Abstract
We have searched for fluctuations in the steady-state tension developed by stimulated single muscle fibers. Such tension "noise" is expected to be present as a result of the statistical fluctuations in the number and/or state of myosin cross-bridges interacting with thin filament sites at any time. A sensitive electro-optical tension transducer capable of resolving the expected fluctuations in magnitude and frequency was constructed to search for the fluctuations. The noise was analyzed by computing the power spectra and amplitude of stochastic fluctuations in the photomultiplier counting rate, which was made proportional to muscle force. The optical system and electronic instrumentation together with the minicomputer software are described. Tensions were measured in single skinned glycerinated rabbit psoas muscle fibers in rigor and during contraction and relaxation. The results indicate the presence of fluctuations in contracting muscles and a complete absence of tension noise in eith rigor or relaxation. Also, a numerical method was developed to simulate the power spectra and amplitude of fluctuations, given the rate constants for association and dissociation of the cross-bridges and actin. The simulated power spectra and the frequency distributions observed experimentally are similar.
Full text
PDF






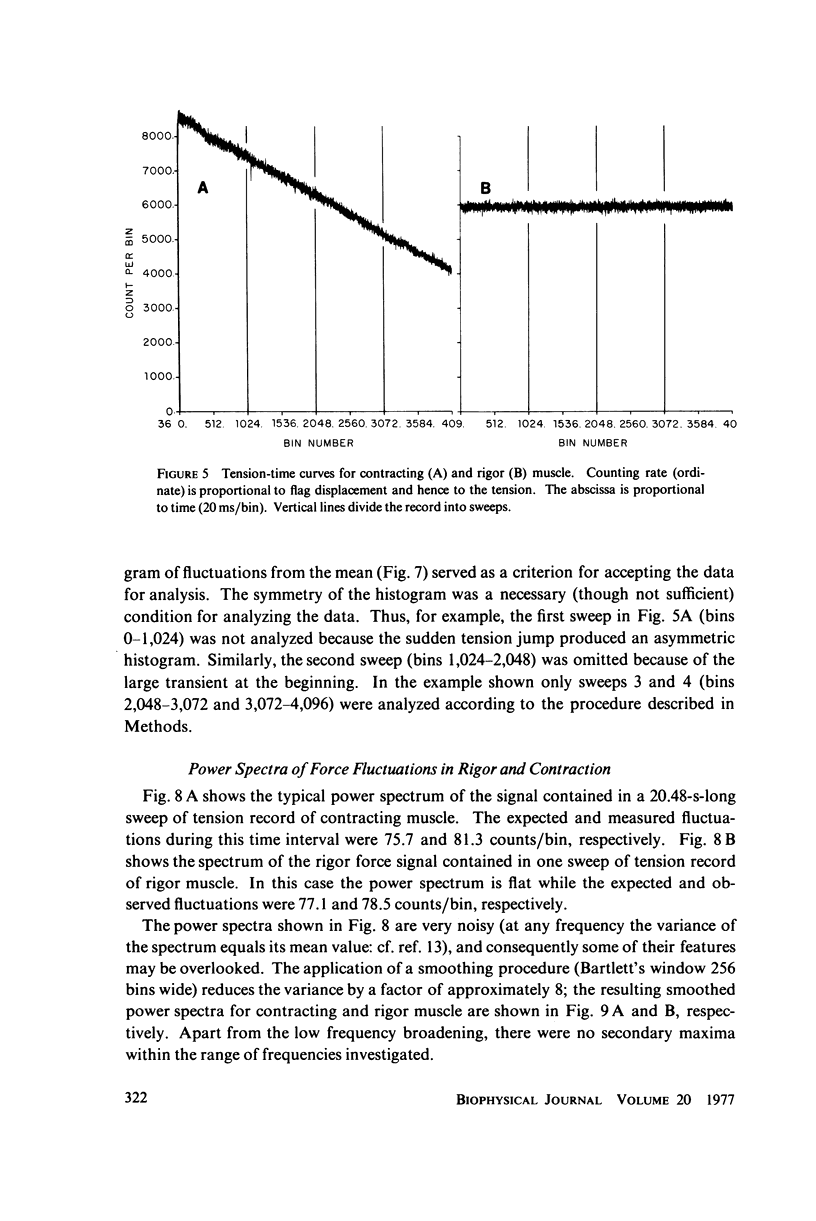







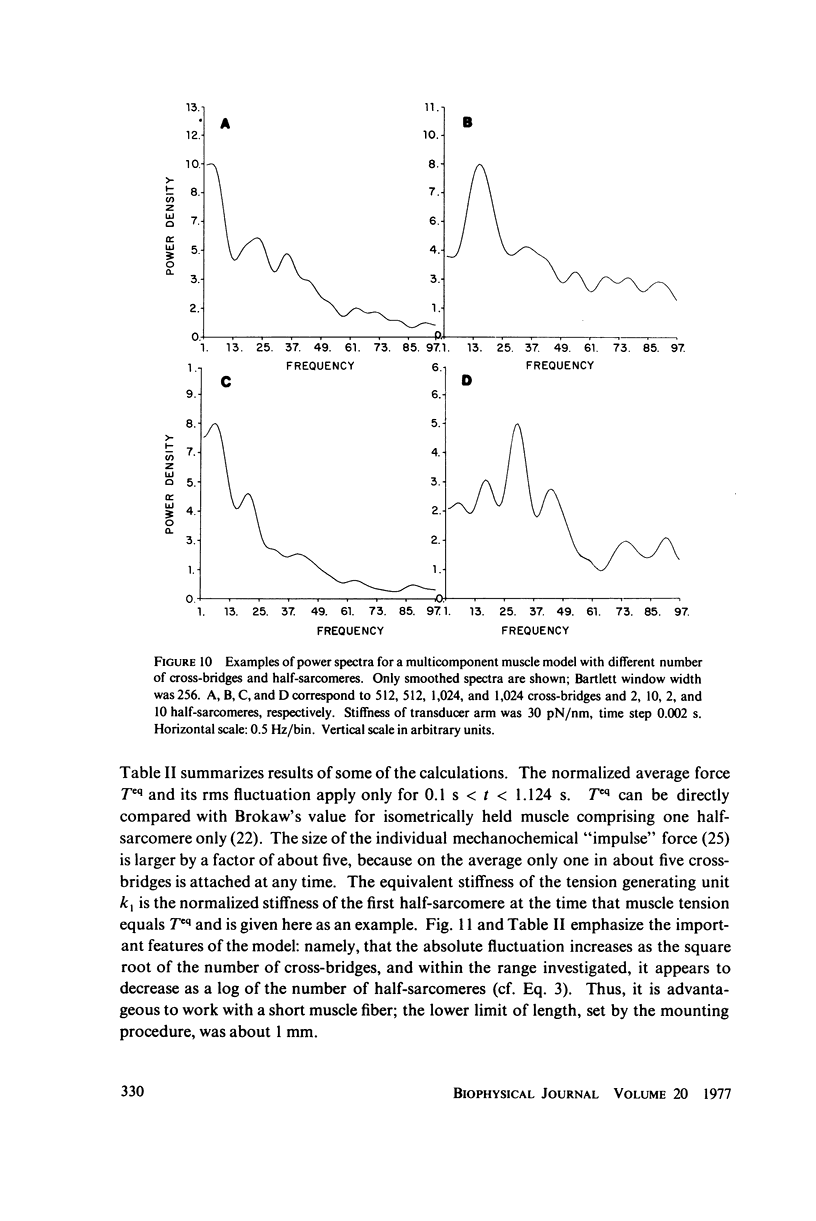
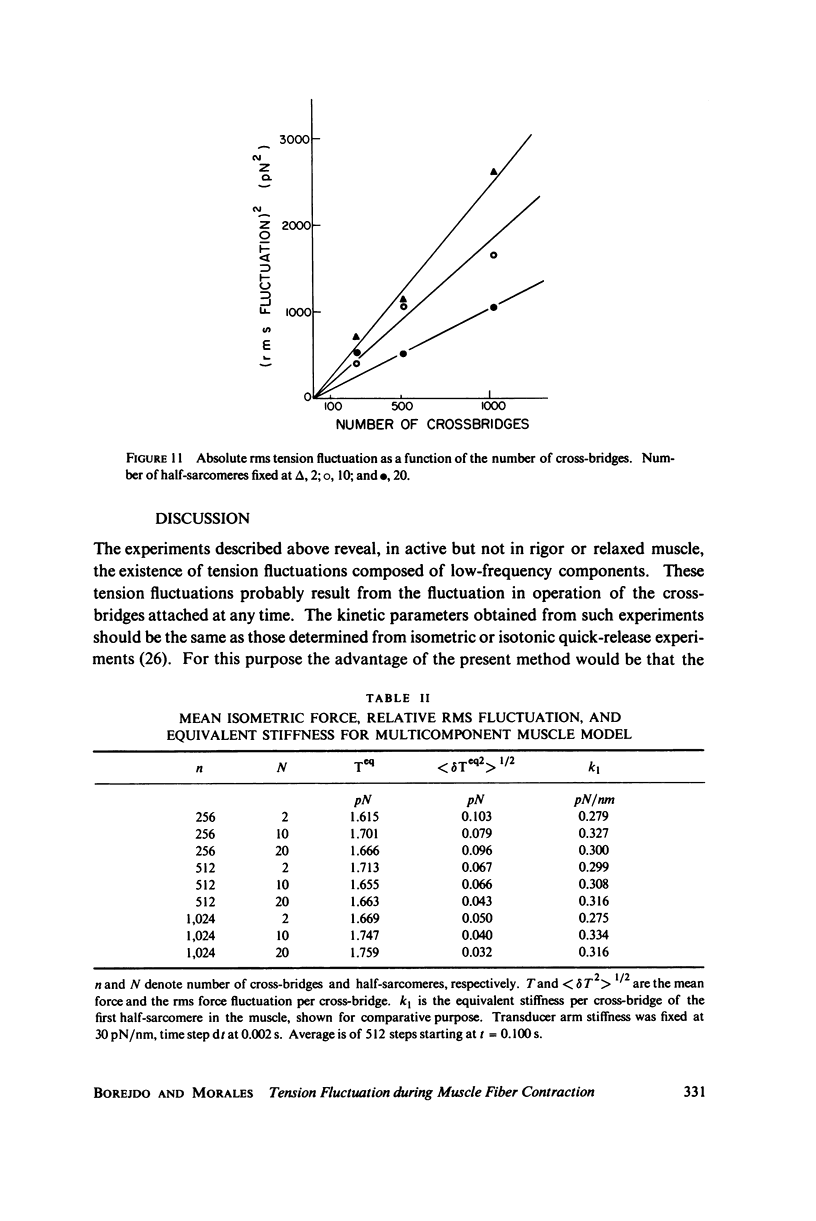



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner R. F., Carlson F. D. Structural dynamics of frog muscle during isometric contraction. J Gen Physiol. 1975 May;65(5):555–581. doi: 10.1085/jgp.65.5.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borejdo J., Oplatka A. Tension development in skinned glycerinated rabbit psoas fiber segments irrigated with soluble myosin fragments. Biochim Biophys Acta. 1976 Jul 9;440(1):241–258. doi: 10.1016/0005-2728(76)90127-4. [DOI] [PubMed] [Google Scholar]
- Borejdo J., Putnam S. Polarization of fluorescence from single skinned glycerinated rabbit psoas fibers in rigor and relaxation. Biochim Biophys Acta. 1977 Mar 11;459(3):578–595. doi: 10.1016/0005-2728(77)90056-1. [DOI] [PubMed] [Google Scholar]
- Brokaw C. J. Computer simulation of movement-generating cross-bridges. Biophys J. 1976 Sep;16(9):1013–1027. doi: 10.1016/S0006-3495(76)85752-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson F. D. Structural fluctuations in the steady state of muscular contraction. Biophys J. 1975 Jul;15(7):633–649. doi: 10.1016/S0006-3495(75)85845-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. D., Hill T. L. Fluctuations and noise in kinetic systems. Application to K+ channels in the squid axon. Biophys J. 1973 Dec;13(12):1276–1295. doi: 10.1016/S0006-3495(73)86062-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966 May;184(1):170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Hill T. L., Eisenberg E., Chen Y. D., Podolsky R. J. Some self-consistent two-state sliding filament models of muscle contraction. Biophys J. 1975 Apr;15(4):335–372. doi: 10.1016/S0006-3495(75)85823-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L. Theoretical formalism for the sliding filament model of contraction of striated muscle. Part II. Prog Biophys Mol Biol. 1975;29(2):105–159. doi: 10.1016/0079-6107(76)90021-3. [DOI] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Jackson J. L., Oplatka A. A mechanochemical theory of fluctuations in muscles. I. Introduction to the theory. Biorheology. 1974 Sep;11(5):315–322. doi: 10.3233/bir-1974-11502. [DOI] [PubMed] [Google Scholar]
- Julian F. J. Activation in a skeletal muscle contraction model with a modification for insect fibrillar muscle. Biophys J. 1969 Apr;9(4):547–570. doi: 10.1016/S0006-3495(69)86403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T. I., Morales M. F. Application of a one-step procedure for measuring inorganic phosphate in the presence of proteins: the actomyosin ATPase system. Anal Biochem. 1977 Jan;77(1):10–17. doi: 10.1016/0003-2697(77)90284-6. [DOI] [PubMed] [Google Scholar]
- Magde D., Elson E. L., Webb W. W. Fluorescence correlation spectroscopy. II. An experimental realization. Biopolymers. 1974 Jan;13(1):29–61. doi: 10.1002/bip.1974.360130103. [DOI] [PubMed] [Google Scholar]
- Oplatka A. On the mechanochemistry of muscular contraction. J Theor Biol. 1972 Feb;34(2):379–403. doi: 10.1016/0022-5193(72)90169-5. [DOI] [PubMed] [Google Scholar]
- Weber A., Murray J. M. Molecular control mechanisms in muscle contraction. Physiol Rev. 1973 Jul;53(3):612–673. doi: 10.1152/physrev.1973.53.3.612. [DOI] [PubMed] [Google Scholar]
- Wise R. M., Rondinone J. F., Briggs F. N. Effect of calcium on force-velocity characteristics of glycerinated skeletal muscle. Am J Physiol. 1971 Oct;221(4):973–979. doi: 10.1152/ajplegacy.1971.221.4.973. [DOI] [PubMed] [Google Scholar]



