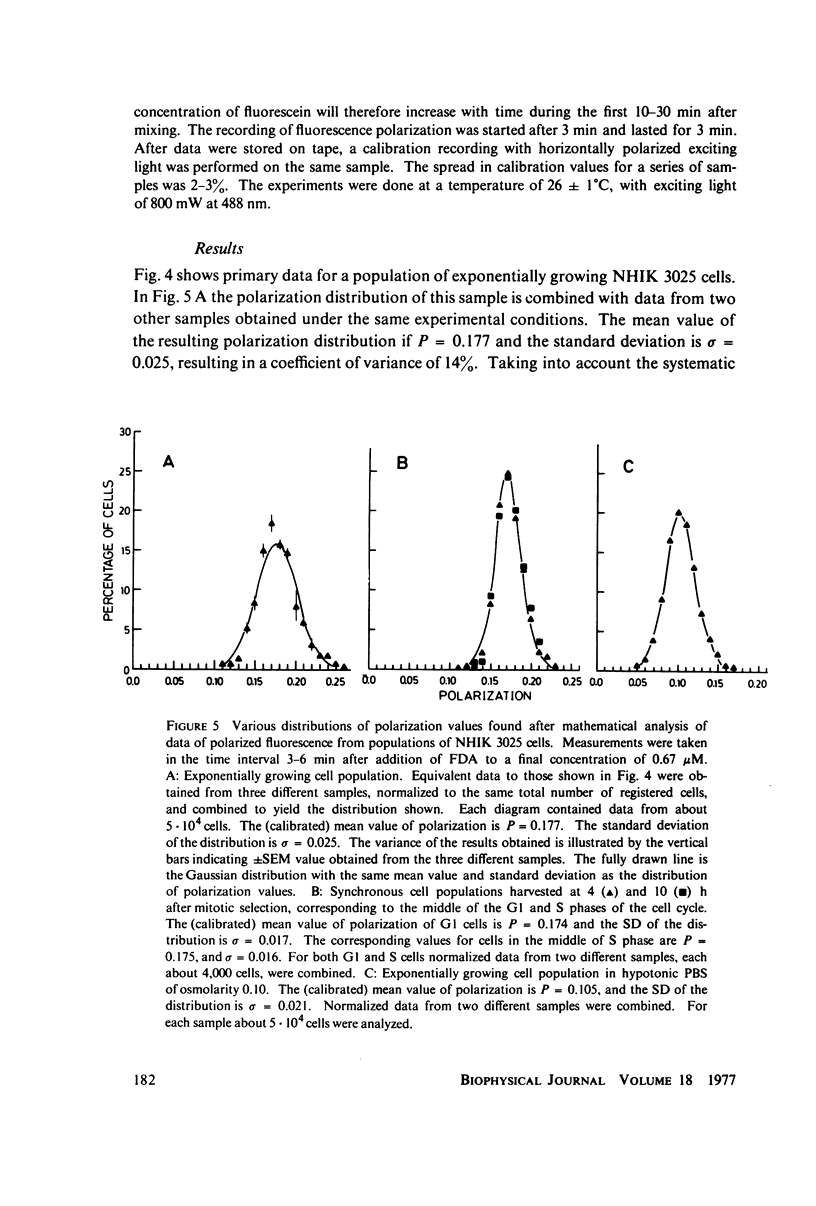
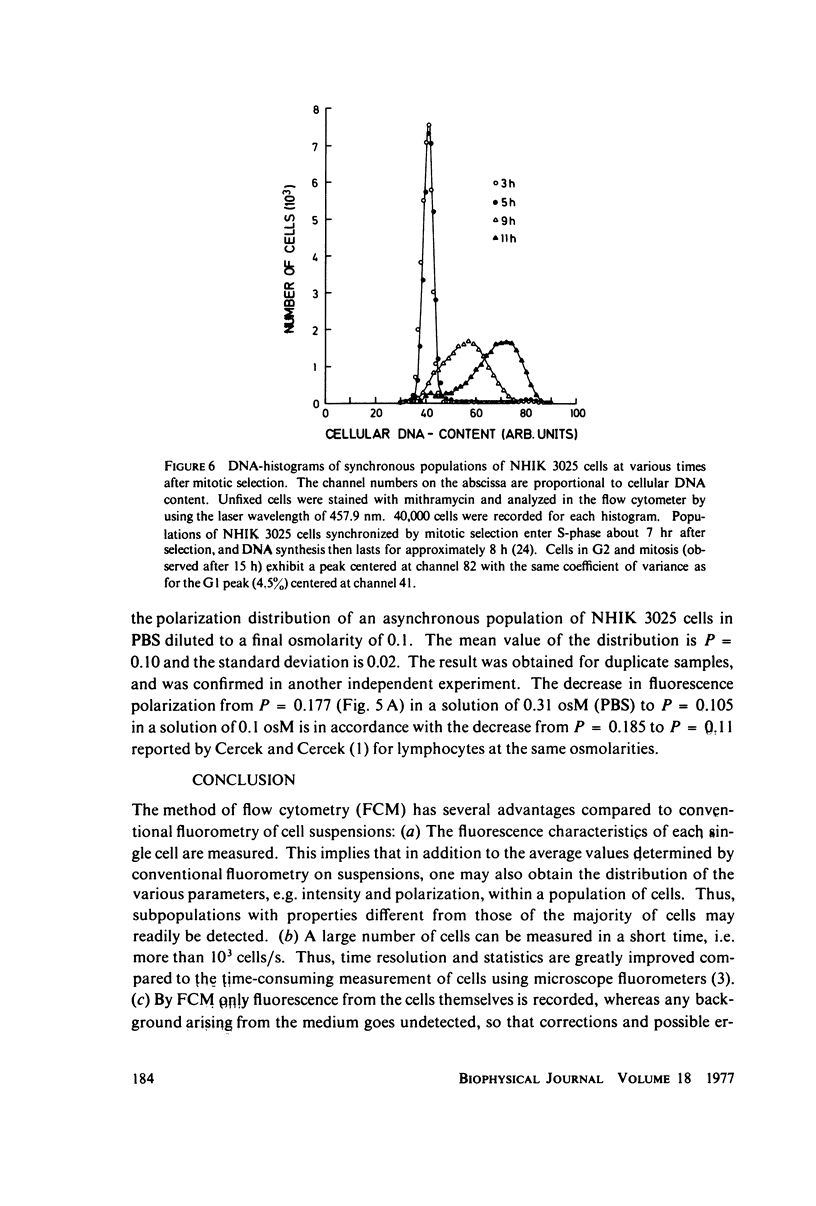
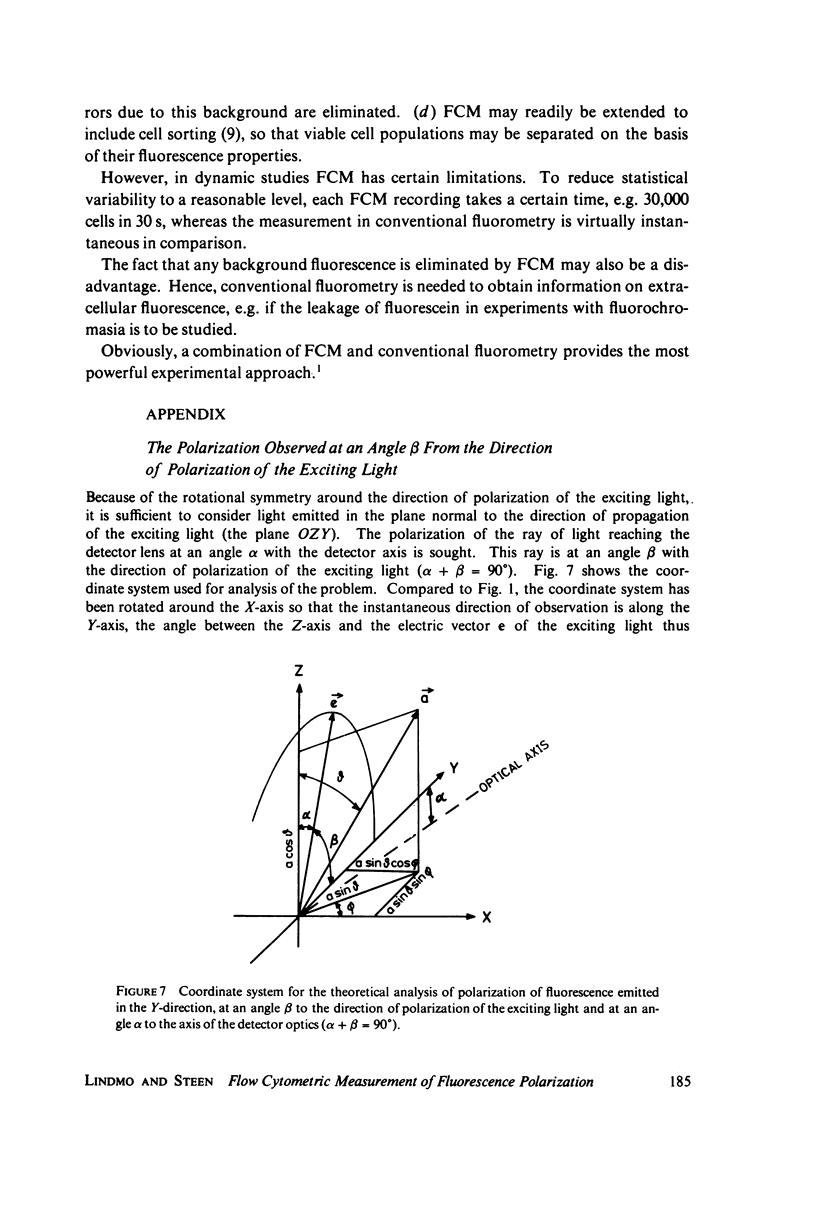
Abstract
Based on the description of a laboratory-built flow cytometer, the necessary modifications of this instrument for the measurement of fluorescence polarization are described. At a maximum rate exceeding 1,000 cells/s, the instrument is capable of measuring simultaneously the horizontally and vertically polarized component of the fluorescence emitted from stained cells excited with vertically polarized light. By mathematical analysis of the accumulated data, the distribution of polarization values in the population is obtained. Various sources of instrumental error have been investigated. The large aperture of the detector optics leads to systematic underestimation of the polarization values. Other errors are negligible, and the instrument is shown to give results consistent with the theory of fluorescence polarization. Application of the instrument is illustrated by experiments with mammalian cells exposed to the fluorogenic substrate fluorescein diacetate (FDA). The polarization of the fluorescence from intracellular fluorescein produced by hydrolysis of FDA is measured, giving information on the cytoplasmic microviscosity. It appears that this microviscosity is constant over the cell cycle. On the other hand, it is significantly affected by the osmolarity of the medium.
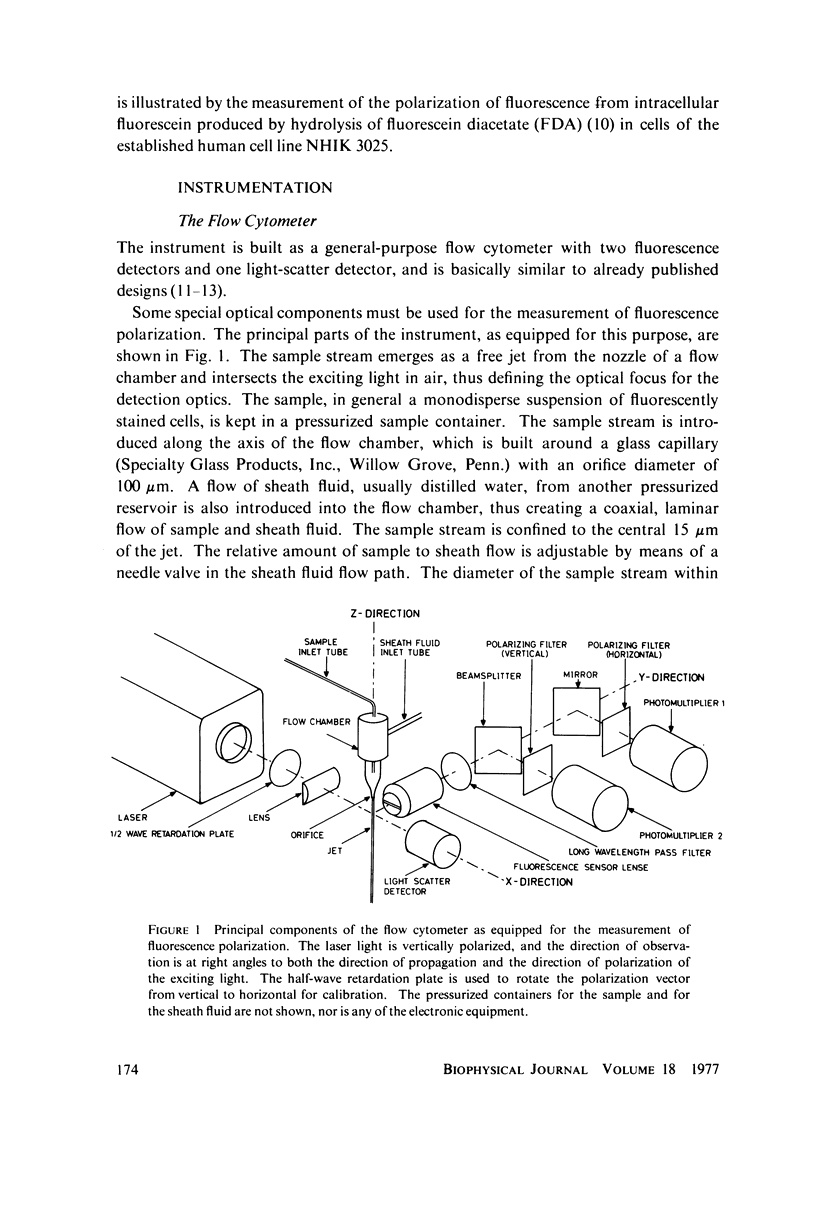
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arndt-Jovin D. J., Ostertag W., Eisen H., Klimek F., Jovin T. M. Studies of cellular differentiation by automated cell separation. Two model systems: Friend virus-transformed cells and Hydra attenuata. J Histochem Cytochem. 1976 Jan;24(1):332–347. doi: 10.1177/24.1.1254928. [DOI] [PubMed] [Google Scholar]
- Cercek L., Cercek B. Changes in the structuredness of cytoplasmic matrix (SCM) in human lymphocytes induced by PHA and cancer basic protein as measured in single cells. Br J Cancer. 1976 May;33(5):539–543. doi: 10.1038/bjc.1976.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cercek L., Cercek B. Changes in the structuredness of cytoplasmic matrix (SCM) induced in mixed lymphocyte interactions. Radiat Environ Biophys. 1976 Mar 30;13(1):71–74. doi: 10.1007/BF01323626. [DOI] [PubMed] [Google Scholar]
- Cercek L., Cercek B. Effects of osmomolarity, calcium and magnesium ions on the structuredness of cytoplasmic matrix (SCM). Radiat Environ Biophys. 1976 Mar 30;13(1):9–12. doi: 10.1007/BF01323618. [DOI] [PubMed] [Google Scholar]
- Cercek L., Cercek B., Franklin C. I. Biophysical differentiation between lymphocytes from healthy donors, patients with malignant diseases and other disorders. Br J Cancer. 1974 May;29(5):345–352. doi: 10.1038/bjc.1974.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cercek L., Cercek B., Ockey C. H. Structuredness of the cytoplasmic matrix and Michaelis-Menten constants for the hydrolysis of FDA during the cell cycle in Chinese hamster ovary cells. Biophysik. 1973;10(3):187–194. doi: 10.1007/BF01190577. [DOI] [PubMed] [Google Scholar]
- Dean P. N., Jett J. H. Mathematical analysis of DNA distributions derived from flow microfluorometry. J Cell Biol. 1974 Feb;60(2):523–527. doi: 10.1083/jcb.60.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulett H. R., Bonner W. A., Sweet R. G., Herzenberg L. A. Development and application of a rapid cell sorter. Clin Chem. 1973 Aug;19(8):813–816. [PubMed] [Google Scholar]
- Inbar M., Shinitzky M., Sachs L. Rotational relaxation time of concanavalin A bound to the surface membrane of normal and malignant transformed cells. J Mol Biol. 1973 Dec 5;81(2):245–253. doi: 10.1016/0022-2836(73)90192-7. [DOI] [PubMed] [Google Scholar]
- PUCK T. T., CIECIURA S. J., FISHER H. W. Clonal growth in vitro of human cells with fibroblastic morphology; comparison of growth and genetic characteristics of single epithelioid and fibroblast-like cells from a variety of human organs. J Exp Med. 1957 Jul 1;106(1):145–158. doi: 10.1084/jem.106.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen E. O., Oftebro R., Brustad T. X-ray inactivation of human cells in tissue culture under aerobic and extremely hypoxic conditions in the presence and absence of TMPN. Int J Radiat Biol Relat Stud Phys Chem Med. 1973 Sep;24(3):285–296. doi: 10.1080/09553007314551121. [DOI] [PubMed] [Google Scholar]
- Rotman B., Papermaster B. W. Membrane properties of living mammalian cells as studied by enzymatic hydrolysis of fluorogenic esters. Proc Natl Acad Sci U S A. 1966 Jan;55(1):134–141. doi: 10.1073/pnas.55.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinitzky M., Inbar M. Microviscosity parameters and protein mobility in biological membranes. Biochim Biophys Acta. 1976 Apr 16;433(1):133–149. doi: 10.1016/0005-2736(76)90183-8. [DOI] [PubMed] [Google Scholar]
- Tobey R. A., Anderson E. C., Petersen D. F. Properties of mitotic cells prepared by mechanically shaking monolayer cultures of Chinese hamster cells. J Cell Physiol. 1967 Aug;70(1):63–68. doi: 10.1002/jcp.1040700109. [DOI] [PubMed] [Google Scholar]




