Abstract
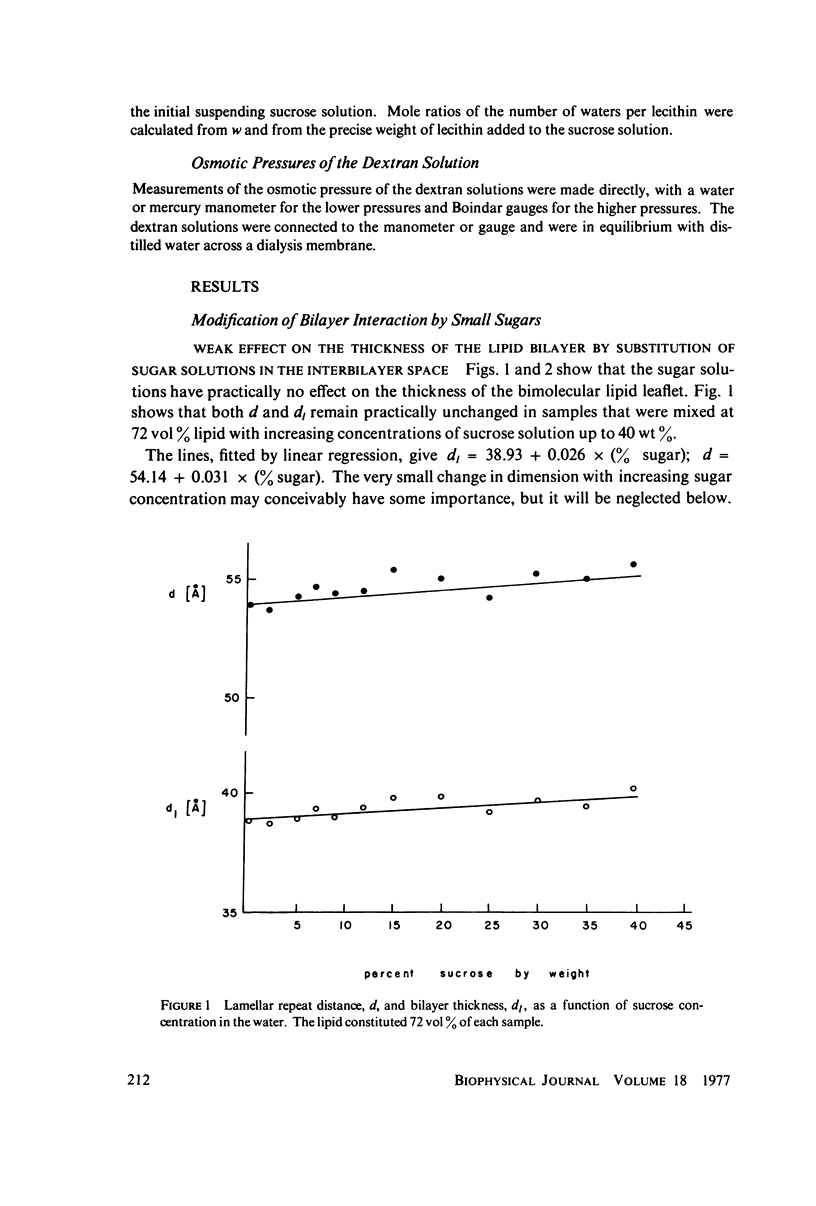
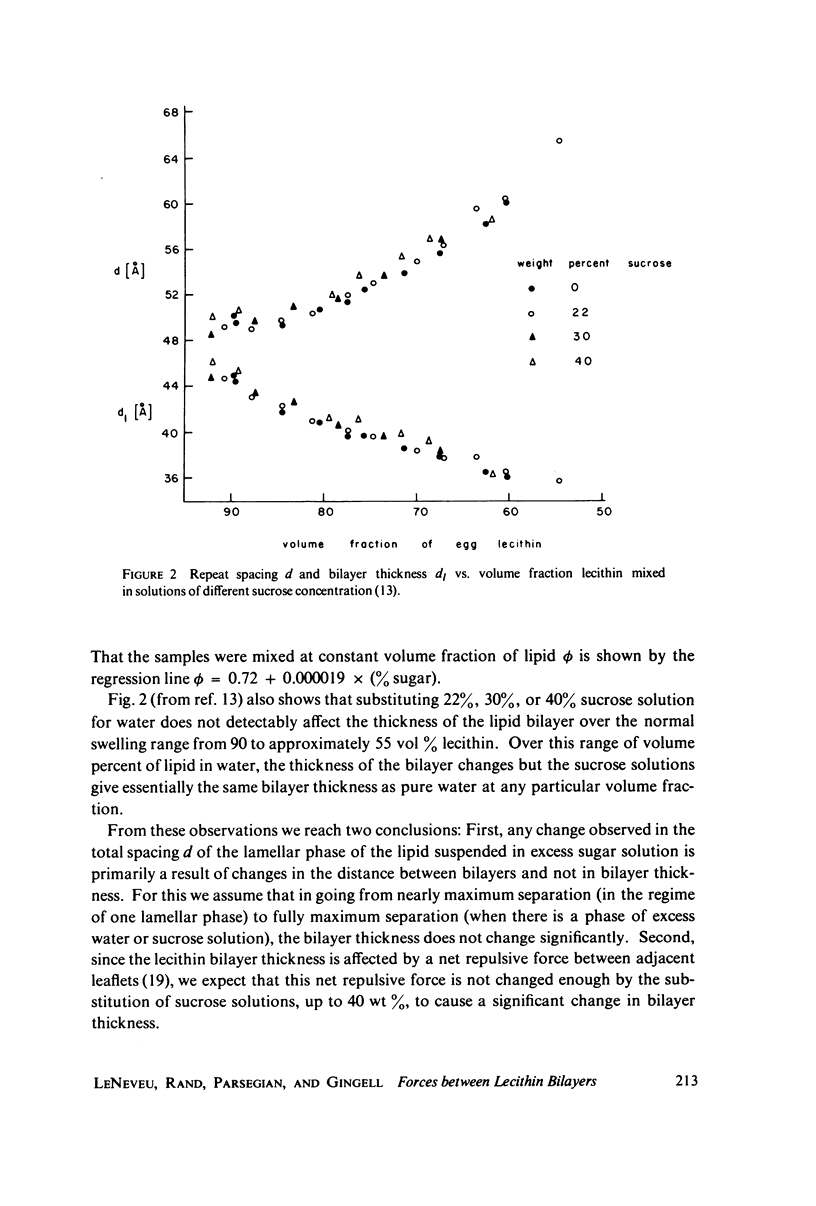
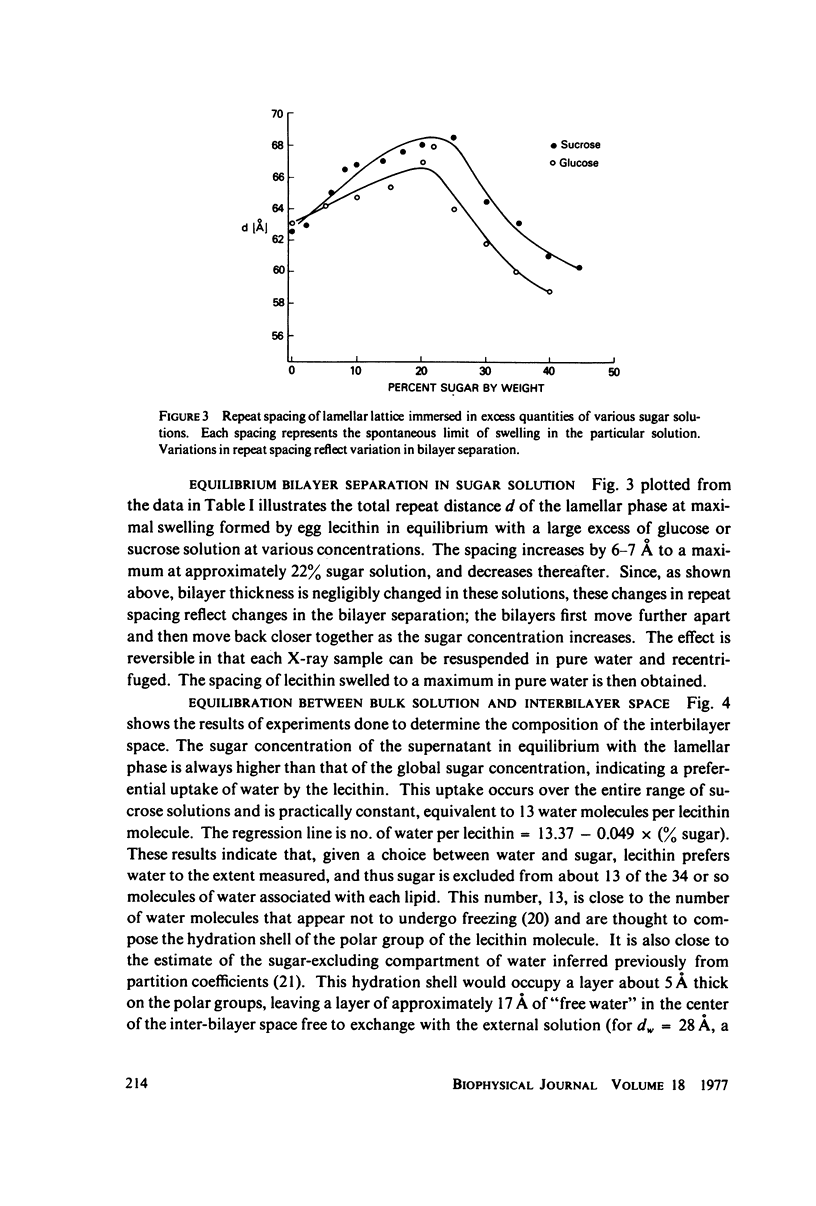
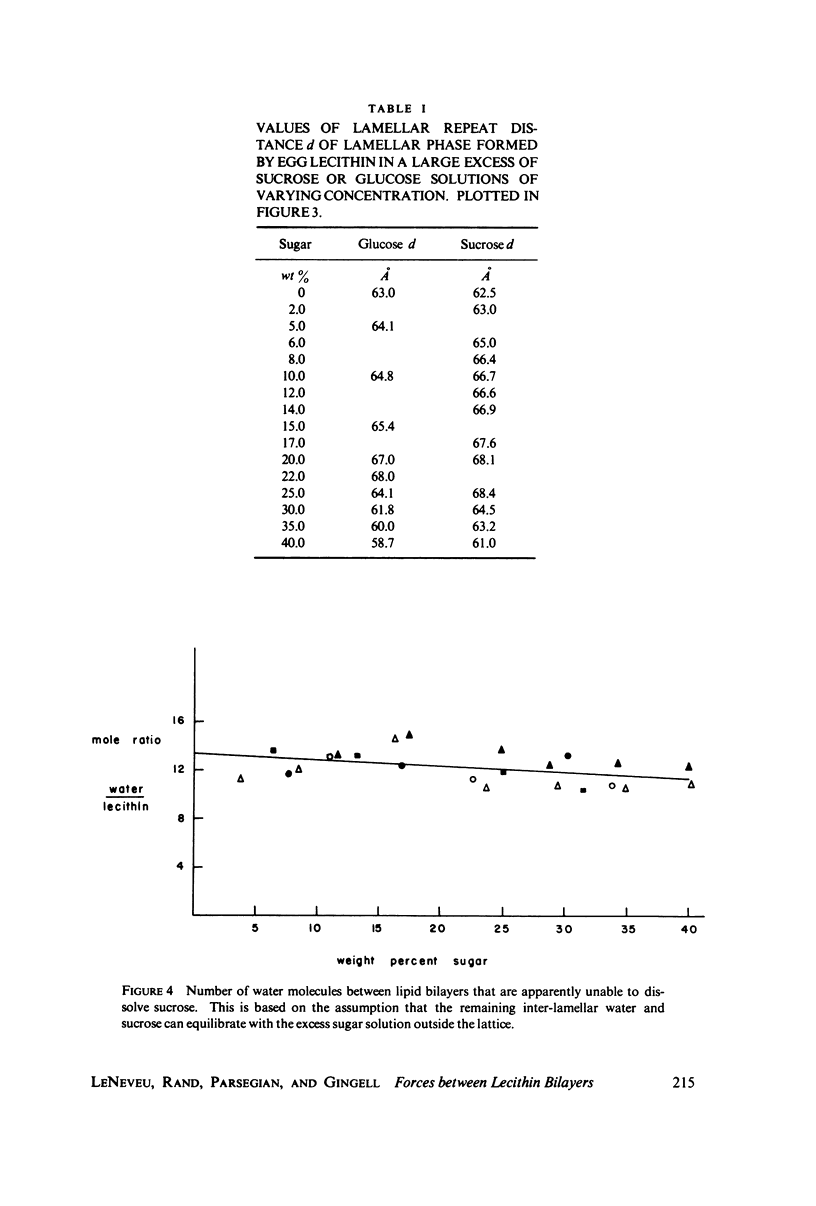
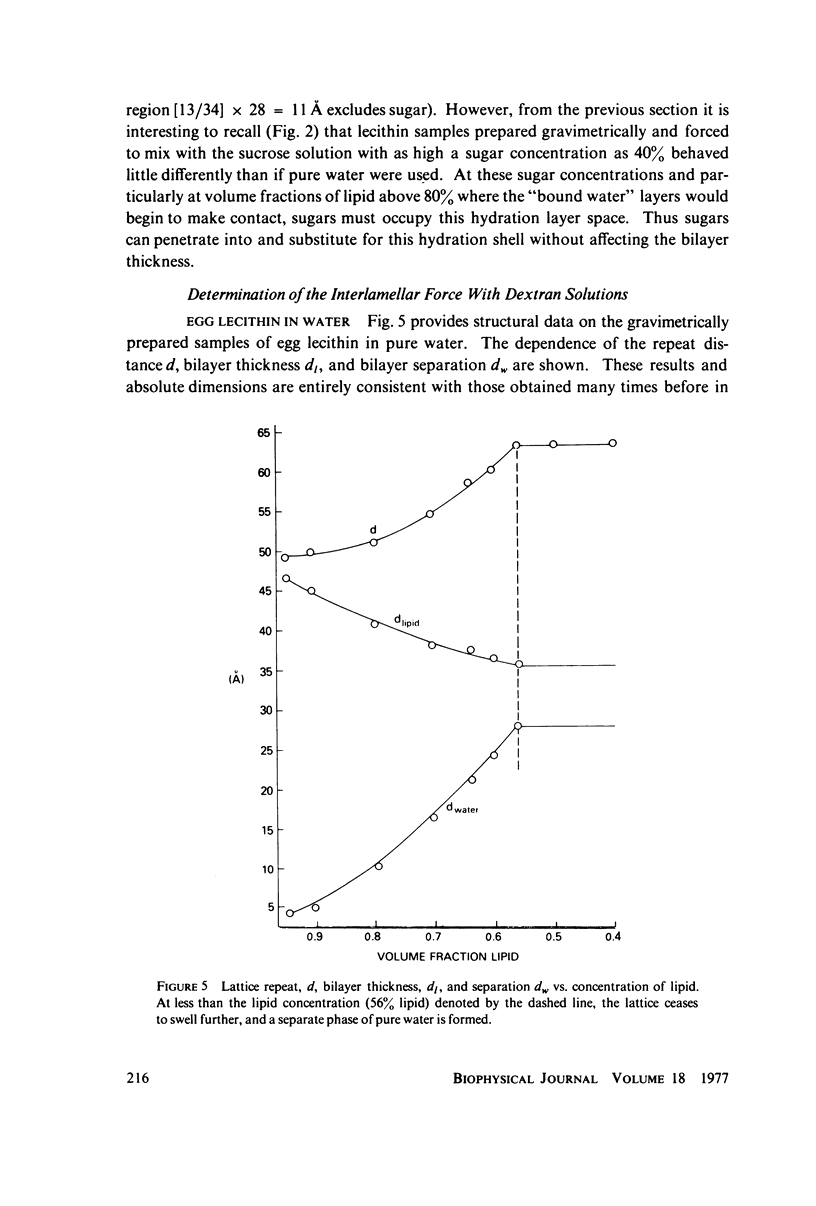
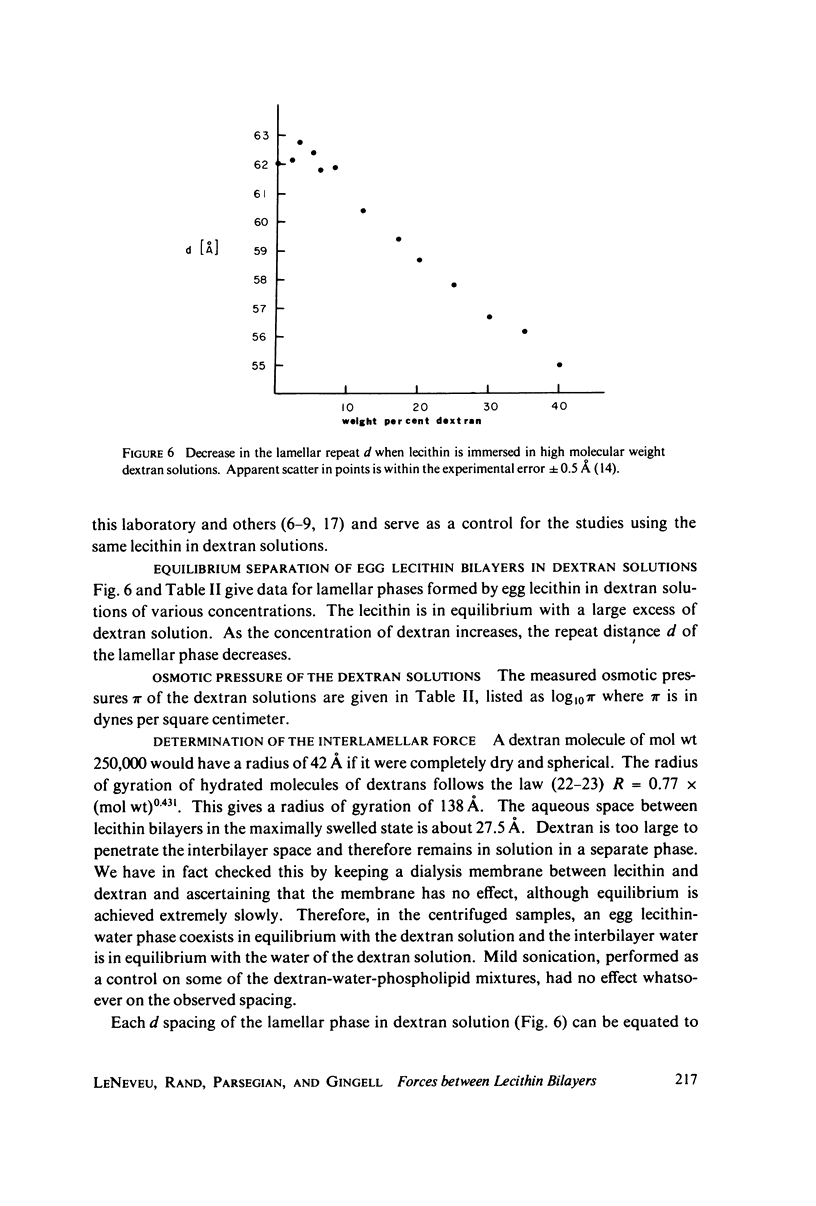
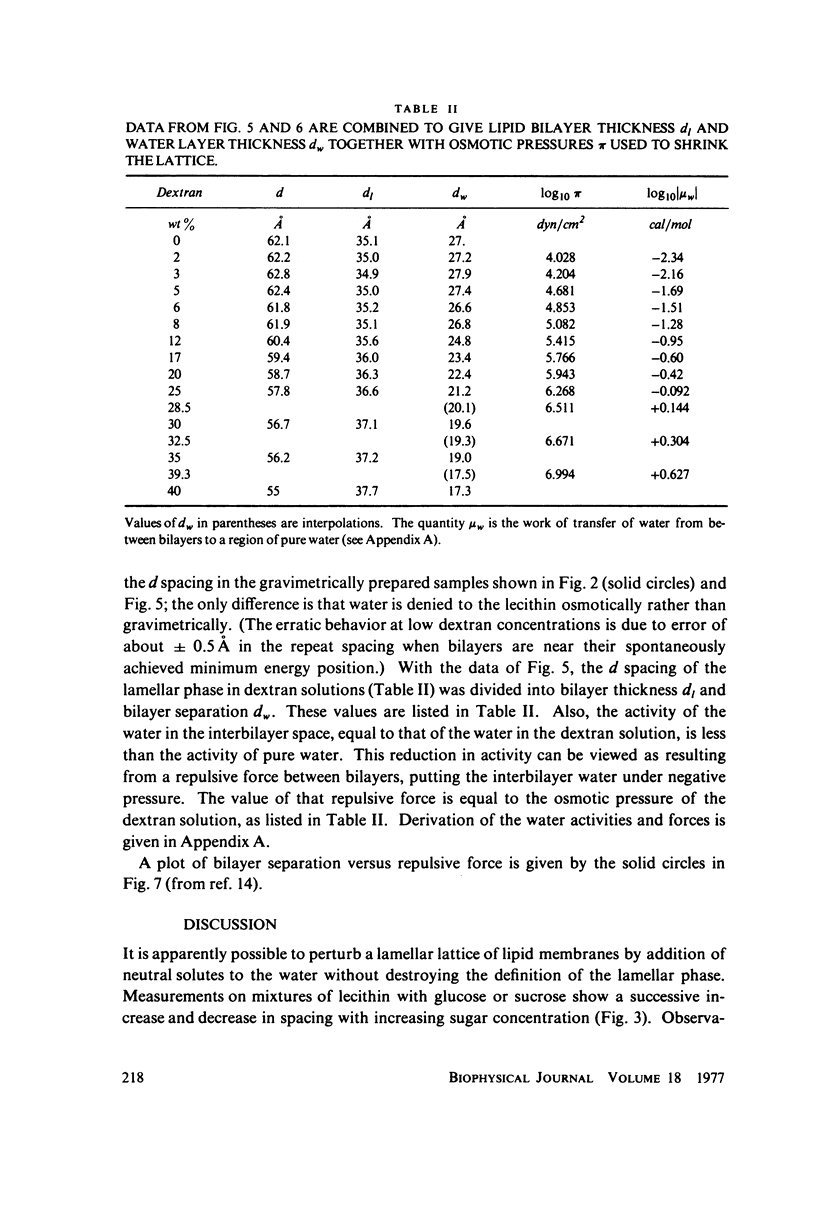
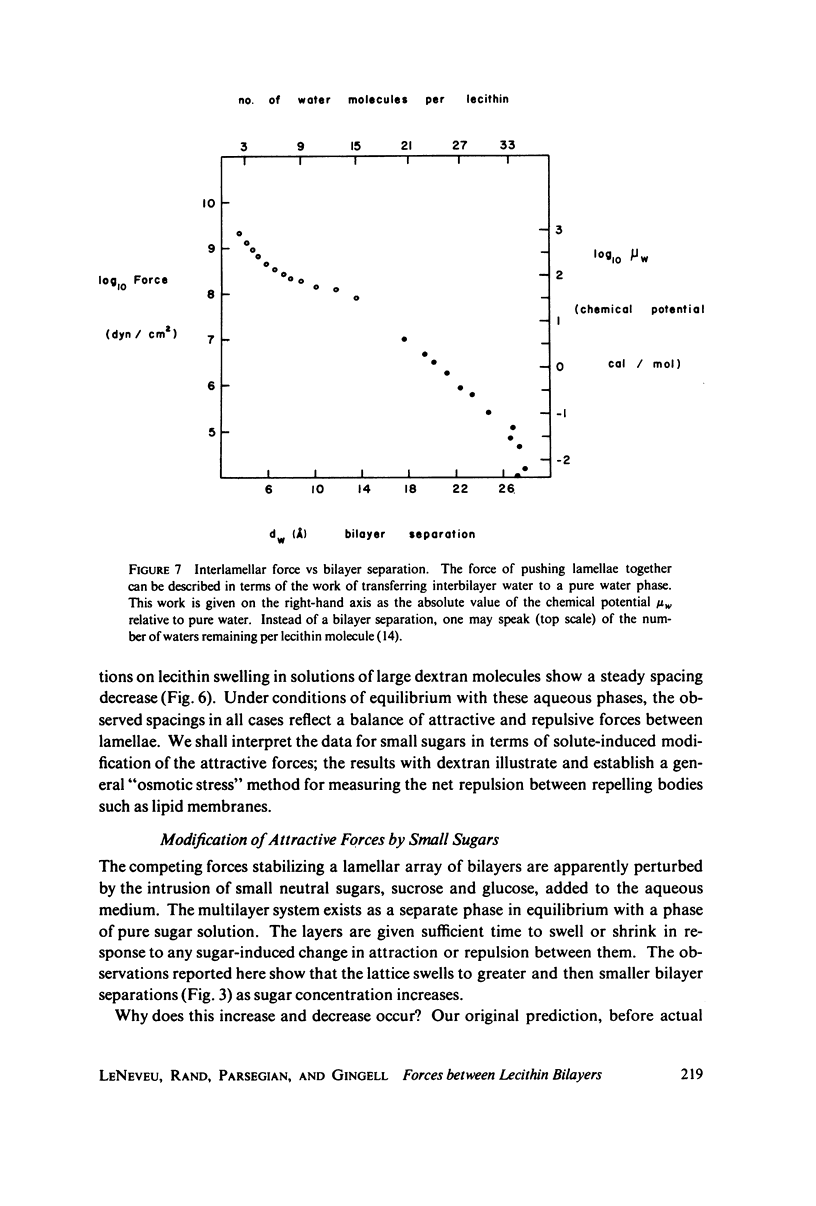
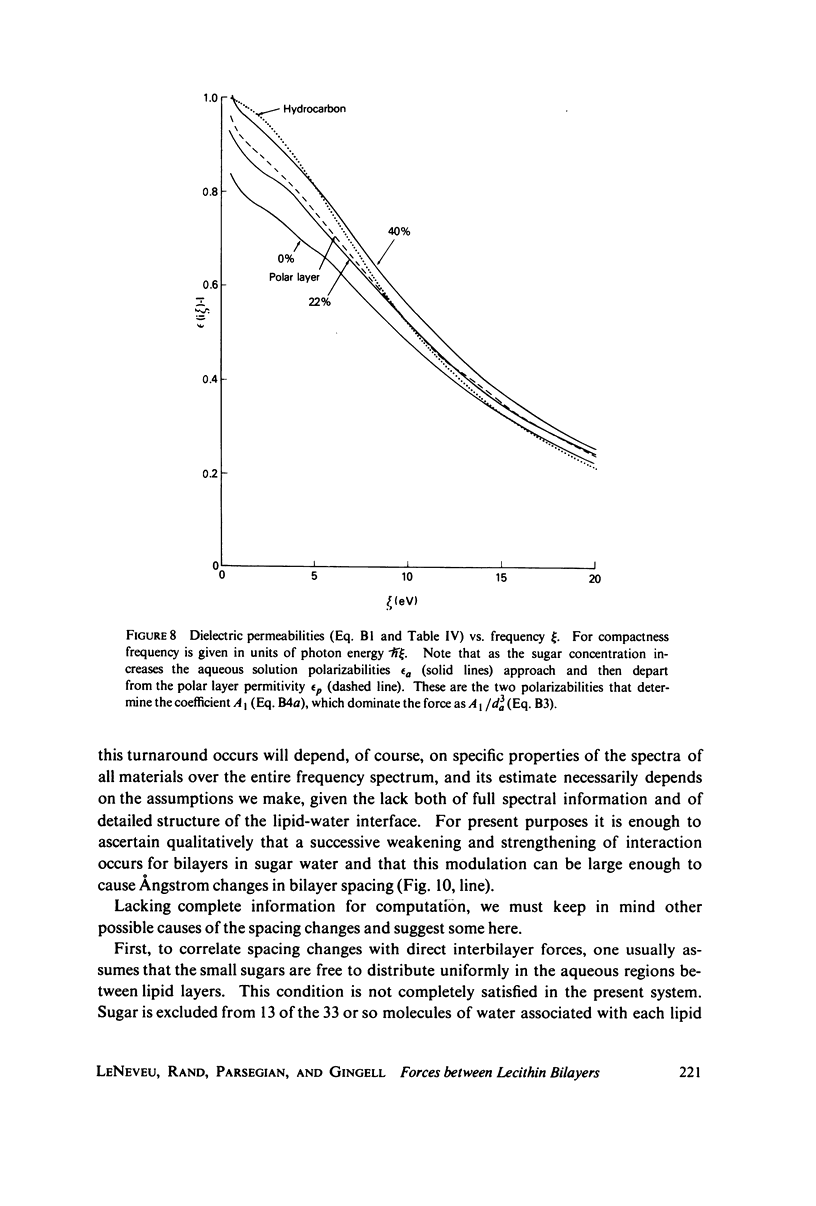
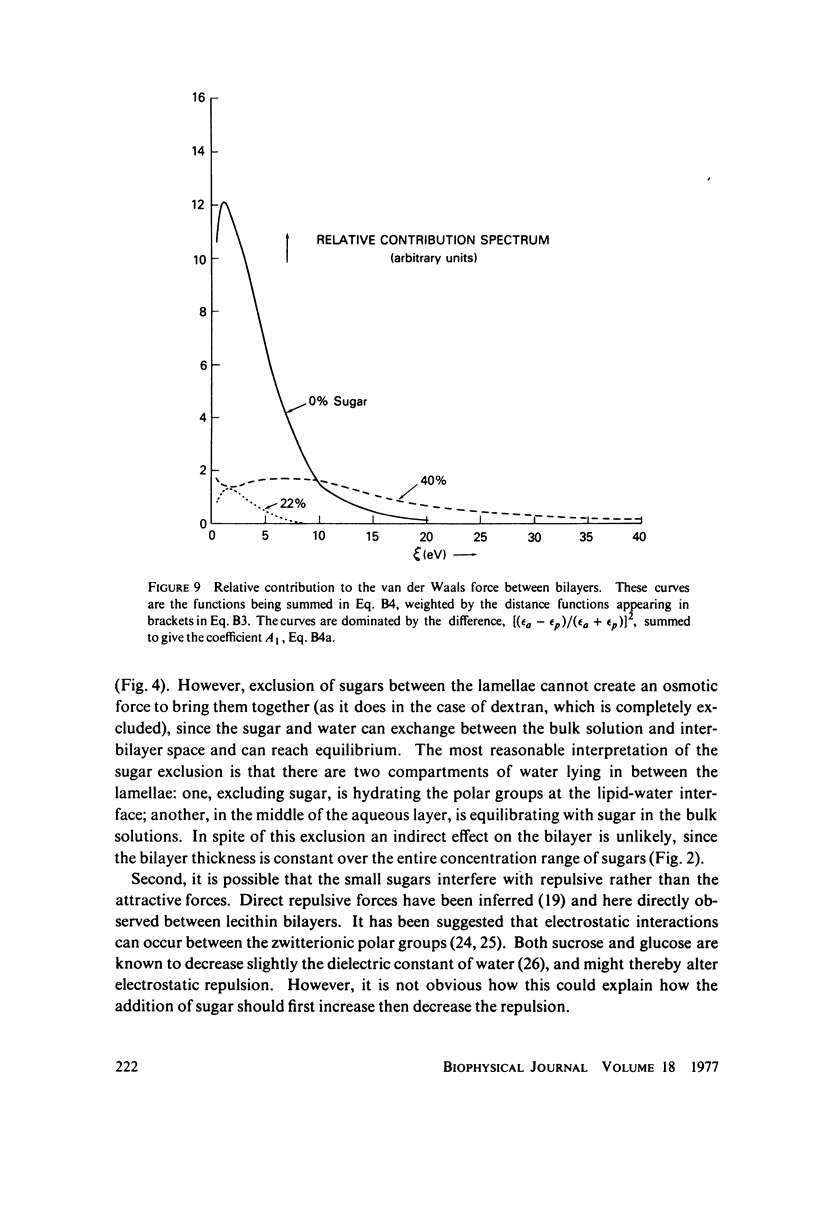
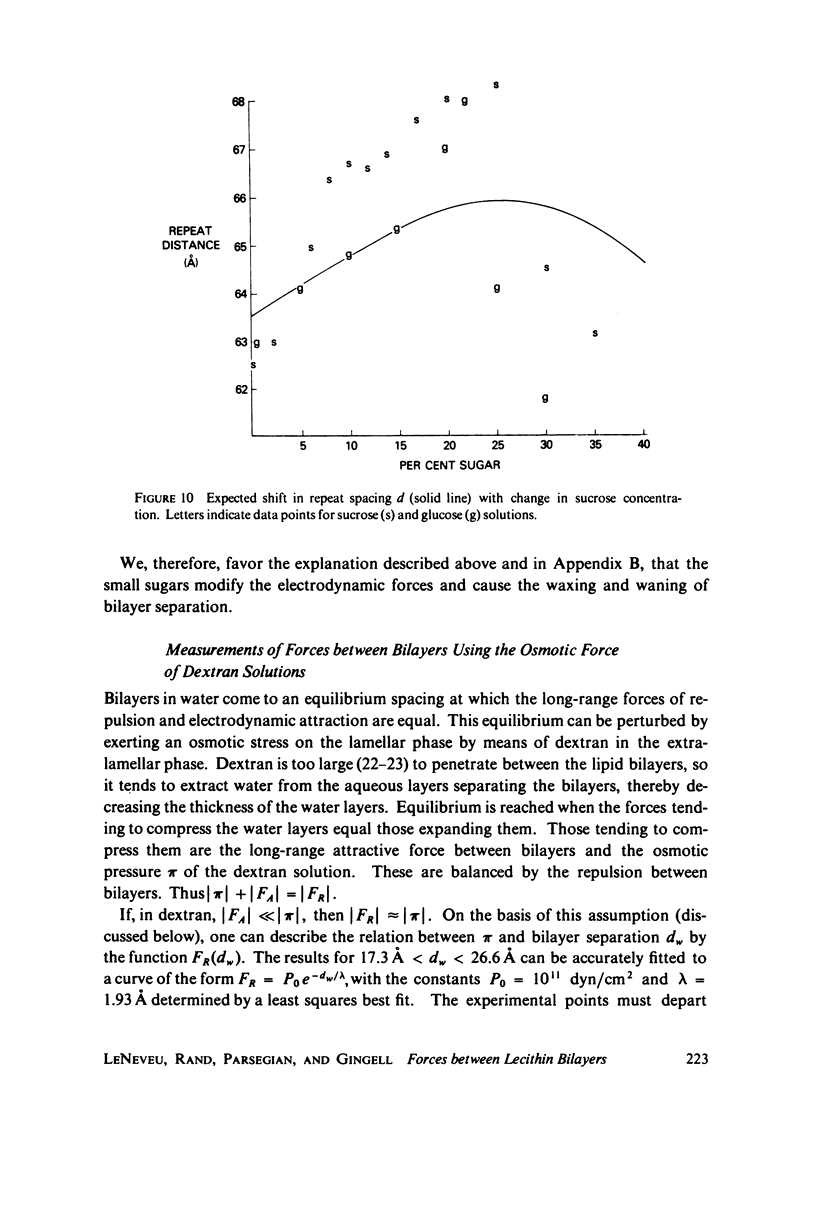
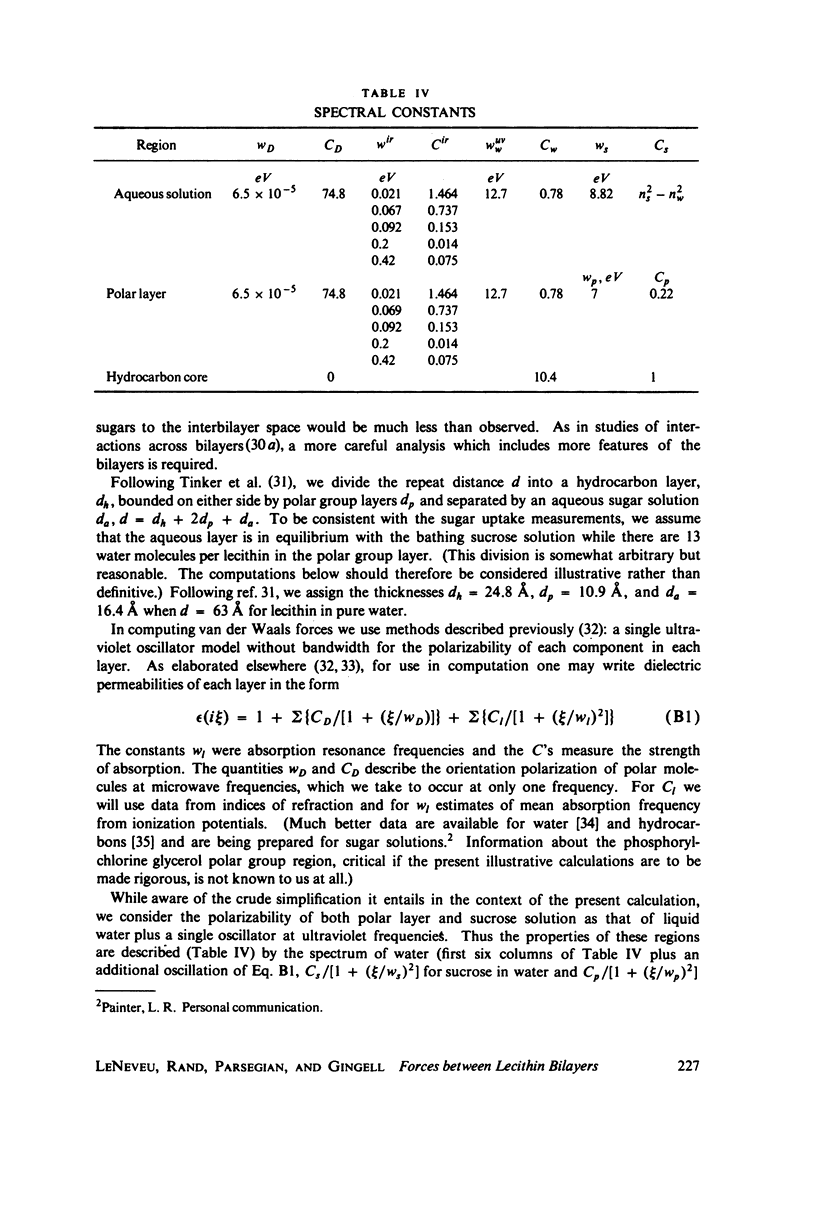
We probe in two different ways the competing attractive and repulsive forces that create lamellar arrays of the phospholipid lecithin when in equilibrium with pure water. The first probe involves the addition of low molecular weight solutes, glucose and sucrose, to a system where the phospholipid is immersed in a large excess of water. Small solutes can enter the aqueous region between bilayers. Their effect is first to increase and then to decrease the separation between bilayers as sugar concentration increases. We interpret this waxing and waning of the lattice spacing in terms of the successive weakening and strengthening of the attractive van der Waals forces originally responsible for creation of a stable lattice. The second probe is an "osmotic stress method," in which very high molecular weight neutral polymer is added to the pure water phase but is unable to enter the multilayers. The polymer competes for water with the lamellar lattice, and thereby compresses it. From the resulting spacing (determined by X-ray diffraction) and the directly measured osmotic pressure, we find a force vs. distance curve for compressing the lattice (or, equivalently, the free energy of transfer to bulk water of water between bilayers. This method reveals a very strong, exponentially varying "hydration force" with a decay distance of about 2 A.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gingell D., Fornes J. A. Demonstration of intermolecular forces in cell adhesion using a new electrochemical technique. Nature. 1975 Jul 17;256(5514):210–211. doi: 10.1038/256210a0. [DOI] [PubMed] [Google Scholar]
- Gingell D., Fornes J. A. Interaction of red blood cells with a polarized electrode: evidence of long-range intermolecular forces. Biophys J. 1976 Oct;16(10):1131–1153. doi: 10.1016/S0006-3495(76)85763-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingell D., Parsegian V. A. Computation of van der Waals interactions in aqueous systems using reflectivity data. J Theor Biol. 1972 Jul;36(1):41–52. doi: 10.1016/0022-5193(72)90175-0. [DOI] [PubMed] [Google Scholar]
- Gingell D., Todd I. Adhesion of red blood cells to charged interfaces between immiscible liquids. A new method. J Cell Sci. 1975 Jul;18(2):227–239. doi: 10.1242/jcs.18.2.227. [DOI] [PubMed] [Google Scholar]
- Katz Y., Diamond J. M. Nonsolvent water in liposomes. J Membr Biol. 1974;17(1):87–100. doi: 10.1007/BF01870174. [DOI] [PubMed] [Google Scholar]
- LeNeveu D. M., Rand R. P., Gingell D., Parsegian V. A. Apparent modification of forces between lecithin bilayers. Science. 1976 Jan 30;191(4225):399–400. doi: 10.1126/science.1246623. [DOI] [PubMed] [Google Scholar]
- LeNeveu D. M., Rand R. P., Parsegian V. A. Measurement of forces between lecithin bilayers. Nature. 1976 Feb 19;259(5544):601–603. doi: 10.1038/259601a0. [DOI] [PubMed] [Google Scholar]
- Parsegian V. A. Evidence for long-range repulsion between bimolecular leaflets of lecithin: an examination of structural data. J Theor Biol. 1967 Apr;15(1):70–74. doi: 10.1016/0022-5193(67)90044-6. [DOI] [PubMed] [Google Scholar]
- Parsegian V. A. Forces between lecithin bimolecular leaflets are due to a disordered surface layer. Science. 1967 May 19;156(3777):939–942. doi: 10.1126/science.156.3777.939. [DOI] [PubMed] [Google Scholar]
- Parsegian V. A., Ninham B. W. Van der Waals forces in many-layered structures: generalizations of the Lifshitz result for two semi-infinite media. J Theor Biol. 1973 Jan;38(1):101–109. doi: 10.1016/0022-5193(73)90227-0. [DOI] [PubMed] [Google Scholar]
- Rand R. P., Luzzati V. X-ray diffraction study in water of lipids extracted from human erythrocytes: the position of cholesterol in the lipid lamellae. Biophys J. 1968 Jan;8(1):125–137. doi: 10.1016/S0006-3495(68)86479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss-Husson F. Structure des phases liquide-cristallines de différents phospholipides, monoglycérides, sphingolipides, anhydres ou en présence d'eau. J Mol Biol. 1967 May 14;25(3):363–382. doi: 10.1016/0022-2836(67)90192-1. [DOI] [PubMed] [Google Scholar]
- SINGLETON W. S., GRAY M. S., BROWN M. L., WHITE J. L. CHROMATOGRAPHICALLY HOMOGENEOUS LECITHIN FROM EGG PHOSPHOLIPIDS. J Am Oil Chem Soc. 1965 Jan;42:53–56. doi: 10.1007/BF02558256. [DOI] [PubMed] [Google Scholar]
- Small D. M. Phase equilibria and structure of dry and hydrated egg lecithin. J Lipid Res. 1967 Nov;8(6):551–557. [PubMed] [Google Scholar]
- Tinker D. O., Pinteric L., Hsia J. C., Rand R. P. Perturbation of lecithin bilayer structure by globoside. Can J Biochem. 1976 Mar;54(3):209–218. doi: 10.1139/o76-033. [DOI] [PubMed] [Google Scholar]


