar]
- Inesi G., Scarpa A. [Fast kinetics of adenosine triphosphate dependent Ca 2+ uptake by fragmented sarcoplasmic reticulum]. Biochemistry. 1972 Feb 1;11(3):356–359. doi: 10.1021/bi00753a008. [DOI] [PubMed] [Google Scholar]
- MARTONOSI A., FERETOS R. SARCOPLASMIC RETICULUM. I. THE UPTAKE OF CA++ BY SARCOPLASMIC RETICULUM FRAGMENTS. J Biol Chem. 1964 Feb;239:648–658. [PubMed] [Google Scholar]
- MacLennan D. H., Holland P. C. Calcium transport in sarcoplasmic reticulum. Annu Rev Biophys Bioeng. 1975;4(00):377–404. doi: 10.1146/annurev.bb.04.060175.002113. [DOI] [PubMed] [Google Scholar]
- Martonosi A., Halpin R. A. Sarcoplasmic reticulum. X. The protein composition of sarcoplasmic reticulum membranes. Arch Biochem Biophys. 1971 May;144(1):66–77. doi: 10.1016/0003-9861(71)90455-3. [DOI] [PubMed] [Google Scholar]
- Meissner G. ATP and Ca2+ binding by the Ca2+ pump protein of sarcoplasmic reticulum. Biochim Biophys Acta. 1973 Apr 16;298(4):906–926. doi: 10.1016/0005-2736(73)90395-7. [DOI] [PubMed] [Google Scholar]
- Meissner G., Conner G. E., Fleischer S. Isolation of sarcoplasmic reticulum by zonal centrifugation and purification of Ca 2+ -pump and Ca 2+ -binding proteins. Biochim Biophys Acta. 1973 Mar 16;298(2):246–269. doi: 10.1016/0005-2736(73)90355-6. [DOI] [PubMed] [Google Scholar]
- Meissner G., Fleischer S. Characterization of sarcoplasmic reticulum from skeletal muscle. Biochim Biophys Acta. 1971 Aug 13;241(2):356–378. doi: 10.1016/0005-2736(71)90036-8. [DOI] [PubMed] [Google Scholar]
- Meissner G., Fleischer S. Characterization of sarcoplasmic reticulum from skeletal muscle. Biochim Biophys Acta. 1971 Aug 13;241(2):356–378. doi: 10.1016/0005-2736(71)90036-8. [DOI] [PubMed] [Google Scholar]
- Meissner G. Isolation and characterization of two types of sarcoplasmic reticulum vesicles. Biochim Biophys Acta. 1975 Apr 21;389(1):51–68. doi: 10.1016/0005-2736(75)90385-5. [DOI] [PubMed] [Google Scholar]
- Ostwald T. J., MacLennan D. H. Isolation of a high affinity calcium-binding protein from sarcoplasmic reticulum. J Biol Chem. 1974 Feb 10;249(3):974–979. [PubMed] [Google Scholar]
- Vale M. G., Carvalho A. P. Utilization of X-537A to distinguish between intravesicular and membrane-bound calcium ions in sarcoplasmic reticulum. Biochim Biophys Acta. 1975 Dec 1;413(2):202–212. doi: 10.1016/0005-2736(75)90104-2. [DOI] [PubMed] [Google Scholar]
- WILBRANDT W., ROSENBERG T. The concept of carrier transport and its corollaries in pharmacology. Pharmacol Rev. 1961 Jun;13:109–183. [PubMed] [Google Scholar]
- Worsfold M., Peter J. B. Kinetics of calcium transport by fragmented sarcoplasmic reticulum. J Biol Chem. 1970 Nov 10;245(21):5545–5552. [PubMed] [Google Scholar]
- Yamada S., Tonomura Y. Reaction mechanism of the Ca 2+ -dependent ATPase of sarcoplasmic reticulum from skeletal muscle. VII. Recognition and release of Ca 2+ ions. J Biochem. 1972 Aug;72(2):417–425. doi: 10.1093/oxfordjournals.jbchem.a129917. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Tonomura Y. Reaction mechanism of the Ca++ -dependent ATPase of sarcoplasmic reticulum from skeletal muscle. I. Kinetic studies. J Biochem. 1967 Nov;62(5):558–575. doi: 10.1093/oxfordjournals.jbchem.a128706. [DOI] [PubMed] [Google Scholar]




