Abstract
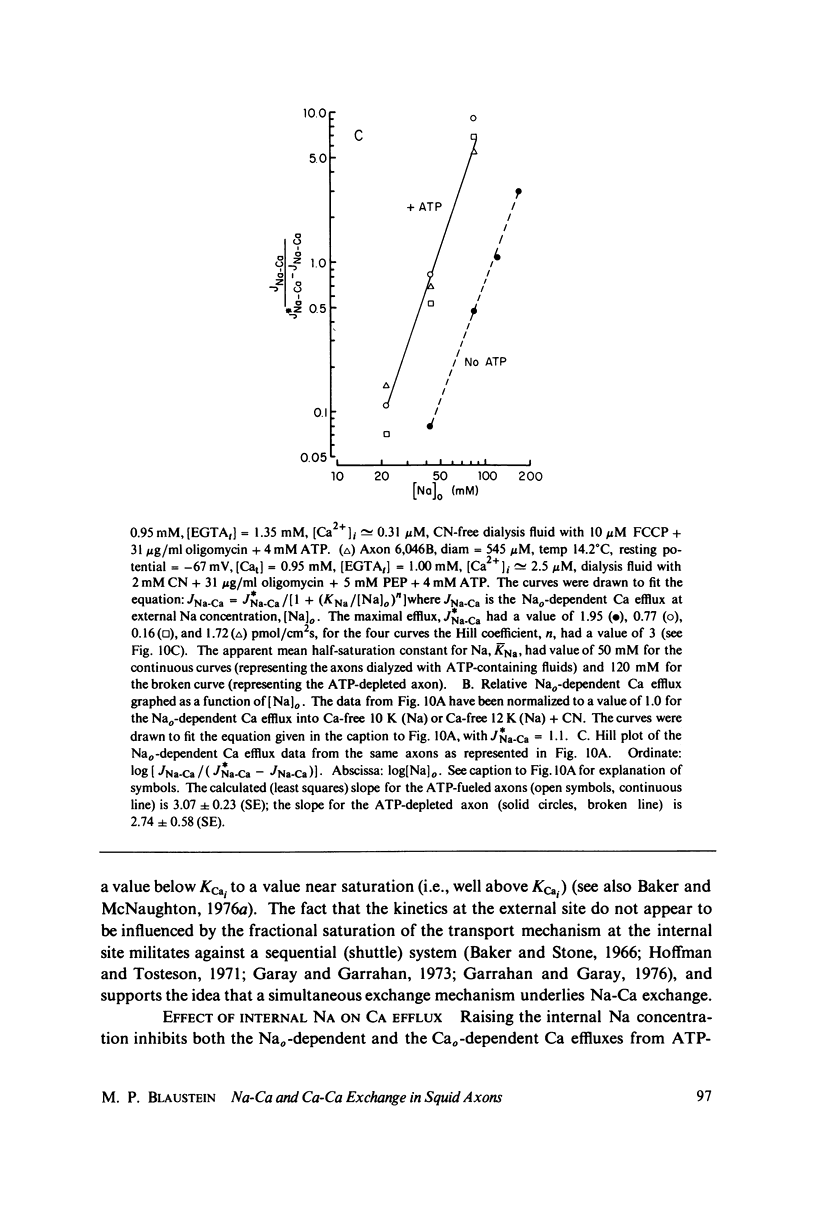
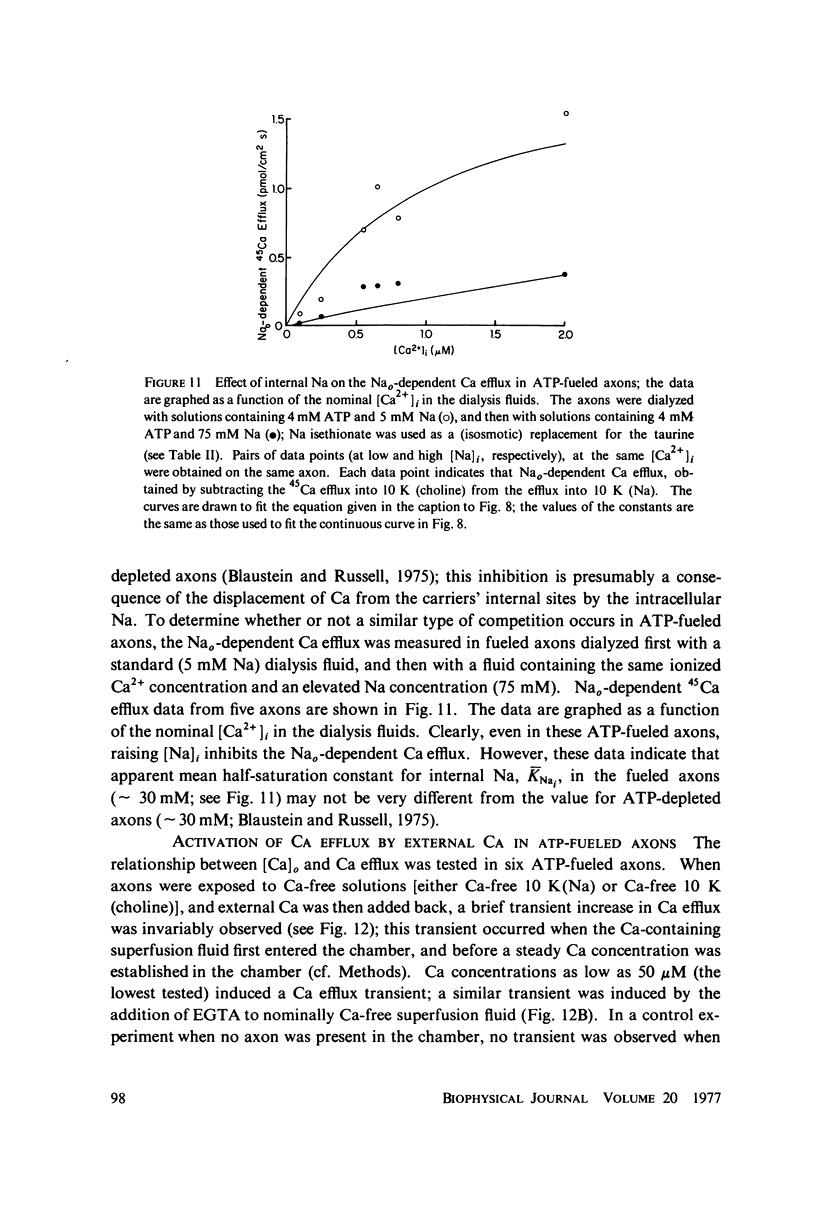
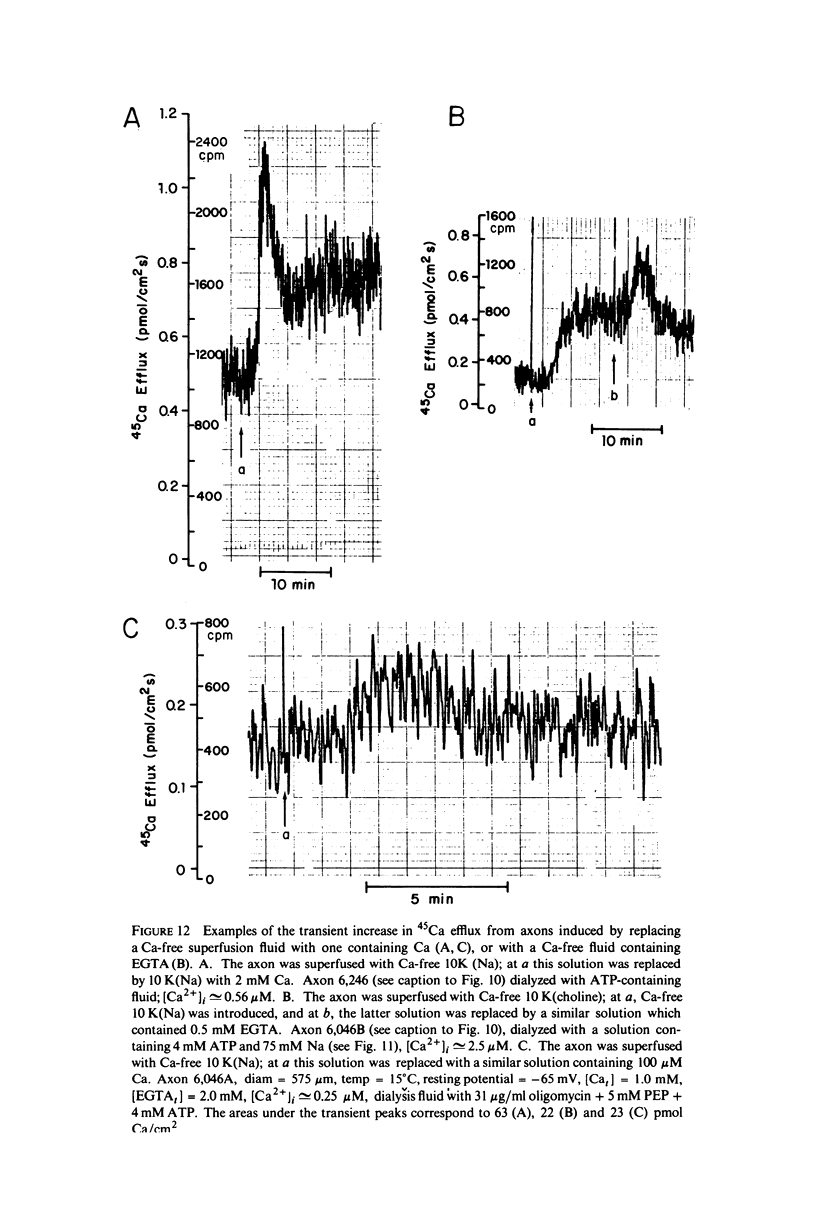
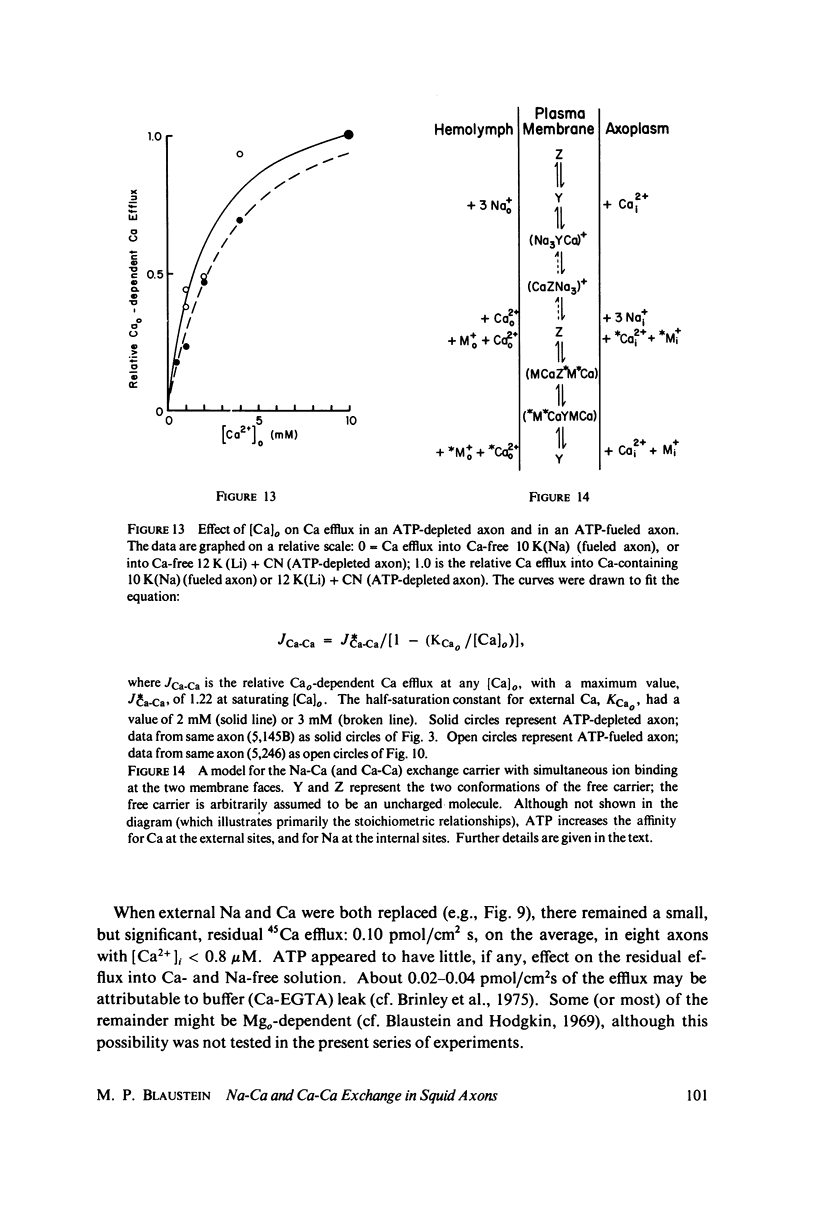
Calcium-45 efflux was measured in squid axons whose internal solute concentration was controlled by internal dialysis. Most of the Ca efflux requires either external Na (Na-Ca exchange) or external Ca plus in alkali metal ion (Ca-Ca exchange; cf. Blaustein & Russell, 1975). Both Na-Ca and Ca-Ca exchange are apparently mediated by a single mechanism because both are inhibited by Sr and Mn, and because addition of Na to an external medium optimal for Ca-Ca exchange inhibits Ca efflux. The transport involves simultaneous (as opposed to sequential) ion counterflow because the fractional saturation by internal Ca (Cai) does not affect the external Na (Nao) activation kinetics; also, Nao promotes Ca efflux whether or not an alkali metal ion is present inside, whereas Ca-Ca exchange requires alkali metal ions both internally and externally (i.e., internal and external sites must be appropriately loaded simultaneously). ATP increases the affinity of the transport mechanism for both Cai and Nao, but it does not affect the maximal transport rate at saturating [Ca2+]i and [Na+]o; this suggest that ATP may be acting as a catalyst of modulator, and not as an energy source. Hill plots of the Nao activation data yield slopes congruent to 3 for both ATP-depleted and ATP-fueled axons, compatible with a 3 Na+-for-1 Ca2+ exchange. With this stoichiometry, the Na electrochemical gradient alone could provide sufficient energy to maintain ionized [Ca2+]i in the physiological range (about 10(-7) M).
Full text
PDF





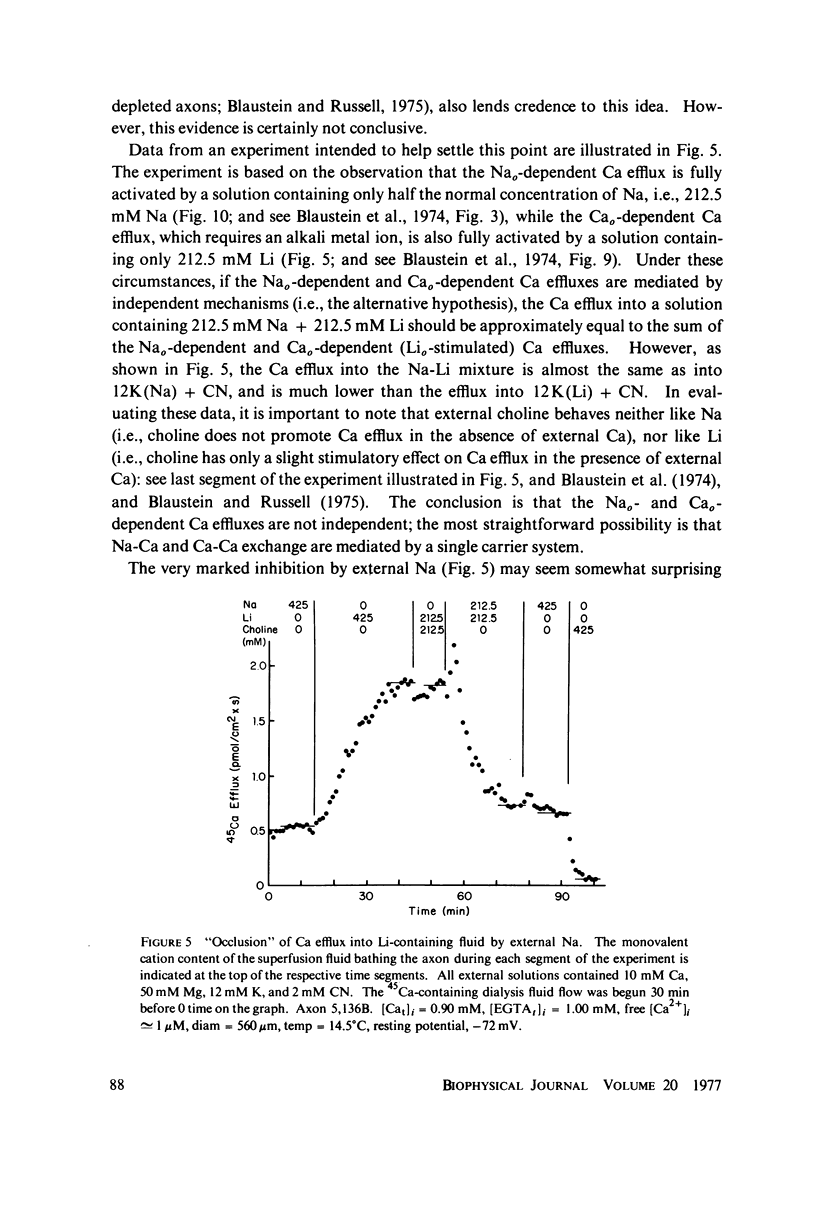
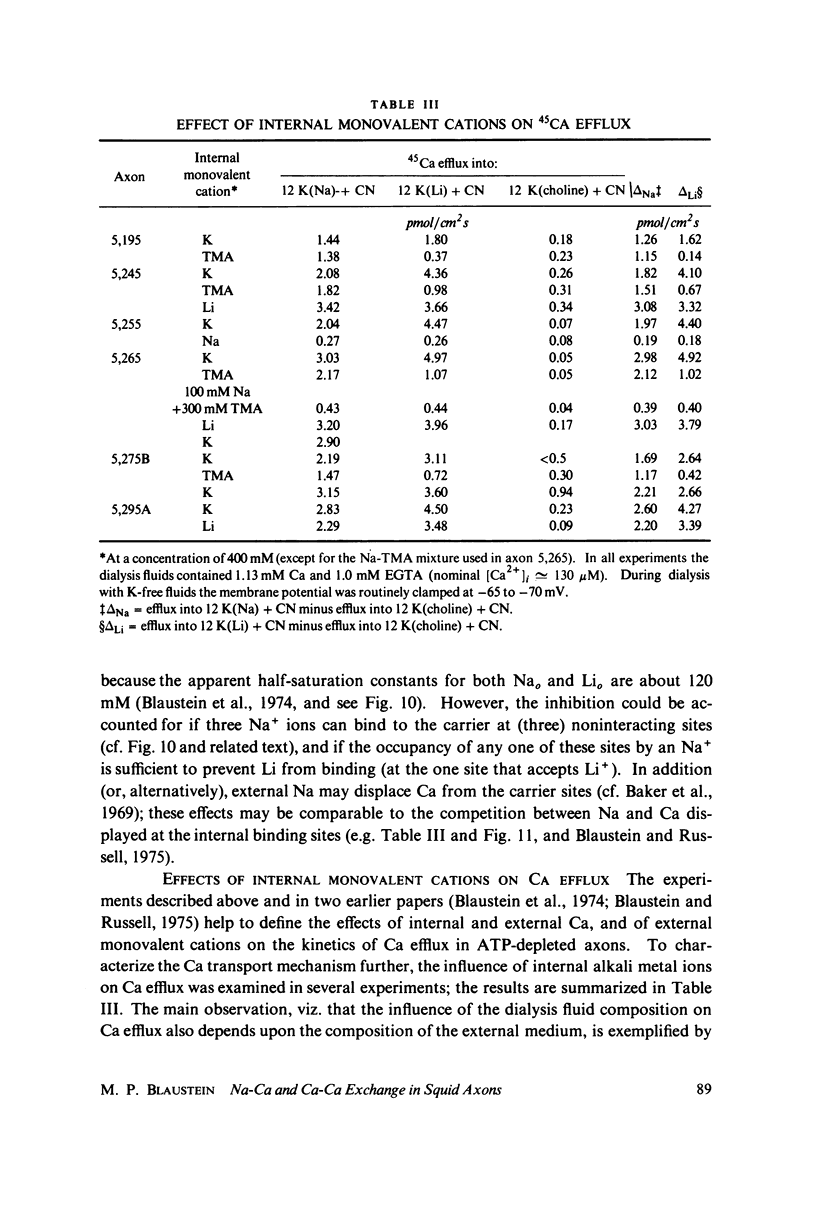
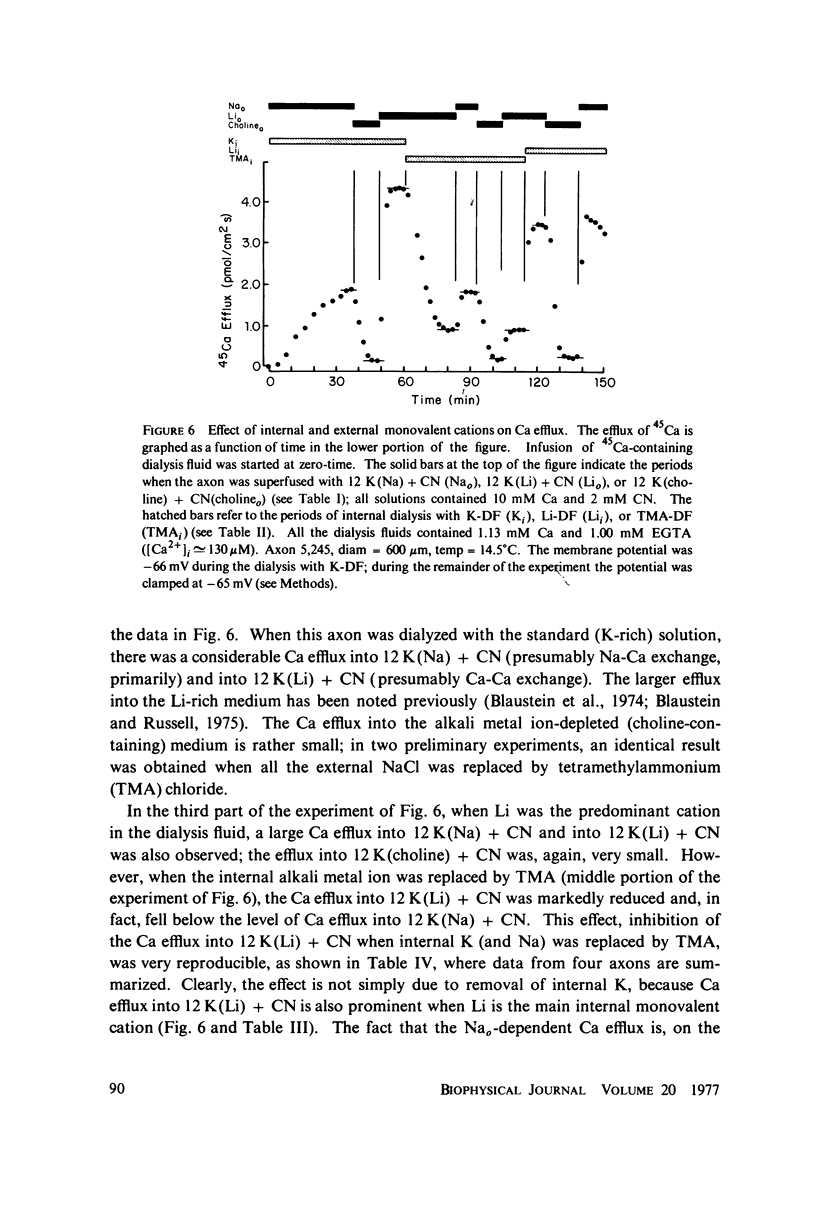
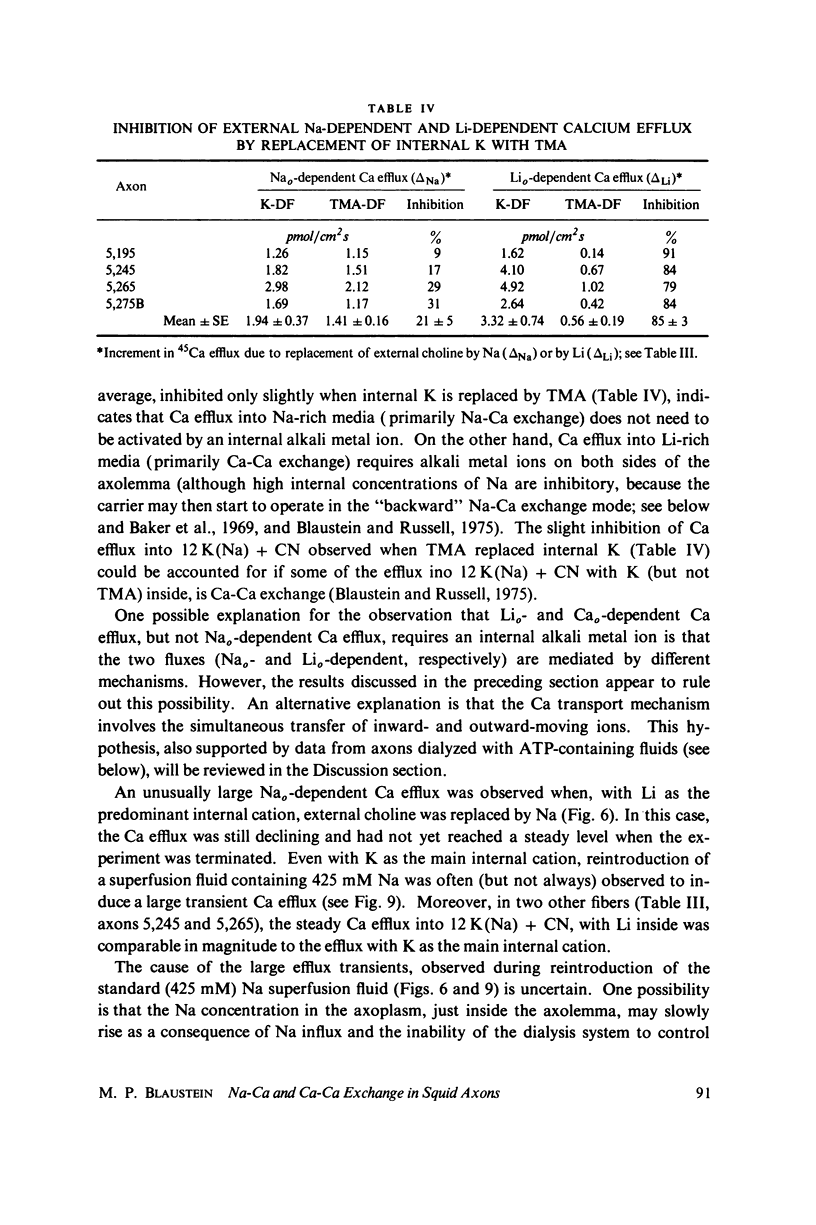












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., McNaughton P. A. Kinetics and energetics of calcium efflux from intact squid giant axons. J Physiol. 1976 Jul;259(1):103–144. doi: 10.1113/jphysiol.1976.sp011457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F. Regulation of intracellular Ca and Mg in squid axons. Fed Proc. 1976 Dec;35(14):2589–2595. [PubMed] [Google Scholar]
- Baker P. F., Stone A. J. A kinetic method for investigating hypothetical models of the sodium pump. Biochim Biophys Acta. 1966 Oct 10;126(2):321–329. doi: 10.1016/0926-6585(66)90069-0. [DOI] [PubMed] [Google Scholar]
- Baker P. F. Transport and metabolism of calcium ions in nerve. Prog Biophys Mol Biol. 1972;24:177–223. doi: 10.1016/0079-6107(72)90007-7. [DOI] [PubMed] [Google Scholar]
- Barlogie B., Hasselbach W., Makinose M. Activation of calcium efflux by ADP and inorganic phosphate. FEBS Lett. 1971 Jan 30;12(5):267–268. doi: 10.1016/0014-5793(71)80194-1. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Ector A. C. Carrier-mediated sodium-dependent and calcium-dependent calcium efflux from pinched-off presynaptic nerve terminals (synaptosomes) in vitro. Biochim Biophys Acta. 1976 Jan 21;419(2):295–308. doi: 10.1016/0005-2736(76)90355-2. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Hodgkin A. L. The effect of cyanide on the efflux of calcium from squid axons. J Physiol. 1969 Feb;200(2):497–527. doi: 10.1113/jphysiol.1969.sp008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Russell J. M. Sodium-calcium exchange and calcium-calcium exchange in internally dialyzed squid giant axons. J Membr Biol. 1975 Jul 24;22(3-4):285–312. doi: 10.1007/BF01868176. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Russell J. M., Weer P. Calcium efflux from internally dialyzed squid axons: the influence of external and internal cations. J Supramol Struct. 1974;2(5-6):558–581. doi: 10.1002/jss.400020505. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P. The ins and outs of calcium transport in squid axons: internal and external ion activation of calcium efflux. Fed Proc. 1976 Dec;35(14):2574–2578. [PubMed] [Google Scholar]
- Blaustein M. P. The interrelationship between sodium and calcium fluxes across cell membranes. Rev Physiol Biochem Pharmacol. 1974;70:33–82. doi: 10.1007/BFb0034293. [DOI] [PubMed] [Google Scholar]
- Boron W. F., De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J Gen Physiol. 1976 Jan;67(1):91–112. doi: 10.1085/jgp.67.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinley F. J., Jr, Mullins L. J. Effects of membrane potential on sodium and potassium fluxes in squid axons. Ann N Y Acad Sci. 1974;242(0):406–433. doi: 10.1111/j.1749-6632.1974.tb19106.x. [DOI] [PubMed] [Google Scholar]
- Brinley F. J., Jr, Mullins L. J. Sodium extrusion by internally dialyzed squid axons. J Gen Physiol. 1967 Nov;50(10):2303–2331. doi: 10.1085/jgp.50.10.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinley F. J., Jr, Mullins L. J. Sodium fluxes in internally dialyzed squid axons. J Gen Physiol. 1968 Aug;52(2):181–211. doi: 10.1085/jgp.52.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinley F. J., Jr, Spangler S. G., Mullins L. J. Calcium and EDTA fluxes in dialyzed squid axons. J Gen Physiol. 1975 Aug;66(2):223–250. doi: 10.1085/jgp.66.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho A. P., Leo B. Effects of ATP on the interaction of Ca++, Mg++, and K+ with fragmented sarcoplasmic reticulum isolated from rabbit skeletal muscle. J Gen Physiol. 1967 May;50(5):1327–1352. doi: 10.1085/jgp.50.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipolo R. Calcium efflux from internally dialyzed squid giant axons. J Gen Physiol. 1973 Nov;62(5):575–589. doi: 10.1085/jgp.62.5.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipolo R., Requena J., Brinley F. J., Jr, Mullins L. J., Scarpa A., Tiffert T. Ionized calcium concentrations in squid axons. J Gen Physiol. 1976 Apr;67(4):433–467. doi: 10.1085/jgp.67.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipolo R. The influence of nucleotides on calcium fluxes. Fed Proc. 1976 Dec;35(14):2579–2582. [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garay R. P., Garrahan P. J. The interaction of sodium and potassium with the sodium pump in red cells. J Physiol. 1973 Jun;231(2):297–325. doi: 10.1113/jphysiol.1973.sp010234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. Facftors affecting the relative magnitudes of the sodium:potassium and sodium:sodium exchanges catalysed by the sodium pump. J Physiol. 1967 Sep;192(1):189–216. doi: 10.1113/jphysiol.1967.sp008296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert D. L., Ehrenstein G. Effect of divalent cations on potassium conductance of squid axons: determination of surface charge. Biophys J. 1969 Mar;9(3):447–463. doi: 10.1016/S0006-3495(69)86396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Karlish S. J. The sodium pump. Annu Rev Physiol. 1975;37:13–55. doi: 10.1146/annurev.ph.37.030175.000305. [DOI] [PubMed] [Google Scholar]
- Glynn I. M., Lew V. L. Synthesis of adenosine triphosphate at the expense of downhill cation movements in intact human red cells. J Physiol. 1970 Apr;207(2):393–402. doi: 10.1113/jphysiol.1970.sp009068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HINKE J. A. The measurement of sodium and potassium activities in the squid axon by means of cation-selective glass micro-electrodes. J Physiol. 1961 Apr;156:314–335. doi: 10.1113/jphysiol.1961.sp006678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz E., Geck P. The efficiency of energetic couping between Na+ flow and amino acid transport in Ehrlich cells-a revised assessment. Biochim Biophys Acta. 1974 Mar 29;339(3):426–431. doi: 10.1016/0005-2736(74)90170-9. [DOI] [PubMed] [Google Scholar]
- Hoffman P. G., Tosteson D. C. Active sodium and potassium transport in high potassium and low potassium sheep red cells. J Gen Physiol. 1971 Oct;58(4):438–466. doi: 10.1085/jgp.58.4.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew V. L., Glynn I. M., Ellory J. C. Net synthesis of ATP by reversal of the sodium pump. Nature. 1970 Feb 28;225(5235):865–866. doi: 10.1038/225865a0. [DOI] [PubMed] [Google Scholar]
- MOORE J. W., COLE K. S. Resting and action potentials of the squid giant axon in vivo. J Gen Physiol. 1960 May;43:961–970. doi: 10.1085/jgp.43.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinose M. Calcium efflux dependent formation of ATP from ADP and orthophosphate by the membranes of the sarcoplasmic vesicles. FEBS Lett. 1971 Jan 30;12(5):269–270. doi: 10.1016/0014-5793(71)80195-3. [DOI] [PubMed] [Google Scholar]
- Makinose M., Hasselbach W. ATP synthesis by the reverse of the sarcoplasmic calcium pump. FEBS Lett. 1971 Jan 30;12(5):271–272. doi: 10.1016/0014-5793(71)80196-5. [DOI] [PubMed] [Google Scholar]
- Mullins L. J., Brinley F. J., Jr Sensitivity of calcium efflux from squid axons to changes in membrane potential. J Gen Physiol. 1975 Feb;65(2):135–152. doi: 10.1085/jgp.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins L. J., Brinley F. J., Jr Some factors influencing sodium extrusion by internally dialyzed squid axons. J Gen Physiol. 1967 Nov;50(10):2333–2355. doi: 10.1085/jgp.50.10.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadarajah A., Leese B., Joplin G. F. Triton X-100 scintillant for counting calcium-45 in biological fluids. Int J Appl Radiat Isot. 1969 Oct;20(10):733–735. doi: 10.1016/0020-708x(69)90071-4. [DOI] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Russell J. M., Blaustein M. P. Calcium efflux from barnacle muscle fibers. Dependence on external cations. J Gen Physiol. 1974 Feb;63(2):144–167. doi: 10.1085/jgp.63.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzmann H. J. Dependence on calcium concentration and stoichiometry of the calcium pump in human red cells. J Physiol. 1973 Dec;235(2):551–569. doi: 10.1113/jphysiol.1973.sp010403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzmann H. J., Vincenzi F. F. Calcium movements across the membrane of human red cells. J Physiol. 1969 Apr;201(2):369–395. doi: 10.1113/jphysiol.1969.sp008761. [DOI] [PMC free article] [PubMed] [Google Scholar]



