Abstract
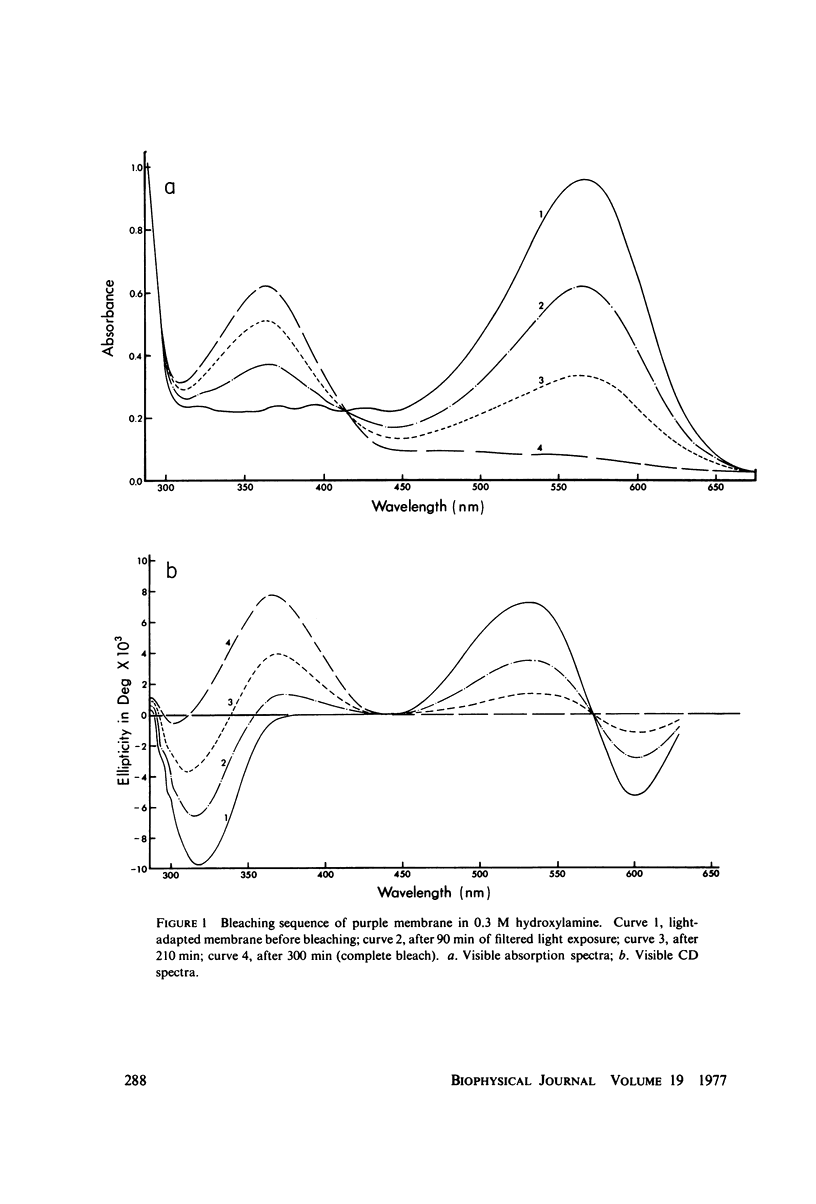
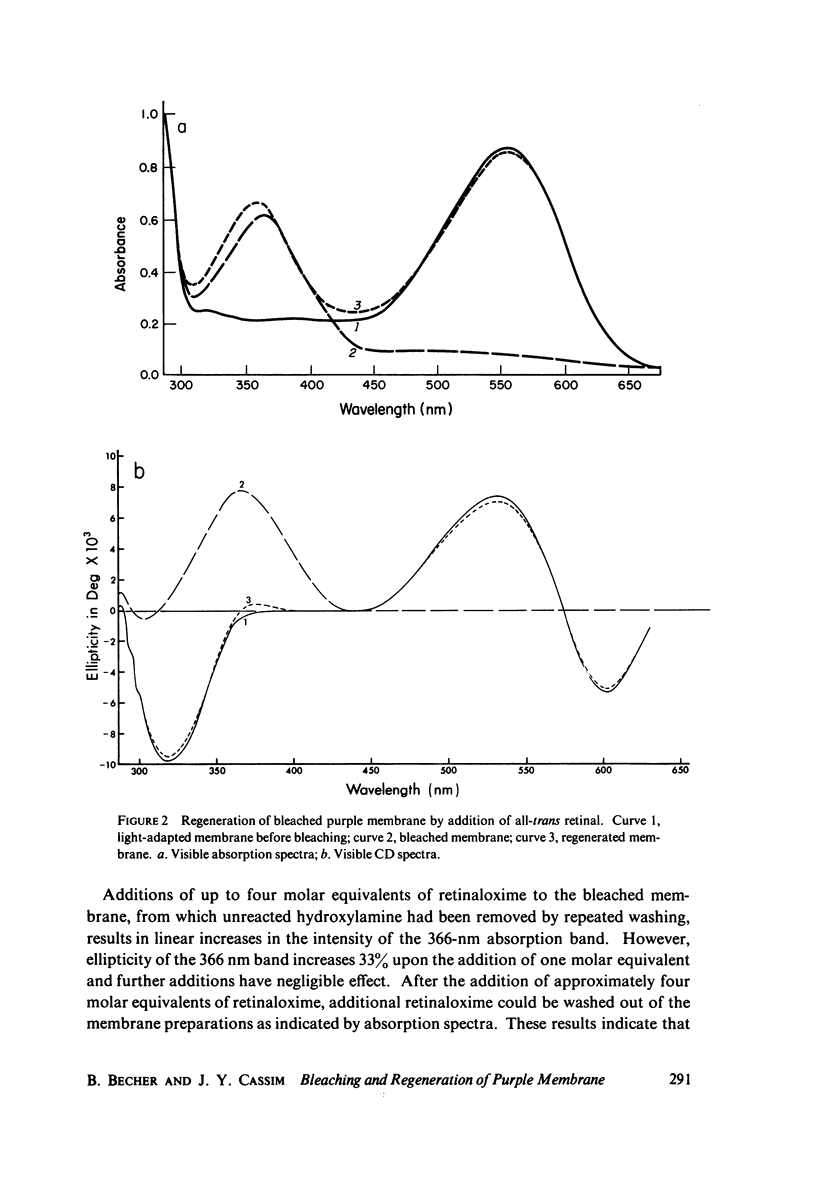
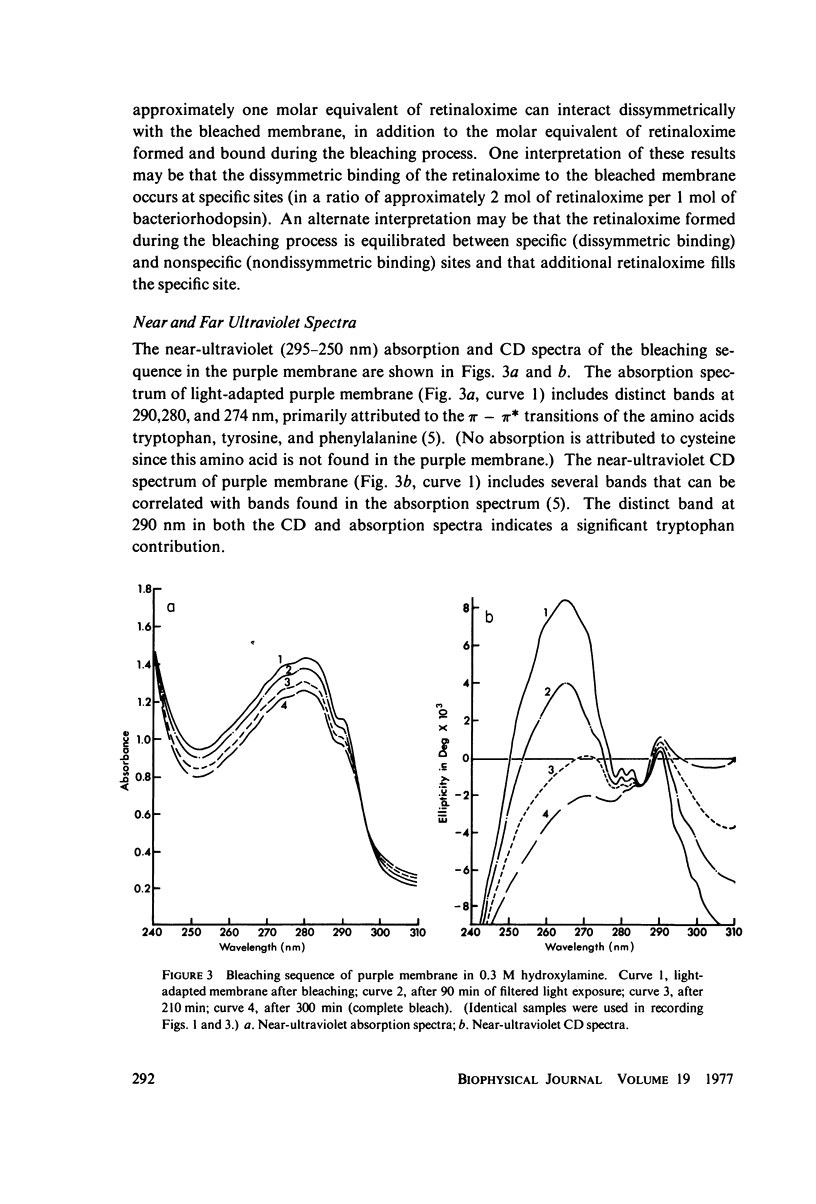
Sequential bleaching in the presence of hydroxylamine and subsequent regeneration of the purple membrane of Halobacterium halobium was studied by concomitant monitoring of its absorption and circular dichroic spectra in order to ascertain its effects on protein interaction(s) (which may result in possible excitonic interaction between the retinal chromophores), chromophore-apoprotein interaction(s), and protein conformational stability in the membrane. It was concluded that (a) although experimental results are consistent with an exciton mechanism for the interaction between retinal π - π* (NV1) transition movements in the purple membrane, no evidence for such a mechanism for interaction between retinaloxime transition moments is apparent in the case of the bleached membrane; (b) the bacteriorhodopsin molecules organized in clusters of three in the membrane appear to bleach simultaneously; (c) the retinaloxime produced on bleaching the purple membrane in the presence of hydroxylamine is strongly optically active, because of dissymmetry-inducing and/or -selecting constraints on the chromophore by a component of the membrane (most likely the apoprotein), and when the membrane is regenerated by the addition of retinal, these constraints are lost; and (d) evidence from ultraviolet absorption and circular dichroic spectra suggests that the membrane apoprotein undergoes appreciable conformational changes involving tertiary structure on bleaching with no significant secondary structure involvement. These results are compared with recently reported results from this laboratory on the effects of bleaching on the bovine rod outer segment disk membrane structure.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer P. J., Dencher N. A., Heyn M. P. Evidence for chromophore-chromophore interactions in the purple membrane from reconstitution experiments of the chromophore-free membrane. Biophys Struct Mech. 1976 Apr 15;2(1):79–92. doi: 10.1007/BF00535654. [DOI] [PubMed] [Google Scholar]
- Becher B. M., Cassim J. Y. Improved isolation procedures for the purple membrane of Halobacterium halobium. Prep Biochem. 1975;5(2):161–178. doi: 10.1080/00327487508061568. [DOI] [PubMed] [Google Scholar]
- Becher B., Cassim J. Y. Effects of light adaptation on the purple membrane structure of Halobacterium halobium. Biophys J. 1976 Oct;16(10):1183–1200. doi: 10.1016/S0006-3495(76)85767-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher B., Ebrey T. G. Evidence for chromophore-chromophore (exciton) interaction in the purple membrane of Halobacterium halobium. Biochem Biophys Res Commun. 1976 Mar 8;69(1):1–6. doi: 10.1016/s0006-291x(76)80263-x. [DOI] [PubMed] [Google Scholar]
- Blaurock A. E. Bacteriorhodospin: a trans-membrane pump containing alpha-helix. J Mol Biol. 1975 Apr 5;93(2):139–158. doi: 10.1016/0022-2836(75)90124-2. [DOI] [PubMed] [Google Scholar]
- Bridgen J., Walker I. D. Photoreceptor protein from the purple membrane of Halobacterium halobium. Molecular weight and retinal binding site. Biochemistry. 1976 Feb 24;15(4):792–798. doi: 10.1021/bi00649a010. [DOI] [PubMed] [Google Scholar]
- Burke M. J., Pratt D. C., Faulkner T. R., Moscowitz A. An analysis of the absorption and circular dichroism of some visual pigments. Exp Eye Res. 1973 Dec 24;17(6):557–572. doi: 10.1016/0014-4835(73)90085-7. [DOI] [PubMed] [Google Scholar]
- Cassim J. Y., Lin T. I. Does myosin-substrate interaction in vitro result in a delocalized conformation change? J Supramol Struct. 1975;3(5-6):510–519. doi: 10.1002/jss.400030510. [DOI] [PubMed] [Google Scholar]
- Chignell C. F., Chignell D. A. A spin label study of purple membranes from Halobacterium halobium. Biochem Biophys Res Commun. 1975 Jan 6;62(1):136–143. doi: 10.1016/s0006-291x(75)80415-3. [DOI] [PubMed] [Google Scholar]
- Cone R. A. Rotational diffusion of rhodopsin in the visual receptor membrane. Nat New Biol. 1972 Mar 15;236(63):39–43. doi: 10.1038/newbio236039a0. [DOI] [PubMed] [Google Scholar]
- Crescitelli F., Mommaerts W. F., Shaw T. I. Circular dichroism of visual pigments in the visible and ultraviolet spectral regions. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1729–1734. doi: 10.1073/pnas.56.6.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrey T. G., Honig B. Ultraviolet chromophore transitions in the rhodopsin spectrum. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1897–1899. doi: 10.1073/pnas.69.7.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R. The structure of the purple membrane from Halobacterium hallobium: analysis of the X-ray diffraction pattern. J Mol Biol. 1975 Apr 5;93(2):123–138. doi: 10.1016/0022-2836(75)90123-0. [DOI] [PubMed] [Google Scholar]
- Heyn M. P., Bauer P. J., Dencher N. A. A natural CD label to probe the structure of the purple membrane from Halobacterium halobium by means of exciton coupling effects. Biochem Biophys Res Commun. 1975 Dec 1;67(3):897–903. doi: 10.1016/0006-291x(75)90761-5. [DOI] [PubMed] [Google Scholar]
- Honig B., Kahn P., Ebrey T. G. Intrinsic optical activity of retinal isomers. Implications for the circular dichroism spectrum of rhodopsin. Biochemistry. 1973 Apr 10;12(8):1637–1643. doi: 10.1021/bi00732a027. [DOI] [PubMed] [Google Scholar]
- Hsu M. C., Woody R. W. The origin of the heme Cotton effects in myoglobin and hemoglobin. J Am Chem Soc. 1971 Jul 14;93(14):3515–3525. doi: 10.1021/ja00743a036. [DOI] [PubMed] [Google Scholar]
- Jan L. Y. The isomeric configuration of the bacteriorhodopsin chromophore. Vision Res. 1975 Oct;15:1081–1086. doi: 10.1016/0042-6989(75)90004-8. [DOI] [PubMed] [Google Scholar]
- Johnston E. M., Zand R. A coupled oscillator calculation of the induced circular dichroism of the visual pigment analog (S)-N-all-trans-retinylidene-alpha-(1-naphthyl)ethylamine. Biochemistry. 1973 Nov 6;12(23):4637–4643. doi: 10.1021/bi00747a015. [DOI] [PubMed] [Google Scholar]
- Johnston E. M., Zand R. Extrinsic Cotton effects in retinaldehyde Schiff's bases. Biochemistry. 1973 Nov 6;12(23):4631–4636. doi: 10.1021/bi00747a014. [DOI] [PubMed] [Google Scholar]
- Johnston E. M., Zand R. Induced optical activity in rhodopsin analogs. Biochem Biophys Res Commun. 1972 May 26;47(4):712–719. doi: 10.1016/0006-291x(72)90550-5. [DOI] [PubMed] [Google Scholar]
- Kemper D. L., Everse J. Active enzyme centrifugation. Methods Enzymol. 1973;27:67–82. doi: 10.1016/s0076-6879(73)27005-2. [DOI] [PubMed] [Google Scholar]
- Kropf A., Whittenberger B. P., Goff S. P., Waggoner A. S. The spectral properties of some visual pigment analogs. Exp Eye Res. 1973 Dec 24;17(6):591–606. doi: 10.1016/0014-4835(73)90088-2. [DOI] [PubMed] [Google Scholar]
- Lewis A., Spoonhower J., Bogomolni R. A., Lozier R. H., Stoeckenius W. Tunable laser resonance raman spectroscopy of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4462–4466. doi: 10.1073/pnas.71.11.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozier R. H., Bogomolni R. A., Stoeckenius W. Bacteriorhodopsin: a light-driven proton pump in Halobacterium Halobium. Biophys J. 1975 Sep;15(9):955–962. doi: 10.1016/S0006-3495(75)85875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Meentzen M., Schuhmann L. Reversible dissociation of the purple complex in bacteriorhodopsin and identification of 13-cis and all-trans-retinal as its chromophores. Eur J Biochem. 1973 Dec 17;40(2):453–463. doi: 10.1111/j.1432-1033.1973.tb03214.x. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Functions of a new photoreceptor membrane. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2853–2857. doi: 10.1073/pnas.70.10.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971 Sep 29;233(39):149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- Waggoner A. S., Stryer L. Induced optical activity of the metarhodopsins. Biochemistry. 1971 Aug 17;10(17):3250–3254. doi: 10.1021/bi00793a014. [DOI] [PubMed] [Google Scholar]



