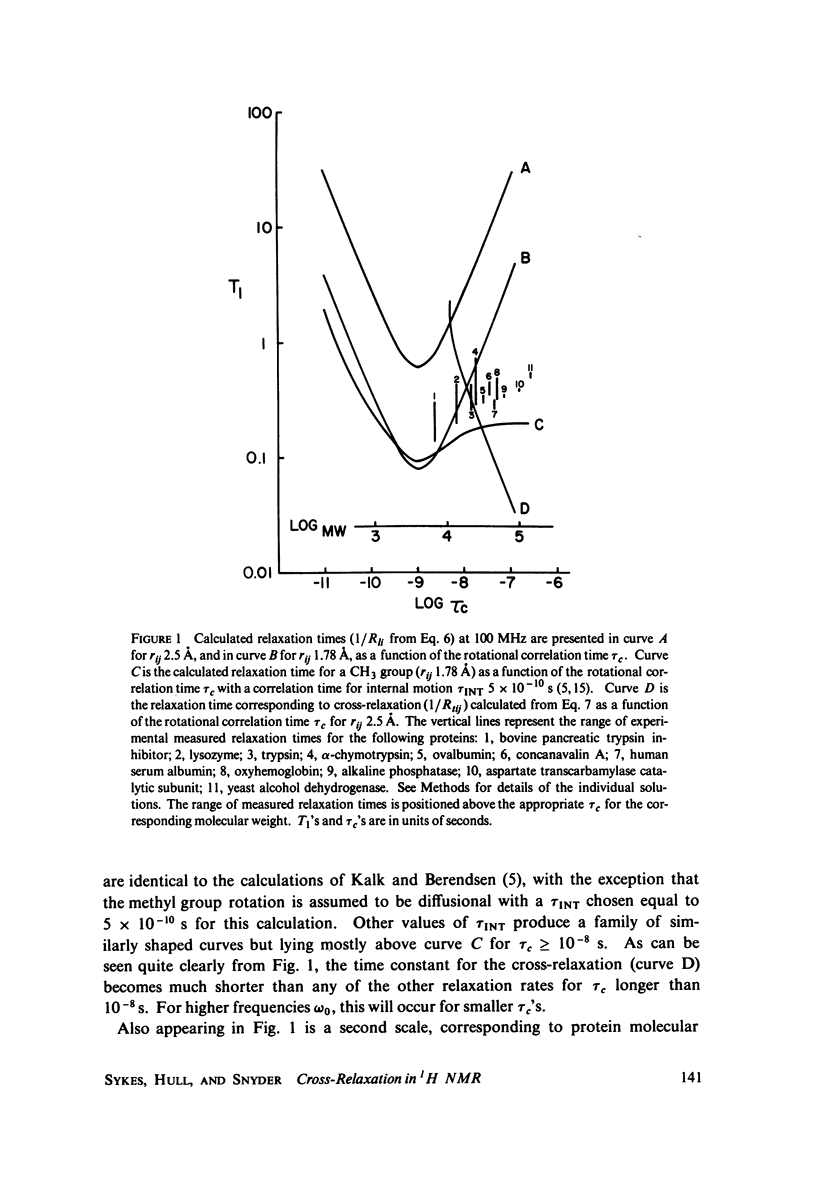
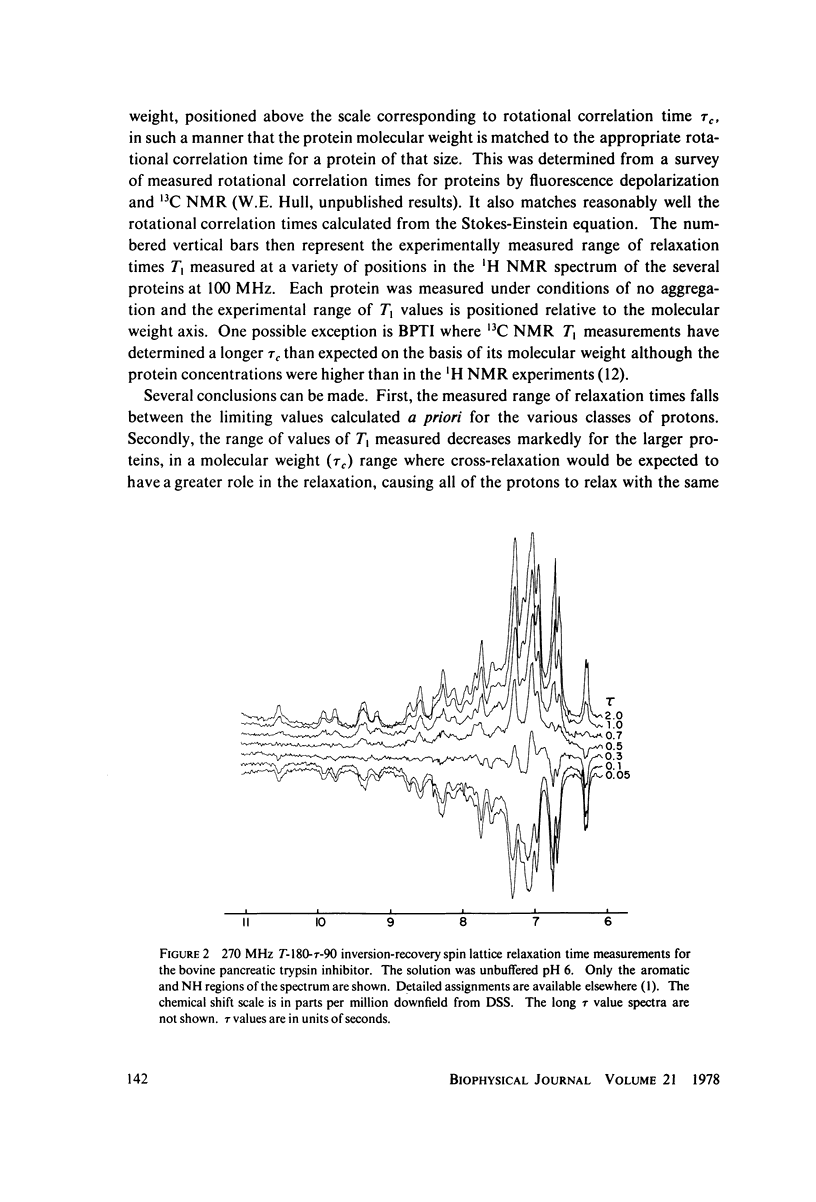
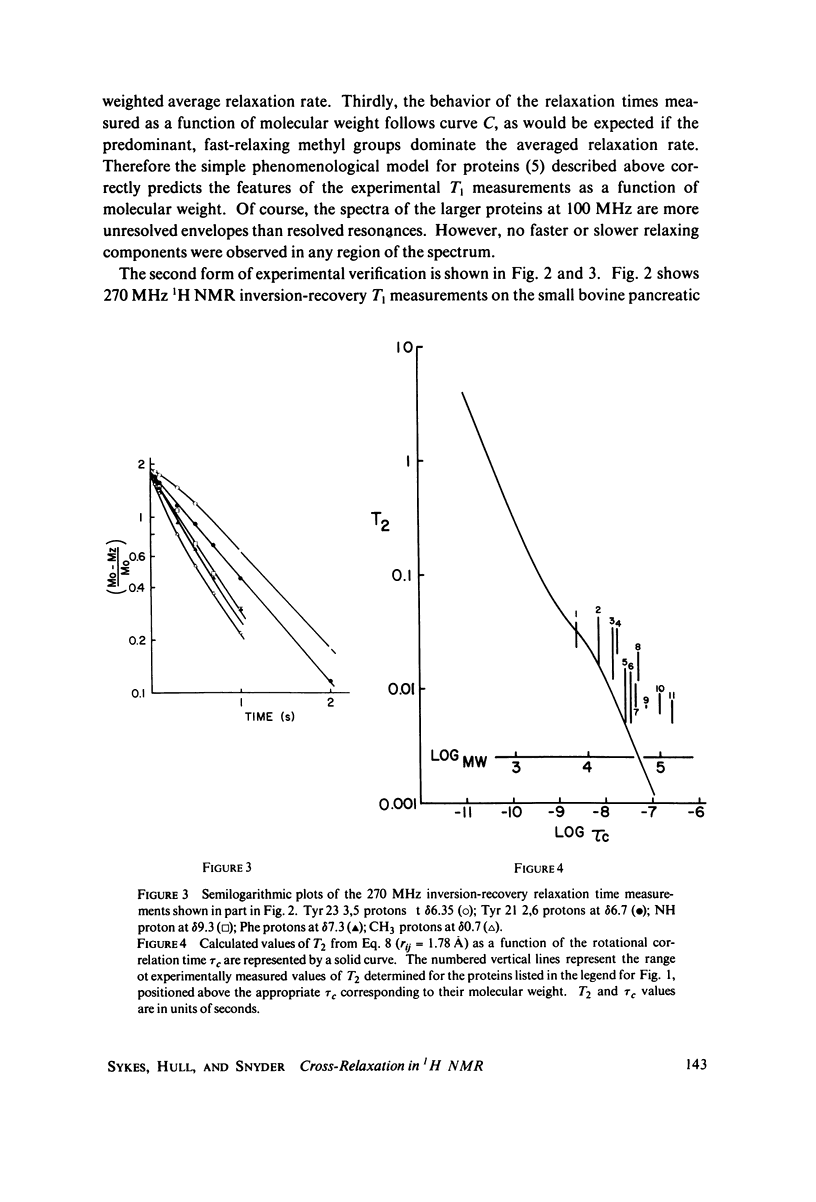
Abstract
Proton nuclear magnetic resonance (NMR) spin lattice relaxation time (T1) and spin-spin relaxation time (T2) measurements are presented for a number of proteins with molecular weights spanning the range of 6,500-150,000 daltons. These measurements provide experimental evidence for the role of cross-relaxation in 1H NMR T1 measurements in proteins. The relationship between these measurements and the theory recently presented by Kalk and Berendsen is discussed. The results indicate that cross-relaxation dominates the T1 measurements for the larger proteins, even at relatively low resonance frequencies such as 100 MHz.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Edzes H. T., Samulski E. T. Cross relaxation and spin diffusion in the proton NMR or hydrated collagen. Nature. 1977 Feb 10;265(5594):521–523. doi: 10.1038/265521a0. [DOI] [PubMed] [Google Scholar]
- Grimaldi J. J., Sykes B. D. Concanavalin A: a stopped flow nuclear magnetic resonance study of conformational changes induced by Mn++, Ca++, and alpha-methyl-D-mannoside. J Biol Chem. 1975 Mar 10;250(5):1618–1624. [PubMed] [Google Scholar]
- Hull W. E., Sykes B. D. Fluorotyrosine alkaline phosphatase: internal mobility of individual tyrosines and the role of chemical shift anisotropy as a 19F nuclear spin relaxation mechanism in proteins. J Mol Biol. 1975 Oct 15;98(1):121–153. doi: 10.1016/s0022-2836(75)80105-7. [DOI] [PubMed] [Google Scholar]
- Manuck B. A., Maloney J. G., Jr, Sykes B. D. Kinetics of the interaction of methyl isonitrile with hemoglobin beta chains: measurement by nuclear magnetic resonance. J Mol Biol. 1973 Dec 5;81(2):199–205. doi: 10.1016/0022-2836(73)90189-7. [DOI] [PubMed] [Google Scholar]
- Marshall A. G., Schmidt P. G., Sykes B. D. Effect of internal rotation on nuclear magnetic relaxation times for macromolecules. Biochemistry. 1972 Oct 10;11(21):3875–3879. doi: 10.1021/bi00771a006. [DOI] [PubMed] [Google Scholar]
- Rosenbusch J. P., Weber K. Subunit structure of aspartate transcarbamylase from Escherichia coli. J Biol Chem. 1971 Mar 25;246(6):1644–1657. [PubMed] [Google Scholar]
- Snyder G. H., Rowan R., 3rd, Karplus S., Sykes B. D. Complete tyrosine assignments in the high field 1H nuclear magnetic resonance spectrum of the bovine pancreatic trypsin inhibitor. Biochemistry. 1975 Aug 26;14(17):3765–3777. doi: 10.1021/bi00688a008. [DOI] [PubMed] [Google Scholar]
- Sykes B. D., Weingarten H. I., Schlesinger M. J. Fluorotyrosine alkaline phosphatase from Escherichia coli: preparation, properties, and fluorine-19 nuclear magnetic resonance spectrum. Proc Natl Acad Sci U S A. 1974 Feb;71(2):469–473. doi: 10.1073/pnas.71.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchia D. A., Lyerla J. R., Jr, Quattrone A. J. Molecular dynamics and structure of the random coil and helical states of the collagen peptide, alpha 1-CB2, as determined by 13C magnetic resonance. Biochemistry. 1975 Mar 11;14(5):887–900. doi: 10.1021/bi00676a004. [DOI] [PubMed] [Google Scholar]



