Abstract
Rationale
Corticotropin releasing factor (CRF) produces anxiety-like and aversive effects when infused directly into the various regions of the brain, including the bed nucleus of the stria terminalis (BNST). However, the CRF receptor subtypes within the BNST mediating these phenomena have not been established.
Objectives
We used selective CRF receptor antagonists to determine the receptor subtypes involved in the anxiogenic-like and aversive effects CRF in the BNST.
Methods
Male Long-Evans rats were bilaterally infused with CRF (0.2 or 1.0 nmol) either alone or in combination with the CRF1 receptor antagonist CP154,526 or the CRF2 receptor antagonist anti-sauvagine 30 (AS30) prior to behavioral testing in the elevated plus maze or place conditioning paradigms.
Results
Intra-BNST administration of CRF produced a dose-dependent reduction in open arm entries and open arm time in the elevated plus maze, indicating an anxiogenic-like effect. These effects were inhibited by co-infusion of CP154,526 but not AS30, indicating that the anxiogenic-like effects of CRF in the BNST are mediated by CRF1 receptors. Place conditioning with intra-BNST administration of CRF produced a dose-dependent aversion to the CRF-paired environment that was prevented by co-infusion of either CP154,526 or AS30, indicating that both CRF receptor subtypes mediate the aversive effects of this peptide. Intra-BNST infusions of the CRF receptor antagonists alone produced no effects in either behavioral paradigm.
Conclusions
CRF1 receptors in the BNST mediate the anxiogenic-like effects CRF in this region, whereas both CRF1 and CRF2 receptor subtypes mediate the conditioned aversive effects of this peptide within the BNST.
Keywords: corticotropin releasing factor, CRF, receptor, limbic system, anxiety, aversion, rat, behavior, conditioning, stress
INTRODUCTION
The extended amygdala, consisting of various limbic and basal forebrain structures including the bed nucleus of the stria terminalis (BNST), is known to mediate behavioral and autonomic responses to stressors, including fear and anxiety (Alheid 2003; Alheid et al. 1995; Davis 1998; Heimer 2003; Schulkin et al. 2005; Walker et al. 2003). For example, electrical stimulation of the BNST in rats produces behavioral and endocrine changes similar to those evoked by stress (Casada and Dafny 1991; Dunn 1987), whereas lesions or pharmacological inactivation of the BNST decrease behavioral and endocrine responses to conditioned or unconditioned stressful stimuli (Bangasser et al. 2005; Davis et al. 1997; Fendt et al. 2003; Gewirtz et al. 1998; Gray et al. 1993; Hammack et al. 2004; Henke 1984; Schulz and Canbeyli 1999; Sullivan et al. 2004; Walker and Davis 1997; but see Treit et al. 1998). Lesions of the BNST also enhance learned despair (Schulz and Canbeyli 2000), and thus this brain region may also mediate coping mechanisms in response to uncontrollable stress.
The BNST contains numerous cell bodies that synthesize corticotropin releasing factor (CRF) (Cummings et al. 1983; Ju et al. 1989; Moga et al. 1989; Morin et al. 1999; Olschowka et al. 1982; Sakanaka et al. 1987; Swanson et al. 1983), a 41 amino acid peptide that mediates anxiety- and stress-like responses (see Bale and Vale 2004 for recent review). In addition to these CRF-containing cell bodies, the BNST also contains CRF-immunopositive fibers and terminals (Cummings et al. 1983; Morin et al. 1999; Olschowka et al. 1982; Sakanaka et al. 1987; Swanson et al. 1983), which originate from the central nucleus of the amygdala (Sakanaka et al. 1986).
Various investigators have shown significant stress- or anxiety-like behavioral and autonomic effects of infusions of CRF into the BNST in rodents. For example, intra-BNST administration of CRF facilitates the acoustic startle reflex (Lee and Davis 1997), enhances retention of an inhibitory avoidance task (Liang et al. 2001), produces anorectic effects (Ciccocioppo et al. 2003) and tachycardia (Nijsen et al. 2001). Conversely, infusions of nonselective CRF antagonists into the BNST produce anxiolytic-like and anti-stress effects, such as attenuated defensive burying behavior (Greenwell et al. 2004), reduced conditioned freezing and stress-induced cardiovascular abnormalities (Nijsen et al. 2001), and reduced conditioned defensive-submissive behavior in response to social defeat (Jasnow et al. 2004). However, the BNST contains both CRF1 and CRF2 receptor subtypes (Chalmers et al. 1995; Chen et al. 2000; Rominger et al. 1998; Rybnikova et al. 2003; Van Pett et al. 2000), and although CRF has a higher affinity for CRF1 over CRF2 receptors (Vaughan et al. 1995), it currently unclear which of these two receptors mediates the behavioral effects of CRF in the BNST.
The goal of the present study was to use subtype-selective CRF receptor antagonists to determine which of the two primary CRF receptor subtypes mediate the anxiogenic-like effects of intra-BNST infusion of CRF. In addition, since intracerebroventricular (icv) administration CRF can produce a conditioned place aversion (Cador et al. 1992), we sought to determine if intra-BNST administration of CRF would also induce a conditioned place aversion, and which CRF receptor subtypes might be involved.
MATERIALS AND METHODS
Subjects
All experimental procedures were conducted in accordance with the National Research Council’s Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (2003), and with the approval of an Institutional Animal Care and Use Committee. Male Long-Evans rats (Harlan, Indianapolis, IN; 250–300 g upon arrival) were individually housed in standard polycarbonate cages. Animals were maintained on a 12:12 light-dark cycle (lights on at 0600 h) with ad libitum access to food and water except during behavioral testing. All experimentation was conducted during the light portion of the light-dark cycle.
Drugs
Synthetic rat/human CRF was obtained from American Peptide Company (Sunnyvale, CA) and was dissolved in artificial cerebrospinal fluid (aCSF, Harvard Apparatus, Holliston, MA). The selective CRF1 receptor antagonist (butylethyl[2,5-dimethyl-7-(2,4,6-trimethylphenyl)-7H-pyrrolo [2,3-d]pyrimidin-4-yl]amine) hydrochloride (Chen et al. 1997; Seymour et al. 2003) was a generous gift from Pfizer, Inc. (Groton, CT) and was dissolved in sterile water containing 0.1% (v/v) acetic acid prior to dilution to the appropriate concentration in aCSF. The selective CRF2 receptor antagonist antisauvagine-30 (AS30, (Ruhmann et al. 1998) was obtained from Polypeptide Laboratories (Torrance, CA) and dissolved in aCSF.
Surgical Procedures
Rats were anesthetized with 5% (v/v) isoflurane vaporized in oxygen at a flow rate of 0.4 L/min and placed into a stereotaxic frame (David Kopf Instruments, Tujunga, CA). Anesthesia was maintained throughout the surgery at 1–2% (v/v). An incision was made to expose the skull and rats were bilaterally implanted with microinjection guide cannulae (26 ga OD, Plastics1, Roanoke, VA) aimed to terminate 1 mm dorsal to the BNST (AP −0.26 mm, ML ±1.5 and DV −6.2 mm from bregma and skull surface according to the atlas of Paxinos and Watson (2005). Guide cannulae were fitted with dummy injectors to prevent obstruction and contamination, and were secured to the skull with stainless steel screws and dental cement. The wound was treated with topical bacitracin (2% w/v) and xylocaine (5% w/v) ointments and sutured closed with 4-0 Vicryl sutures. Animals were allowed at least 5 days of recovery in the home cage prior to subsequent procedures.
Elevated Plus Maze Procedures
Following surgical procedures, animals were randomly assigned to one of the various treatment groups (vehicle, CRF alone, CRF + antagonists, CRF antagonists alone) for elevated plus maze testing. Prior to testing, animals were handled for approximately 5–10 min daily for at least one week, during which dummy injectors were removed and immediately replaced to keep the cannulae free of obstruction, and to habituate the animals to microinjection handling procedures. On the day of experimentation, animals were transferred to a darkened testing room containing the elevated plus maze apparatus (approximately 10–20 lux at the center of the maze) and allowed to habituate for at least 1 hr prior to testing. Animals then had their dummy injectors removed and were inserted with clean microinjection needles (33 ga OD) that extended 1 mm beyond the ventral tip of the guide cannulae. Drug solutions containing CRF (0.2 or 1.0 nmol) and/or CP154,526 (0.2 or 1.0 nmol) or AS30 (0.2 or 1.0 nmol) were then infused at a rate of 0.5 μl/min over a period of 1 min while the animal was placed in a 35 cm diameter cylindrical Plexiglas container. Following drug infusion, microinjection needles remain in place for an additional 1 min to allow for diffusion of the substances into the surrounding tissue, and then the injector was removed and replaced with the dummy injector. Animals were then returned to the home cage for 5 min. Next, animals were placed onto the center of the elevated plus maze apparatus (SciPro, Sanborn, NY) for 10 min while being videotaped. The videotapes were later scored for time spent in the open arms, time spent in the closed arms, and number and open and closed arm entries by one of three investigators blind to the experimental condition. Each investigator was trained to score elevated plus maze videos until inter-observer reliability was >90%. The elevated plus maze apparatus was constructed of white polyvinylchloride plastic and consisted of two open arms (44 cm long × 15 cm wide) bisecting at a 90° angle two closed arms (44 cm long × 14 cm wide with 22.5 cm high walls). The maze was placed on an aluminum frame so that the arms were elevated 75 cm from the ground. All animals were tested on the elevated plus maze apparatus only once.
Place Conditioning Procedures
At least one week following elevated plus maze procedures, a randomly chosen subset of animals were selected for place conditioning procedures and assigned to one of the various treatment groups (CRF alone, CRF + antagonists, CRF antagonists alone). Briefly, rats were placed into a three-compartment place preference apparatus (Med Associates, St. Albans, VT) equipped with two distinct conditioning compartments (28 × 21 × 21 cm) that differed in color, lighting, and floor texture, separated by a smaller central neutral gray compartment (12 × 21 × 21 cm). Access to one or both of the conditioning compartments was controlled by automatic guillotine-type doors via a remote computer. The position of the animal in the apparatus was determined by computer-interfaced infrared photobeams in each of the three compartments placed 2 cm above the floor 5 cm apart. During a 30 min pre-conditioning test, rats had access to both conditioning compartments in order to establish baseline compartment preference. No innate bias towards either of the compartments was observed. Then, over the next 6 days, animals received alternating infusions of CRF (0.2 or 1.0 nmol) and/or the CRF antagonists CP154,526 or AS30 (0.2 nmol each) or CSF vehicle into the BNST (as described above) and confined to one of the two conditioning compartments for 20 min. One conditioning session was conducted per day, and the CRF/drug-paired compartment was counterbalanced across animals. On the day following the last conditioning session, rats were placed in the center gray compartment and given free access to both conditioning compartments for 30 min to ascertain the development of a conditioned place aversion.
Histology Verification of Cannulae Placement
Following experimental procedures, animals were deeply anesthetized with sodium pentobarbital (150 mg/kg i.p.) and perfused transcardially with saline followed by 10% formalin. Brains were then removed, immersed in the fixative solution for at least 24 hr, and then immersed in a 30% (w/v) sucrose solution for at least 48 hr. Brains were then cut into 40 μm coronal sections on a Leica CM1900 cryostat (Leica Microsystems, Bannockburn, IL), mounted onto microscope slides, and stained with cresyl violet for histological verification of cannula and injector placement.
Assessment of CRF Diffusion from the Injection Site
To assess the extent of diffusion of CRF from the tip of the injector, a subset of animals (n=3) received bilateral infusions of CRF that had been labeled with fluorescein isothiocyanate (FITC) using an EZ-Label protein labeling kit (Pierce Biotechnology) according to the manufacturer’s directions. The resulting FITC-labeled CRF solution was then evaporated under a vacuum and resuspended in aCSF at a concentration of 1.0 nmol/μl. Microinjections of FITC-labeled CRF into the BNST were conducted as described above. Fifteen minutes following the infusion, rats were deeply anesthestized with sodium pentobarbital (150 mg/kg i.p.) and perfused transcardially with saline followed by 4% (w/v) paraformaldehyde (Sigma-Aldrich, St. Louis, MO). Brains were then removed, immersed in the fixative solution for at least 24 hr, and then immersed in a 30% (w/v) sucrose solution for at least 48 hours. Brains were then cut into 40 μm coronal sections on a cryostat, mounted onto microscope slides, coverslipped with GelMount mounting media (Biomeda, Foster City, CA) containing an antifade reagent, and viewed under fluorescence (488 nm excitation) using a Leica DMLB microscope equipped with mercury lamp and a digital camera.
Data Analysis
For the elevated plus maze experiments, entries onto the open or closed arms were counted only if the animal placed all four paws onto the arm. Partial arm entries and entries into the intersection of the arms were not counted. Percent open arm entries was calculated by dividing the number of open arm entries by the total number of arm entries, multiplied by 100. Likewise, percent open time was calculated as the time spent on the open arms divided by total time spent on the open and closed arms, multiplied by 100. Statistical analyses were performed using SigmaStat version 3.0 (SPSS Inc., Chicago, IL). Data from experiments analyzing the effects of infusions of CRF (with or without co-infusion of specific antagonists) on behavior in the elevated plus maze were analyzed across all treatment conditions by a one-way analysis of variance (ANOVA) followed by Holm-Sidak post-hoc multiple comparisons against vehicle-treated animals. Similarly, data from experiments analyzing the effects of CRF antagonists alone on behavior in the elevated plus maze were analyzed across all treatment conditions by a one-way analysis of variance (ANOVA) followed by Holm-Sidak post-hoc multiple comparisons against vehicle-treated animals. When data normality tests failed, a Kruskal-Wallis one-way ANOVA on ranks was performed. In place conditioning experiments examining the effects of intra-BNST infusions of CRF alone, data were analyzed for each dose by one-way repeated measures ANOVA (pre- versus post-conditioning) with Holm-Sidak post-hoc multiple comparisons. To assess the effects of co-infusion of specific CRF antagonists on place conditioning, post-conditioning data from the high dose of CRF (1.0 nmol) were compared with those of co-infusion with CP154,526 or AS30 by one-way ANOVA with Holm-Sidak post-hoc multiple comparisons. P-values less than 0.05 were considered statistically significant for all tests.
RESULTS
Cannula placement
The locations of the tips of the injectors in the BNST for each of the animals with histologically verified correct guide cannulae placement are shown in Fig. 1 (open circles). The location of injector tips from animals that received the 1.0 nmol dose of CRF but were subsequently determined to have erroneous injector placement outside of the BNST are also shown in Fig. 1 (filled circles). Such brain regions containing erroneously placed injectors included the nucleus accumbens, lateral hypothalamus, and thalamus. This group of animals (n=4) served as a negative anatomical control for the anxiogenic effects of CRF when infused into the BNST.
Fig. 1.
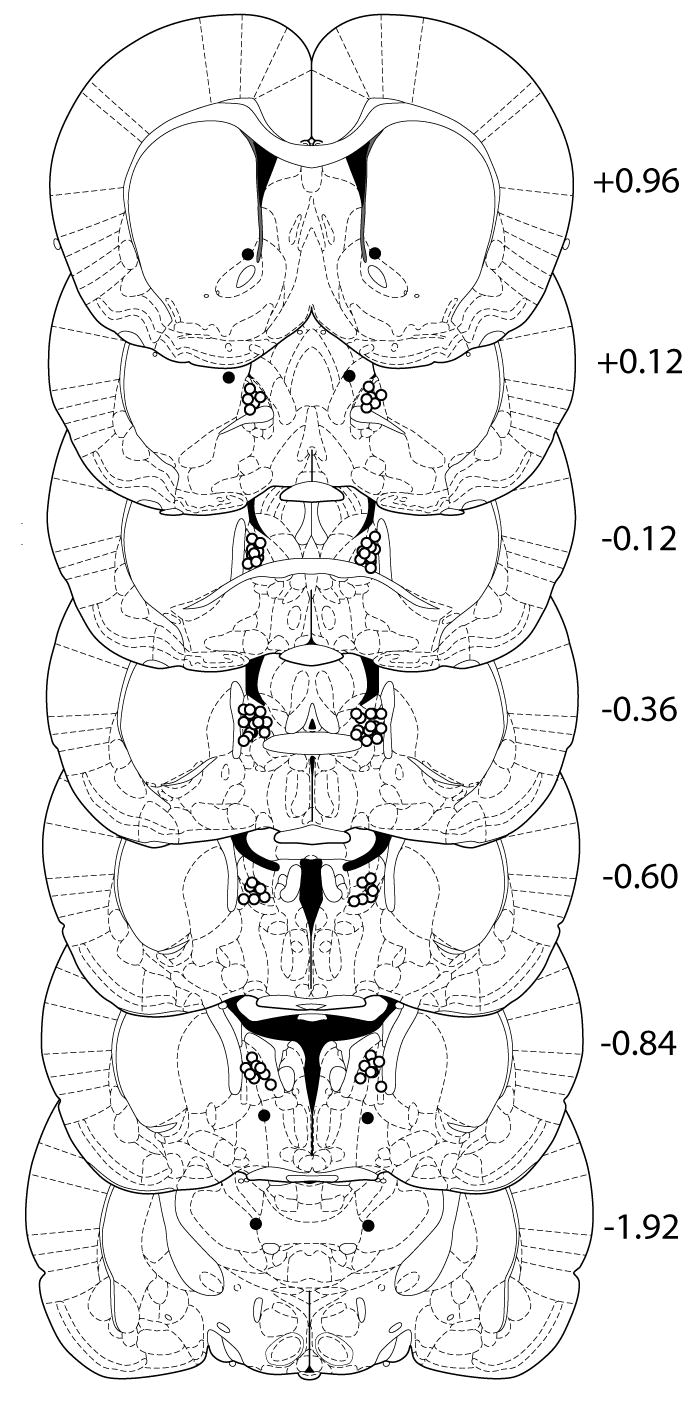
Diagrams of coronal sections of the rat brain containing the BNST. Locations of the tips of the injectors that were histologically verified to reside within the BNST are shown as open circles. Locations of the tips of the injectors in animals that were infused with the high (1 nmol) dose of CRF but whose injector placement was subsequently shown to be outside of the BNST are shown as filled circles. Numbers to the right of each coronal section represent the distance (in mm) of that section from bregma. Diagrams were adapted from the atlas of Paxinos and Watson (2005).
Diffusion of CRF from the injection site
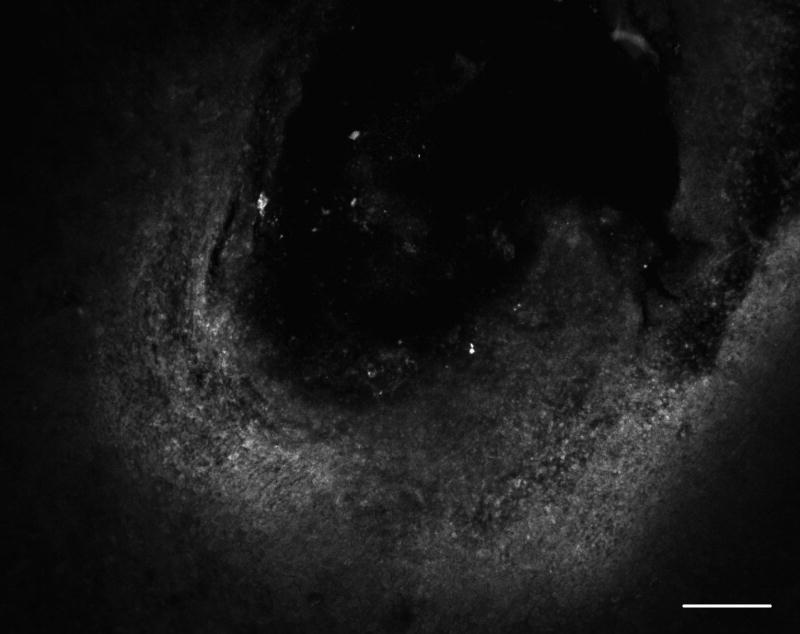
To assess the extent to which CRF diffuses away from the site of injection, we injected FITC-labeled CRF at a concentration of 1.0 nmol/μl (equal to the highest concentration used in behavioral testing) into the BNST and sacrificed the animals 15 min later (equal to the time at which plus maze testing was ended for each subject). As seen in Fig. 2, the majority of labeled CRF diffused approximately 100–150 μm from the tip of the injector, suggesting that the effects of CRF when injected into the BNST were mediated locally and not by significant diffusion of the peptide into surrounding regions.
Fig. 2.
Assessment of the diffusion of CRF from the tip of the injector. A subset of rats (n=3) used in the behavioral experiments were euthanized 15 min following infusion of FITC-labeled CRF (1.0 nmol/μl) into the BNST. Scale bar = 50 μm.
CRF subtypes involved in the anxiogenic-like effects of intra-BNST administered CRF
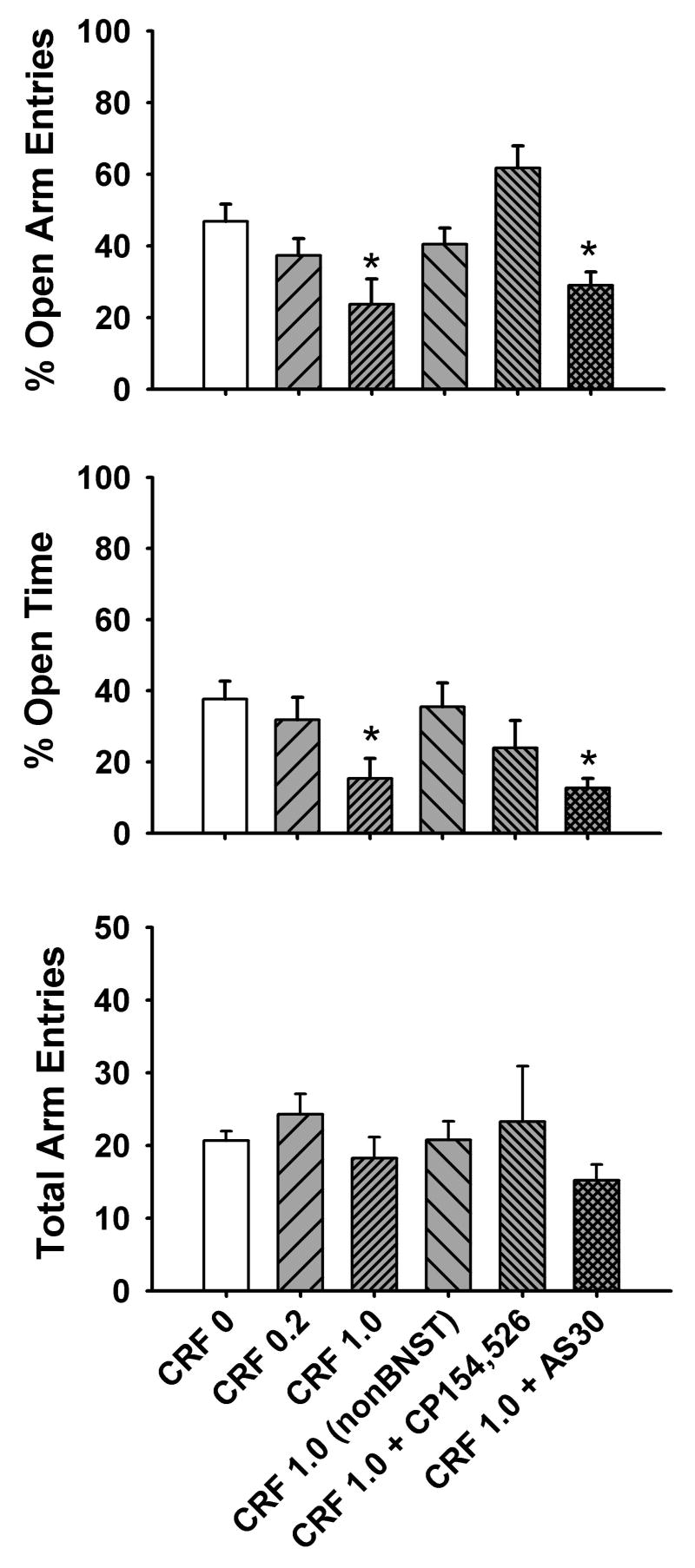
As seen in Fig. 3, infusion of CRF into the BNST resulted in a dose-dependent increase in anxiety-like behaviors in the elevated plus maze, as indicated by a decrease in % open arm entries [F(5,27)=5.29, p<0.005] and % open time [F(5,27)=3.32, p<0.05] following infusion of the 1.0 nmol dose of CRF (n=8) but not the 0.2 nmol dose of CRF (n=7), compared with vehicle-treated animals (n=6). Total arm entries, an index of general locomotor activity, was not affected by CRF infusion [H(5)=4.79, p>0.05]. Post-hoc multiple comparisons revealed that the anxiogenic-like effects of CRF were not observed when guide cannula placement was outside the BNST (i.e., nucleus accumbens, lateral hypothalamus or thalamus, n=4 total) or when CRF was co-infused with the CRF1 receptor antagonist CP154,526 (1.0 nmol, n=4). However, anxiogenic-like effects were still observed when CRF was co-infused with the CRF2 receptor antagonist AS30 (1.0 nmol, n=5). Lower (0.2 nmol) doses of CP154,526 or AS30 (n=5–6 per group) were ineffective at blocking the anxiogenic-like effects of intra-BNST administered CRF (data not shown).
Fig. 3.
Dose-dependent increase in anxiety-like behavior in the elevated plus maze produced by intra-BNST infusion of CRF, as indicated by a decrease in % open arm entries (top panel) and % open time (middle panel). Dose of CRF (0, 0.2 or 1.0 nmol) is given along the x-axis. The CRF 1.0 (nonBNST) group represents animals that received the 1 nmol dose of CRF but later were histologically determined to have injector placement outside the BNST. The anxiogenic-like effects of intra-BNST administered CRF (1 nmol) were attenuated by co-infusion of the CRF1-selective antagonist CP154,526 (1.0 nmol, CRF + CP154,526) but not the CRF2-selective antagonist AS30 (1 nmol, CRF + AS30). No effect of any treatment condition on total arm entries was observed (bottom panel). n=6–8 animals per group for CRF treatment and n=4–5 animals per group for CRF (nonBNST) and CRF + antagonist treatment. *p<0.05 vs. vehicle (CRF 0) group.
Effects of intra-BNST administration of CRF receptor antagonists alone on anxiety-like behavior
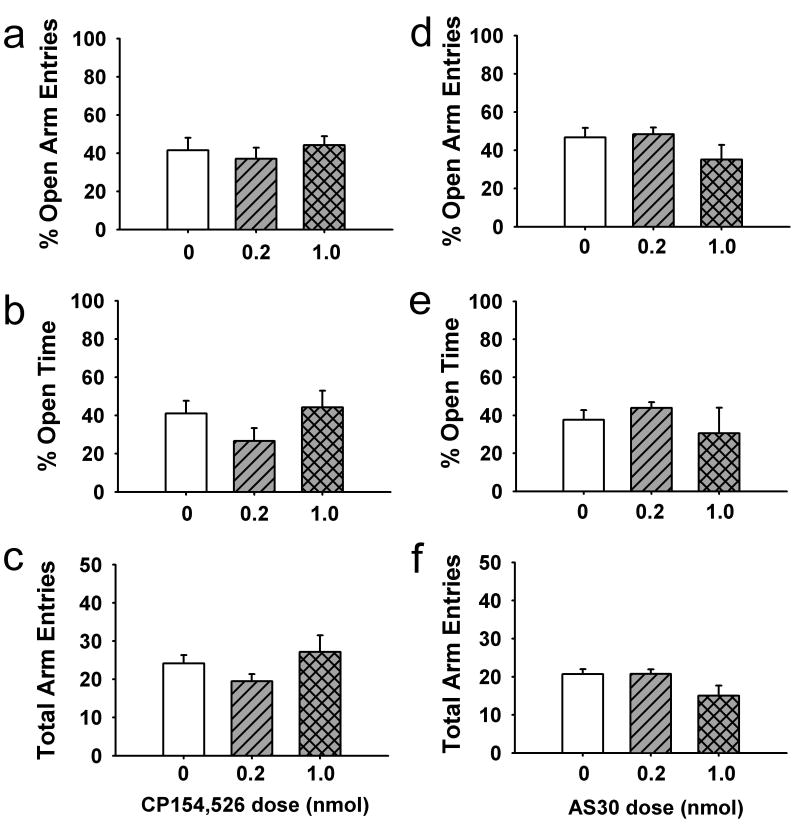
To determine if CRF subtype-selective antagonists possessed any intrinsic anxiogenic-like or anxiolytic-like activity, we assessed the effects of intra-BNST administration of CP154,526 or AS30 alone on anxiety-like behaviors in the elevated plus maze. Neither the 0.2 nmol (n=6) nor the 1.0 nmol (n=7) dose of the CRF1-selective antagonist CP154,526 produced any changes in % open arm entries [F(2,16)=0.42, p>0.05; Fig. 4a], % open time [F(2,16)=1.52, p>0.05; Fig. 4b] or total arm entries [F(2,16)=1.45, p>0.05; Fig. 4c] as compared with vehicle-treated animals (n=6). Similarly, neither the 0.2 nmol (n=8) nor the 1.0 nmol (n=5) dose of CRF2-selective antagonist AS30 produced any changes in % open arm entries [F(2,16)=1.83, p>0.05; Fig. 4d], % open time [H(4)=0.71, p>0.05; Fig. 4e] or total arm entries [F(2,16)=3.50, p>0.05; Fig. 4f] as compared with vehicle-treated animals (n=6).
Fig. 4.
Lack of effect of infusion of the CRF1 antagonist CP154,526 (a–c) or the CRF2 antagonist AS30 (d–f) alone into the BNST on anxiety-like behaviors in the elevated plus maze. n=5–8 per group.
Conditioned place aversion produced by infusions of CRF into the BNST
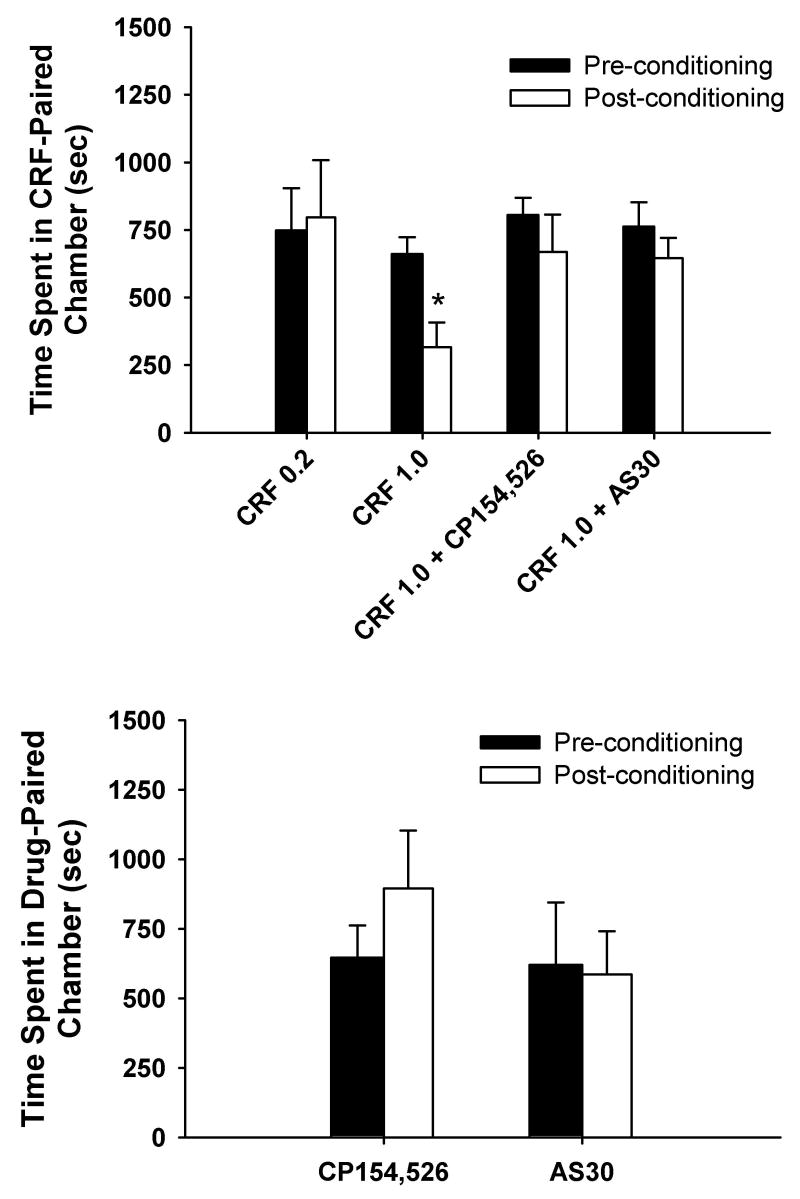
To determine if intra-BNST administration of CRF produced any subjective aversive effects, we conducted place conditioning in a randomly chosen subset of animals at least 1 week following elevated plus maze procedures. The results of these studies are shown in Fig. 5. While infusions of the 0.2 nmol dose (n=3) of CRF into the BNST did not produce any evidence of conditioned place aversion [F(1,2)=0.05, p>0.05], the 1.0 nmol dose (n=5) of CRF produce a significant reduction in preference for the compartment previously paired with CRF infusions [F(1,4)=18.98, p<0.05].
Fig. 5.
Top panel, Dose-dependent induction of a conditioned place aversion to intra-BNST administered CRF. Dose of CRF (0.2 or 1.0 nmol) is given along the x-axis. The conditioned aversive effects of intra-BNST administered CRF (1 nmol) were not observed when CRF was co-infused with the CRF1-selective antagonist CP154,526 (0.2 nmol, CRF + CP154,526) or the CRF2-selective antagonist AS30 (0.2 nmol, CRF + AS30). n=3–6 animals per group. *p<0.05 vs. preconditioning values from animals treated with 1.0 snmol CRF. Bottom panel, Lack of aversive effects of intra-BNST administration of the CRF1-selective antagonist CP154,526 (0.2 nmol) or the CRF2-selective antagonist AS30 (0.2 nmol) along during place conditioning. n=3–4 animals per group.
Co-infusion of either the CRF1-selective antagonist CP154,526 (0.2 nmol, n=5) or the CRF2-selective antagonist AS30 (0.2 nmol, n=6) with CRF (1.0 nmol) during the conditioning phase prevented the subsequent development of a conditioned place aversion to the CRF-paired compartment [F(1,4)=1.39, p>0.05 for CRF + CP154,526; F(1,6)=4.58, p>0.05 for CRF + AS30]. When compared with time spent in the CRF (1.0 nmol)-paired compartment following conditioning, co-infusion of either CP154,526 or AS30 along with CRF during conditioning produced a trend toward increasing the time spent in the drug-paired compartment, although these data just missed achieving statistical significance [F(2,13)=3.61, p=0.06]. The doses of the CRF antagonists employed (0.2 nmol) failed to produce any conditioned place preference or aversion when administered alone into the BNST [F(1,3)=2.42, p>0.05 for CP154,526 (n=4); F(1,2)=0.21, p>0.05 for AS30 (n=3)].
DISCUSSION
We have shown that when administered into the BNST, CRF produces dose-dependent anxiogenic-like effects in rats as measured by the elevated plus maze. These observations are consistent with those of other reports showing increases in stress-like behaviors following intra-BNST infusions of CRF (Ciccocioppo et al. 2003; Lee and Davis 1997; Liang et al. 2001; Nijsen et al. 2001). Anxiogenic-like effects of CRF were not observed in animals with erroneously placed microinjection needles outside of the BNST in regions such as the lateral hypothalamus, thalamus and nucleus accumbens, indicating a degree of anatomical specificity for the observed effects of CRF.
Through the use of CRF subtype selective antagonists, we demonstrated that the anxiogenic-like effects of CRF in the BNST are mediated by CRF1 and not CRF2 receptors. These findings are consistent with studies showing that the anxiogenic-like effects of CRF are primarily mediated by CRF1 receptors (Deak et al. 1999; Griebel et al. 1998, 2002; Heinrichs et al. 1997a; Schulz et al. 1996; Tezval et al. 2004; Todorovic et al. 2005), and are also consistent with studies in genetically altered mice in which deletion of the CRF1 receptor gene produces anxiolytic-like effects (Contarino et al. 1999; Muller et al. 2003; Risbrough et al. 2004; Smith et al. 1998; Timpl et al. 1998). Our data also suggest that the anxiogenic-like effects of centrally (i.e., icv) administered CRF may be mediated, at least in part, by actions within the BNST.
The finding that the anxiogenic-like effects of intra-BNST infusions of CRF were not attenuated by co-infusion of the CRF2 antagonist AS30, despite the presence of moderate amounts of CRF2 receptors within the BNST (Chalmers et al. 1995; Chen et al. 2000; Rominger et al. 1998; Rybnikova et al. 2003; Van Pett et al. 2000), is worthy of discussion. This lack of effect could be attributed to the higher affinity of CRF for CRF1 over CRF2 receptors (Vaughan et al. 1995). However, there is a growing (albeit conflicting) body of evidence that CRF2 receptors, in addition to CRF1 receptors, mediate anxiety-like and stress-related behaviors. For example, genetic deletion of the CRF2 receptor gene in mice has been shown to produce increase in anxiety-like behaviors in the elevated plus maze (Bale et al. 2000; Kishimoto et al. 2000), although Kishimoto and colleagues demonstrated this effect to be gender-specific whereas Bale et al. did not. Others have shown that there is no difference in elevated plus maze behavior between CRF2 receptor-deficient mice and wild-type littermates (Coste et al. 2000). Pharmacological studies on the role of CRF2 receptors in anxiety- and stress-like responses have yielded equally conflicting results. For example, antagonism of CRF2 receptors by icv administration of AS30 has been shown to reduce the facilitation of the acoustic startle reflex produced by central administration of CRF (Risbrough et al. 2003) and produce anxiolytic-like effects in the elevated plus maze (Pelleymounter et al. 2002; Takahashi et al. 2001), whereas activation of CRF2 receptors by icv infusions of the endogenous CRF2-preferring putative neuropeptide urocortin 2 produce anxiogenic-like effects (Pelleymounter et al. 2002, 2004). On the other hand, this latter study showed that the anxiogenic-like effects of urocortin 2 were not attenuated by co-infusion of AS30, and also showed that icv administration CRF2-preferring ligand urocortin 3 had no effect on behavior in the elevated plus maze (Pelleymounter et al. 2004), questioning the role of CRF2 receptors in the behavioral actions of urocortin 2 and 3. Further adding to the confusion to these conflicting reports, some investigators have reported that icv infusions of urocortin 2 or 3 actually produce anxiolytic-like effects in some behavioral paradigms, including the elevated plus maze (Valdez et al. 2002, 2003, 2004; but see Venihaki et al. 2004) and acoustic startle response (Risbrough et al. 2004). Thus, there is no current general consensus as to the precise role that CRF2 receptors play in anxiety-like behaviors and responses to stressors when numerous brain regions are examined at once (i.e., via icv infusions or embryonic gene deletion).
When brain regions are examined individually (i.e., via site-specific microinjections), the role of CRF2 receptors in governing anxiety-like behaviors becomes slightly more clear. Using a fear conditioning paradigm, Radulovic and coworkers demonstrated that infusions of AS30 into the lateral septum but not the hippocampus facilitated the acquisition of conditioned freezing behavior (Radulovic et al. 1999), suggesting that CRF2 receptor function in this region has an inhibitory role in conditioned fear responses. Subsequently, Bakshi and colleagues showed that infusions of the dual CRF1/CRF2 receptor antagonist D-Phe-CRF(12–41), but not the selective CRF1 antagonist NBI27914, actually attenuated unconditioned shock-induced freezing when infused into the lateral septum (Bakshi et al. 2002), suggesting that CRF2 receptor function in the lateral septum has a permissive role in the expression of unconditioned anxiety-like behavior. It is therefore possible that CRF2 receptors in the lateral septum play a dichotomous role mediating conditioned versus unconditioned anxiety responses. If the same phenomenon were true in the BNST, this may explain, at least in part, why our experiments showed no effects of CRF2 antagonism on basal or CRF-induced anxiety-like behavior in the elevated plus maze (a measure of unconditioned anxiety-like behavior), whereas CRF2 receptors in the BNST did appear to mediate the conditioned aversive effects of CRF. This hypothesis is further supported by the recent finding that CRF2 receptors in the BNST mediate conditioned behavioral responses to social defeat in hamsters (Cooper and Huhman 2005). Future studies will hopefully further elucidate the role of both CRF1 and CRF2 receptor subtypes in the BNST in mediating conditioned versus unconditioned anxiety-like behaviors and responses to stressors.
We were particularly surprised by our current findings that CRF1 antagonist CP154,526 and the CRF2 antagonist AS30 produced no anxiolytic-like effects when administered alone into the BNST. These data suggest that neither CRF receptor subtype in the BNST mediates basal levels of anxiety-like behaviors under the current experimental conditions. However, it should be noted that in our study, rats that had been well-habituated to handling and microinjection procedures were used as subjects to avoid potential floor effects and hence an inability to discern any anxiogenic-like effects of CRF (for example, see Andrews and File 1993). It is therefore possible that anxiolytic-like effects of either of these drugs in the BNST might be observed under conditions in which animals has been previously exposed to stress (see Hogg 1996). In support of this possibility, intra-BNST administration of the CRF2 antagonist AS30 reduces submissive-defensive behaviors induced by conditioned social defeat in the hamster but not in undefeated animals (Cooper and Huhman 2005). Futher experimentation is needed to address the possibility that blockade of CRF1 or CRF2 receptors in the BNST produces anxiolytic-like or anti-stress effects under different conditions of prior stress exposure.
As with all studies employing exogenous administration of endogenously occurring neuroactive substances into specific brain regions, it is unknown whether the effective concentration of CRF administered (1.0 nmol/μl) in our study is relevant to the extracellular concentrations of this peptide found in the BNST under physiological conditions in either normal or stressed subjects. Likewise, it is unknown if the extracellular levels of CRF antagonists such as CP154,526 following peripheral administration would reach the concentrations in the BNST achieved by local infusion of 0.2 or 1.0 nmol/μl of these compounds. To our knowledge, no-net flux microdialysis studies estimating the extracellular concentrations of CRF or CRF antagonists in the BNST or other brain regions have not been conducted. Nonetheless, the doses of all infused substances in the present study are comparable to those that have been used by other investigators.
Through the use of FITC-labeled CRF, we demonstrated that the majority of diffusion of CRF is limited to approximately 100–150 μm from the tip of the injector at 15 min post-infusion, and thus the anxiogenic-like effects of CRF in the BNST are likely anatomically specific when administered directly into this region. It is possible, however, that conjugation of the FITC molecule (molecular weight ~390) to CRF (molecular weight ~4700), slowed the diffusion of the CRF molecule in comparison with the unlabeled peptide used in our behavioral experiments, resulting in an underestimation of the degree of diffusion of CRF when administered into the BNST. An alternative approach would be to use CRF labeled with a radioactive isotope (i.e., I125) as the injected substance, since this modification would likely produce minimal changes in the diffusion characteristics of this peptide. However, this would require the use of autoradiographic techniques to detect the labeled peptide, which generally have lower spatial resolution than fluorescent labeling techniques.
Intra-BNST administration of CRF also produced a dose-dependent conditioned place aversion, paralleling previous findings that icv administration of this peptide produces such an effect (Cador et al. 1992). Thus, as with CRF-induced anxiety-like behaviors, these data suggest that centrally administered CRF may act within the BNST to produce its aversive effects. Interestingly, we found that co-infusion of either CP154,526 or AS30 blocked the development of a conditioned place aversion to CRF, suggesting that both CRF1 and CRF2 receptor subtypes in the BNST mediate the conditioned aversive effects of this peptide, despite CRF displaying a higher intrinsic affinity for CRF1 over CRF2 receptors (Vaughan et al. 1995). As mentioned earlier, this apparent involvement of the CRF2 receptors in the conditioned aversive effects of intra-BNST administration of CRF, in contrast with the lack of effect of CRF2 antagonism on the anxiogenic-like effects of CRF in the elevated plus maze (a measure of unconditioned anxiety-like behavior), reveal a potentially dichotomous role of CRF2 receptors in mediating conditioned versus unconditioned behaviors. For instance, when administered icv, CRF2 antagonists attenuate CRF-induced potentiation of the acoustic startle reflex (Risbrough et al. 2003, 2004). When administered into the lateral septum, CRF2 receptor antagonism in the lateral septum enhances conditioned freezing (Radulovic et al. 1999) but attenuates unconditioned freezing behavior (Bakshi et al. 2002). Likewise, antagonism of CRF2 receptors in the BNST attenuates conditioned submissive-defensive behaviors in defeated but not non-defeated hamsters (Cooper and Huhman 2005). In light of these findings and our current data, it is possible that CRF2 receptors in the BNST are similarly involved in mediating conditioned versus unconditioned fear-related behaviors.
An alternative explanation for the efficacy of both CRF1 and CRF2 antagonists in reversing the conditioned place aversion produced by intra-BNST infusion of CRF is that CRF antagonists may interfere with normal learning and memory processes. For example, CRF has been shown to increase hippocampal long-term potentiation (Blank et al. 2002; Wang et al. 1998, 2000) and facilitate learning of certain behavioral tasks, including aversive learning (Blank et al. 2002; Garcia-Lecumberri and Ambrosio 2000; Heinrichs et al. 1997b; Hung et al. 1992; Lee 1995; Lee and Sung 1989; Liang et al. 2001; Liang and Lee 1988; Ma et al. 1999; Radulovic et al. 1999; Zorrilla et al. 2002), while disruption of CRF receptor function can attenuate certain forms of learning (Contarino et al. 1999; Heinrichs 2003). Thus, the apparent blockade of intra-BNST CRF-induced conditioned place aversion by both CRF1 and CRF2 antagonist could be a result of an attenuated ability of the animal to associate the aversive effects of CRF in the BNST with the environment in which CRF is administered, rather than an attenuation of the actual aversive effects of CRF itself. Further studies are needed to dissociate potential learning and memory substrates of CRF receptor antagonists from the aversive properties of CRF in the BNST.
The current findings may have relevance to the neurobiological mechanisms underlying psychiatric disorders such as depression, anxiety, post-traumatic stress disorder (PTSD) and drug addiction. For example, the BNST is activated by acute stressors (Bonaz and Tache 1994; Campeau and Watson 1997; Kollack-Walker et al. 1997; Martinez et al. 1998, 2002), likely from excitatory glutamatergic and/or CRF-containing inputs from the amygdala, ventral subiculum and limbic cortices, and is essential for stress-induced learning (Bangasser et al. 2005). Chronic stress, which is known to produce depressive-like symptoms such as anhedonia, elevates levels of CRF in the BNST (Chappell et al. 1986; Stout et al. 2000). In light of the current findings, these elevated levels of CRF in this region may therefore contribute to the symptoms of anxiety that often co-occur with depression. In addition, two of the hallmark symptoms of PTSD are anxiety and avoidance of people, objects or places associated with a severe stressor (Association 1994), and since the present study demonstrates that intra-BNST administration of CRF induces both anxiogenic-like effects and a conditioned place aversion, these findings may represent a biological substrate of PTSD. Furthermore, we have previously shown using in vivo microdialysis procedures that extracellular levels of CRF are elevated in the BNST during acute alcohol withdrawal and are reduced by subsequent alcohol self-adminstration (Olive et al. 2002). Taken together with the current findings, it is likely that CRF, acting through CRF1 receptors within the BNST, contributes to the anxiogenic-like effects of acute alcohol withdrawal, whereas both CRF1 and CRF2 receptor contribute to the aversive consequences of this withdrawal state. Finally, other investigators have shown that administration of CRF into the BNST (but not the central nucleus of the amygdala) reinstates previously extinguished cocaine-seeking behavior (Erb and Stewart 1999), whereas blockade of CRF receptors in this region (but not the central nucleus of the amygdala or nucleus accumbens) attenuates the ability of stress to reinstate previously extinguished cocaine-seeking behavior (Erb et al. 2001; Erb and Stewart 1999) or morphine conditioned place preference (Wang et al. 2006). Thus, CRF in the BNST appears to contribute to the reinstatement of drug-seeking behavior as well withdrawal-induced anxiety-like behaviors.
In conclusion, our results show that in the BNST, CRF acts primarily on CRF1 receptors to produce anxiety-like behaviors. However, the conditioned aversive effects of CRF in the BNST appear to be mediated by both CRF1 and CRF2 receptor subtypes. These results provide a neuroanatomical and neurochemical substrates by which CRF produces anxiogenic-like and aversive effects, and hopefully this knowledge will lead to improved therapeutic strategies for the treatment of various psychiatric conditions that involve excessive CRF signaling, such as anxiety, depression, PTSD, alcohol withdrawal, and relapse to drug addiction.
Acknowledgments
This work was supported by Public Health Service grants AA13276 and AA013852 to MFO, the Charleston Alcohol Research Center (AA010761), and funds provided by the State of California for medical research on alcohol and substance abuse through the University of California at San Francisco. The authors wish to thank Drs. Tom Greenberg and Eric Zorrilla of The Scripps Research Institute for technical advice on elevated plus maze procedures. The experiments described herein were conducted in accordance with the current laws of the Food and Drug Administration of the United States of America. Portions of these data have been presented previously in abstract form (Sahuque et al. 2004).
References
- Alheid GF. Extended amygdala and basal forebrain. Ann N Y Acad Sci. 2003;985:185–205. doi: 10.1111/j.1749-6632.2003.tb07082.x. [DOI] [PubMed] [Google Scholar]
- Alheid GF, de Olmos JS, Beltramino CA (1995) Amygdala and extended amygdala. In: Paxinos G (ed) The Rat Nervous System. Academic Press, San Diego, pp 495–578
- Andrews N, File SE. Handling history of rats modifies behavioural effects of drugs in the elevated plus-maze test of anxiety. Eur J Pharmacol. 1993;235:109–12. doi: 10.1016/0014-2999(93)90827-5. [DOI] [PubMed] [Google Scholar]
- Association AP (1994) Diagnostic and Statistical Manual of Mental Disorders, 4th ed. American Psychiatric Press, Washington DC
- Bakshi VP, Smith-Roe S, Newman SM, Grigoriadis DE, Kalin NH. Reduction of stress-induced behavior by antagonism of corticotropin-releasing hormone 2 (CRH2) receptors in lateral septum or CRH1 receptors in amygdala. J Neurosci. 2002;22:2926–2935. doi: 10.1523/JNEUROSCI.22-07-02926.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GF, Vale WW, Lee K-F. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nature Genet. 2000;24:410–414. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–57. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Santollo J, Shors TJ. The bed nucleus of the stria terminalis is critically involved in enhancing associative learning after stressful experience. Behav Neurosci. 2005;119:1459–66. doi: 10.1037/0735-7044.119.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank T, Nijholt I, Eckart K, Spiess J. Priming of long-term potentiation in mouse hippocampus by corticotropin-releasing factor and acute stress: implications for hippocampus-dependent learning. J Neurosci. 2002;22:3788–94. doi: 10.1523/JNEUROSCI.22-09-03788.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaz B, Tache Y. Induction of Fos immunoreactivity in the rat brain after cold-restraint induced gastric lesions and fecal excretion. Brain Res. 1994;652:56–64. doi: 10.1016/0006-8993(94)90316-6. [DOI] [PubMed] [Google Scholar]
- Cador M, Ahmed SH, Koob GF, Le Moal M, Stinus L. Corticotropin-releasing factor induces a place aversion independent of its neuroendocrine role. Brain Res. 1992;597:304–9. doi: 10.1016/0006-8993(92)91487-y. [DOI] [PubMed] [Google Scholar]
- Campeau S, Watson SJ. Neuroendocrine and behavioral responses and brain pattern of c-fos induction associated with audiogenic stress. J Neuroendocrinol. 1997;9:577–88. doi: 10.1046/j.1365-2826.1997.00593.x. [DOI] [PubMed] [Google Scholar]
- Casada JH, Dafny N. Restraint and stimulation of bed nucleus of the stria terminalis produce similar stress-like behaviors. Brain Res Bull. 1991;27:207–12. doi: 10.1016/0361-9230(91)90069-v. [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell PB, Smith MA, Kilts CD, Bissette G, Ritchie J, Anderson C, Nemeroff CB. Alterations in corticotropin-releasing factor-like immunoreactivity in discrete rat brain regions after acute and chronic stress. J Neurosci. 1986;6:2908–14. doi: 10.1523/JNEUROSCI.06-10-02908.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Brunson KL, Müller MB, Cariaga W, Baram TZ. Immunocytochemical distribution of corticotropin-releasing hormone receptor type-1 (CRF1)-like immunoreactivity in the mouse brain: Light microscopy analysis using an antibody directed against the C-terminus. J Comp Neurol. 2000;420:305–323. doi: 10.1002/(sici)1096-9861(20000508)420:3<305::aid-cne3>3.0.co;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YL, Mansbach RS, Winter SM, Brooks E, Collins J, Corman ML, Dunaiskis AR, Faraci WS, Gallaschun RJ, Schmidt A, Schulz DW. Synthesis and oral efficacy of a 4-(butylethylamino)pyrrolo[2,3-d]pyrimidine: a centrally active corticotropin-releasing factor1 receptor antagonist. J Med Chem. 1997;40:1749–1754. doi: 10.1021/jm960861b. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Fedeli A, Economidou D, Policani F, Weiss F, Massi M. The bed nucleus is a neuroanatomical substrate for the anorectic effect of corticotropin-releasing factor and for its reversal by nociceptin/orphanin FQ. J Neurosci. 2003;23:9445–9451. doi: 10.1523/JNEUROSCI.23-28-09445.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contarino A, Dellu F, Koob GF, Smith GW, Lee K-F, Vale W, Gold LH. Reduced anxiety-like and cognitive performance in mice lacking the corticotropin-releasing factor receptor 1. Brain Res. 1999;835:1–9. doi: 10.1016/s0006-8993(98)01158-5. [DOI] [PubMed] [Google Scholar]
- Cooper MA, Huhman KL. Corticotropin-releasing factor type II (CRF2) receptors in the bed nucleus of the stria terminalis modulate conditioned defeat in Syrian hamsters (Mesocricetus auratus) Behav Neurosci. 2005;119:1042–1051. doi: 10.1037/0735-7044.119.4.1042. [DOI] [PubMed] [Google Scholar]
- Coste SC, Kesterson RA, Heldwein KA, Stevens SL, Heard AD, Hollis JH, Murray SE, Hill JK, Pantely GA, Hohimer AR, Hatton DC, Phillips TJ, Finn DA, Low MJ, Rittenberg MB, Stenzel P, Stenzel-Poore MP. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nature Genet. 2000;24:403–409. doi: 10.1038/74255. [DOI] [PubMed] [Google Scholar]
- Cummings S, Elde R, Ells J, Lindall A. Corticotropin-releasing factor immunoreactivity is widely distributed within the central nervous system of the rat: an immunohistochemical study. J Neurosci. 1983;3:1355–68. doi: 10.1523/JNEUROSCI.03-07-01355.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. Are different parts of the extended amygdala involved in fear versus anxiety? Biol Psychiatry. 1998;44:1239–47. doi: 10.1016/s0006-3223(98)00288-1. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Lee Y. Roles of the amygdala and bed nucleus of the stria terminalis in fear and anxiety measured with the acoustic startle reflex. Possible relevance to PTSD. Ann N Y Acad Sci. 1997;821:305–31. doi: 10.1111/j.1749-6632.1997.tb48289.x. [DOI] [PubMed] [Google Scholar]
- Deak T, Nguyen KT, Ehrlich AL, Watkins LR, Spencer RL, Maier SF, Licinio J, Wong ML, Chrousos GP, Webster E, Gold PW. The impact of the nonpeptide corticotropin-releasing hormone antagonist antalarmin on behavioral and endocrine responses to stress. Endocrinology. 1999;140:79–86. doi: 10.1210/endo.140.1.6415. [DOI] [PubMed] [Google Scholar]
- Dunn JD. Plasma corticosterone responses to electrical stimulation of the bed nucleus of the stria terminalis. Brain Res. 1987;407:327–31. doi: 10.1016/0006-8993(87)91111-5. [DOI] [PubMed] [Google Scholar]
- Erb S, Salmaso N, Rodaros D, Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2001;158:360–365. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci. 1999;19: RC35:1–6. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Endres T, Apfelbach R. Temporary inactivation of the bed nucleus of the stria terminalis but not of the amygdala blocks freezing induced by trimethylthiazoline, a component of fox feces. J Neurosci. 2003;23:23–8. doi: 10.1523/JNEUROSCI.23-01-00023.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Lecumberri C, Ambrosio E. Differential effect of low doses of intracerebroventricular corticotropin-releasing factor in forced swimming test. Pharmacol Biochem Behav. 2000;67:519–25. doi: 10.1016/s0091-3057(00)00384-1. [DOI] [PubMed] [Google Scholar]
- Gewirtz JC, McNish KA, Davis M. Lesions of the bed nucleus of the stria terminalis block sensitization of the acoustic startle reflex produced by repeated stress, but not fear-potentiated startle. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:625–48. doi: 10.1016/s0278-5846(98)00028-1. [DOI] [PubMed] [Google Scholar]
- Gray TS, Piechowski RA, Yracheta JM, Rittenhouse PA, Bethea CL, Van de Kar LD. Ibotenic acid lesions in the bed nucleus of the stria terminalis attenuate conditioned stress-induced increases in prolactin, ACTH and corticosterone. Neuroendocrinology. 1993;57:517–24. doi: 10.1159/000126400. [DOI] [PubMed] [Google Scholar]
- Greenwell TN, Zorrilla EP, Koob GF (2004) Microinfusion of a corticotropin-releasing factor receptor antagonist into the bed nucleus of the stria terminalis attenuates defensive burying behavior. Program No 1027.12. 2004 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience. Online.
- Griebel G, Perrault G, Sanger DJ. Characterization of the behavioral profile of the non-peptide CRF receptor antagonist CP-154,526 in anxiety models in rodents: comparison with diazepam and buspirone. Psychopharmacology. 1998;138:55–66. doi: 10.1007/s002130050645. [DOI] [PubMed] [Google Scholar]
- Griebel G, Simiand J, Steinberg R, Jung M, Gully D, Roger P, Geslin M, Scatton B, Maffrand JP, Soubrie P. 4-(2-Chloro-4-methoxy-5-methylphenyl)-N-[(1S)-2-cyclopropyl-1-(3-fluoro-4-methylphenyl)ethyl]5-methyl-N-(2-propynyl)-1, 3-thiazol-2-amine hydrochloride (SSR125543A), a potent and selective corticotrophin-releasing factor(1) receptor antagonist. II. Characterization in rodent models of stress-related disorders. J Pharmacol Exp Ther. 2002;301:333–45. doi: 10.1124/jpet.301.1.333. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Watkins LR, Maier SF. Chemical lesion of the bed nucleus of the stria terminalis blocks the behavioral consequences of uncontrollable stress. Behav Neurosci. 2004;118:443–448. doi: 10.1037/0735-7044.118.2.443. [DOI] [PubMed] [Google Scholar]
- Heimer L. A new anatomical framework for neuropsychiatric disorders and drug abuse. Am J Psychiatry. 2003;160:1726–39. doi: 10.1176/appi.ajp.160.10.1726. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC. Modulation of social learning in rats by brain corticotropin-releasing factor. Brain Res. 2003;994:107–14. doi: 10.1016/j.brainres.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Lapsansky J, Lovenberg TW, De Souza EB, Chalmers DT. Corticotropin-releasing factor CRF1, but not CRF2, receptors mediate anxiogenic-like behavior. Reg Peptides. 1997a;71:15–21. doi: 10.1016/s0167-0115(97)01005-7. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Vale EA, Lapsansky J, Behan DP, McClure LV, Ling N, De Souza EB, Schulteis G. Enhancement of performance in multiple learning tasks by corticotropin-releasing factor-binding protein ligand inhibitors. Peptides. 1997b;18:711–6. doi: 10.1016/s0196-9781(97)00120-4. [DOI] [PubMed] [Google Scholar]
- Henke PG. The bed nucleus of the stria terminalis and immobilization-stress: unit activity, escape behaviour, and gastric pathology in rats. Behav Brain Res. 1984;11:35–45. doi: 10.1016/0166-4328(84)90006-8. [DOI] [PubMed] [Google Scholar]
- Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- Hung HC, Chou CK, Chiu TH, Lee EH. CRF increases protein phosphorylation and enhances retention performance in rats. Neuroreport. 1992;3:181–4. doi: 10.1097/00001756-199202000-00015. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Davis M, Huhman KL. Involvement of central amygdalar and bed nucleus of the stria terminalis corticotropin-releasing factor in behavioral responses to social defeat. Behav Neurosci. 2004;118:1052–61. doi: 10.1037/0735-7044.118.5.1052. [DOI] [PubMed] [Google Scholar]
- Ju G, Swanson LW, Simerly RB. Studies on the cellular architecture of the bed nuclei of the stria terminalis in the rat: II. Chemoarchitecture. J Comp Neurol. 1989;280:603–21. doi: 10.1002/cne.902800410. [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Radulovic J, Radulovic M, Lin CR, Schrick C, Hooshmand F, Hermanson O, Rosenfeld MG, Spiess J. Deletion of Crhr2 reveals an anxiolytic role for corticotropin-releasing hormone receptor-2. Nature Genet. 2000;24:415–419. doi: 10.1038/74271. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Watson SJ, Akil H. Social stress in hamsters: defeat activates specific neurocircuits within the brain. J Neurosci. 1997;17:8842–55. doi: 10.1523/JNEUROSCI.17-22-08842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EH. Corticotropin-releasing factor injected into the lateral septum improves memory function in rats. Chin J Physiol. 1995;38:125–9. [PubMed] [Google Scholar]
- Lee EH, Sung YJ. Differential influences of corticotropin-releasing factor on memory retention of aversive learning and appetitive learning in rats. Behav Neural Biol. 1989;52:285–94. doi: 10.1016/s0163-1047(89)90412-3. [DOI] [PubMed] [Google Scholar]
- Lee Y, Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J Neurosci. 1997;17:6434–6446. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang KC, Chen H-C, Chen D-Y. Posttraining infusion of norepinephrine and corticotropin releasing factor into the bed nucleus of the stria terminalis enhanced retention in an inhibitory avoidance task. Chin J Physiol. 2001;44:33–43. [PubMed] [Google Scholar]
- Liang KC, Lee EH. Intra-amygdala injections of corticotropin releasing factor facilitate inhibitory avoidance learning and reduce exploratory behavior in rats. Psychopharmacology. 1988;96:232–6. doi: 10.1007/BF00177566. [DOI] [PubMed] [Google Scholar]
- Ma YL, Chen KY, Wei CL, Lee EH. Corticotropin-releasing factor enhances brain-derived neurotrophic factor gene expression to facilitate memory retention in rats. Chin J Physiol. 1999;42:73–81. [PubMed] [Google Scholar]
- Martinez M, Calvo-Torrent A, Herbert J. Mapping brain response to social stress in rodents with c-fos expression: a review. Stress. 2002;5:3–13. doi: 10.1080/102538902900012369. [DOI] [PubMed] [Google Scholar]
- Martinez M, Phillips PJ, Herbert J. Adaptation in patterns of c-fos expression in the brain associated with exposure to either single or repeated social stress in male rats. Eur J Neurosci. 1998;10:20–33. doi: 10.1046/j.1460-9568.1998.00011.x. [DOI] [PubMed] [Google Scholar]
- Moga MM, Saper CB, Gray TS. Bed nucleus of the stria terminalis: cytoarchitecture, immunohistochemistry, and projection to the parabrachial nucleus in the rat. J Comp Neurol. 1989;283:315–32. doi: 10.1002/cne.902830302. [DOI] [PubMed] [Google Scholar]
- Morin SM, Ling N, Liu X-J, Kahl SD, Gehlert DR. Differential distribution of urocortin- and corticotropin-releasing factor-like immunoreactivities in the rat brain. Neuroscience. 1999;92:281–291. doi: 10.1016/s0306-4522(98)00732-5. [DOI] [PubMed] [Google Scholar]
- Muller MB, Zimmermann S, Sillaber I, Hagemeyer TP, Deussing JM, Timpl P, Kormann MS, Droste SK, Kuhn R, Reul JM, Holsboer F, Wurst W. Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat Neurosci. 2003;6:1100–7. doi: 10.1038/nn1123. [DOI] [PubMed] [Google Scholar]
- Nijsen MJ, Croiset G, Diamant M, De Wied D, Wiegant VM. CRH signalling in the bed nucleus of the stria terminalis is involved in stress-induced cardiac vagal activation in conscious rats. Neuropsychopharmacology. 2001;24:1–10. doi: 10.1016/S0893-133X(00)00167-6. [DOI] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacol Biochem Behav. 2002;72:213–20. doi: 10.1016/s0091-3057(01)00748-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olschowka JA, O’Donohue TL, Mueller GP, Jacobowitz DM. The distribution of corticotropin releasing factor-like immunoreactive neurons in rat brain. Peptides. 1982;3:995–1015. doi: 10.1016/0196-9781(82)90071-7. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (2005) The Rat Brain in Stereotaxic Coordinates, 5th edn. Academic Press, San Diego
- Pelleymounter MA, Joppa M, Ling N, Foster AC. Pharmacological evidence supporting a role for central corticotropin-releasing factor2 receptors in behavioral, but not endocrine, response to environmental stress. J Pharmacol Exp Ther. 2002;302:145–152. doi: 10.1124/jpet.302.1.145. [DOI] [PubMed] [Google Scholar]
- Pelleymounter MA, Joppa M, Ling N, Foster AC. Behavioral and neuroendocrine effects of the selective CRF2 receptor agonists urocortin II and urocortin III. Peptides. 2004;25:659–666. doi: 10.1016/j.peptides.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Radulovic J, Rühmann A, Liepold T, Spiess J. Modulation of learning and anxiety by corticotropin-releasing Factor (CRF) and stress: differential roles of CRF receptors 1 and 2. J Neurosci. 1999;19:5016–5025. doi: 10.1523/JNEUROSCI.19-12-05016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbrough VB, Hauger RL, Pelleymounter MA, Geyer MA. Role of corticotropin releasing factor (CRF) receptors 1 and 2 in CRF-potentiated acoustic startle in mice. Psychopharmacology. 2003;170:178–87. doi: 10.1007/s00213-003-1535-6. [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Hauger RL, Roberts AL, Vale WW, Geyer MA. Corticotropin-releasing factor receptors CRF1 and CRF2 exert both additive and opposing influences on defensive startle behavior. J Neurosci. 2004;24:6545–52. doi: 10.1523/JNEUROSCI.5760-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rominger DH, Rominger CM, Fitzgerald LW, Grzanna R, Largent BL, Zaczek R. Characterization of [125I]sauvagine binding to CRH2 receptors: membrane homogenate and autoradiographic studies. J Pharmacol Exp Ther. 1998;286:459–68. [PubMed] [Google Scholar]
- Ruhmann A, Bonk I, Lin CR, Rosenfeld MG, Spiess J. Structural requirements for peptidic antagonists of the corticotropin-releasing factor receptor (CRFR): development of CRFR2β-selective antisauvagine-30. Proc Natl Acad Sci USA. 1998;95:15264–15269. doi: 10.1073/pnas.95.26.15264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybnikova EA, Pelto-Huikko M, Rakitskaya VV, Shalyapina VG. Localization of corticoliberin receptors in the rat brain. Neurosci Behav Physiol. 2003;33:399–404. doi: 10.1023/a:1022807926406. [DOI] [PubMed] [Google Scholar]
- Sahuque LL, Mcgeehan AJ, Kinder JR, Janak PH, Olive MF (2004) Anxiogenic and aversive effects of corticotropin releasing factor (CRF) in the bed nucleus of the stria terminalis. Program No. 5789.15. 2004 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience. Online.
- Sakanaka M, Shibasaki T, Lederis K. Distribution and efferent projections of corticotropin-releasing factor-like immunoreactivity in the rat amygdaloid complex. Brain Res. 1986;382:213–238. doi: 10.1016/0006-8993(86)91332-6. [DOI] [PubMed] [Google Scholar]
- Sakanaka M, Shibasaki T, Lederis K. Corticotropin releasing factor-like immunoreactivity in the rat brain as revealed by a modified cobalt-glucose oxidase-diaminobenzidine method. J Comp Neurol. 1987;260:256–98. doi: 10.1002/cne.902600209. [DOI] [PubMed] [Google Scholar]
- Schulkin J, Morgan MA, Rosen JB. A neuroendocrine mechanism for sustaining fear. Trends Neurosci. 2005;28:629–35. doi: 10.1016/j.tins.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Schulz D, Canbeyli R. Freezing behavior in BNST-lesioned Wistar rats. Ann N Y Acad Sci. 1999;877:728–31. doi: 10.1111/j.1749-6632.1999.tb09311.x. [DOI] [PubMed] [Google Scholar]
- Schulz D, Canbeyli RS. Lesion of the bed nucleus of the stria terminalis enhances learned despair. Brain Res Bull. 2000;52:83–7. doi: 10.1016/s0361-9230(00)00235-5. [DOI] [PubMed] [Google Scholar]
- Schulz DW, Mansbach RS, Sprouse J, Braselton JP, Collins J, Corman M, Dunaiskis A, Faraci S, Schmidt AW, Seeger T, Seymour P, Tingley FD, Winston EN, Chen YL, Heym J. CP-154,526: a potent and selective nonpeptide antagonist of corticotropin releasing factor receptors. Proc Natl Acad Sci. 1996;93:10477–10482. doi: 10.1073/pnas.93.19.10477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour PA, Schmidt AW, Schulz DW. The pharmacology of CP-154,526, a non-peptide antagonist of the CRH1 receptor: a review. CNS Drug Rev. 2003;9:57–96. doi: 10.1111/j.1527-3458.2003.tb00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA, Sawchenko PE, Koob GF, Vale W, Lee KF. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- Stout SC, Mortas P, Owens MJ, Nemeroff CB, Moreau J. Increased corticotropin-releasing factor concentrations in the bed nucleus of the stria terminalis of anhedonic rats. Eur J Pharmacol. 2000;401:39–46. doi: 10.1016/s0014-2999(00)00412-x. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Apergis J, Bush DEA, Johnson LR, Hou M, LeDoux JE. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience. 2004;128:7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Ho SP, Livanov V, Graciani N, Arneric SP. Antagonism of CRF2 receptors produces anxiolytic behavior in animal models of anxiety. Brain Res. 2001;902:135–42. doi: 10.1016/s0006-8993(01)02405-2. [DOI] [PubMed] [Google Scholar]
- Tezval H, Jahn O, Todorovic C, Sasse A, Eckart K, Spiess J. Cortagine, a specific agonist of corticotropin- releasing factor receptor subtype 1, is anxiogenic and antidepressive in the mouse model. Proc Natl Acad Sci USA. 2004;101:9468–9473. doi: 10.1073/pnas.0403159101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl P, Spanagel R, Sillaber I, Kresse A, Reul JMHM, Stalla GK, Blanquet V, Steckler T, Holsboer F, Wurst W. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nature Genet. 1998;19:162–166. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- Todorovic C, Jahn O, Tezval H, Hippel C, Spiess J. The role of CRF receptors in anxiety and depression: implications of the novel CRF1 agonist cortagine. Neurosci Biobehav Rev. 2005;29:1323–33. doi: 10.1016/j.neubiorev.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Treit D, Aujla H, Menard J. Does the bed nucleus of the stria terminalis mediate fear behaviors? Behav Neurosci. 1998;112:379–386. doi: 10.1037//0735-7044.112.2.379. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Inoue K, Koob GF, Rivier J, Vale W, Zorrilla EP. Human urocortin II: mild locomotor suppressive and delayed anxiolytic-like effects of a novel corticotropin-releasing factor related peptide. Brain Res. 2002;943:142–50. doi: 10.1016/s0006-8993(02)02707-5. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Sabino V, Koob GF. Increased anxiety-like behavior and ethanol self-administration in dependent rats: reversal via corticotropin-releasing factor-2 receptor activation. Alcohol Clin Exp Res. 2004;28:865–72. doi: 10.1097/01.alc.0000128222.29875.40. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Zorrilla EP, Rivier J, Vale WW, Koob GF. Locomotor suppressive and anxiolytic-like effects of urocortin 3, and highy selective type 2 corticotropoin releasing factor agonist. Brain Res. 2003;980:206–212. doi: 10.1016/s0006-8993(03)02971-8. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C, Rivier J, Sawchenko PE, Vale W. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378:287–92. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- Venihaki M, Sakihara S, Subramanian S, Dikkes P, Weninger SC, Liapakis G, Graf T, Majzoub JA. Urocortin III, a brain neuropeptide of the corticotropin-releasing hormone family: modulation by stress and attenuation of some anxiety-like behaviours. J Neuroendocrinol. 2004;16:411–22. doi: 10.1111/j.1365-2826.2004.01170.x. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci. 1997;17:9375–83. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Wang HL, Tsai LY, Lee EH. Corticotropin-releasing factor produces a protein synthesis--dependent long-lasting potentiation in dentate gyrus neurons. J Neurophysiol. 2000;83:343–9. doi: 10.1152/jn.2000.83.1.343. [DOI] [PubMed] [Google Scholar]
- Wang HL, Wayner MJ, Chai CY, Lee EH. Corticotrophin-releasing factor produces a long-lasting enhancement of synaptic efficacy in the hippocampus. Eur J Neurosci. 1998;10:3428–37. doi: 10.1046/j.1460-9568.1998.00352.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Fang Q, Liu Z, Lu L. Region-specific effects of brain corticotropin-releasing factor receptor type 1 blockade on footshock-stress- or drug-priming-induced reinstatement of morphine conditioned place preference in rats. Psychopharmacology. 2006;185:19–28. doi: 10.1007/s00213-005-0262-6. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Schulteis G, Ormsby A, Klaassen A, Ling N, McCarthy JR, Koob GF, De Souza EB. Urocortin shares the memory modulating effects of corticotropin-releasing factor (CRF): mediation by CRF1 receptors. Brain Res. 2002;952:200–10. doi: 10.1016/s0006-8993(02)03345-0. [DOI] [PubMed] [Google Scholar]