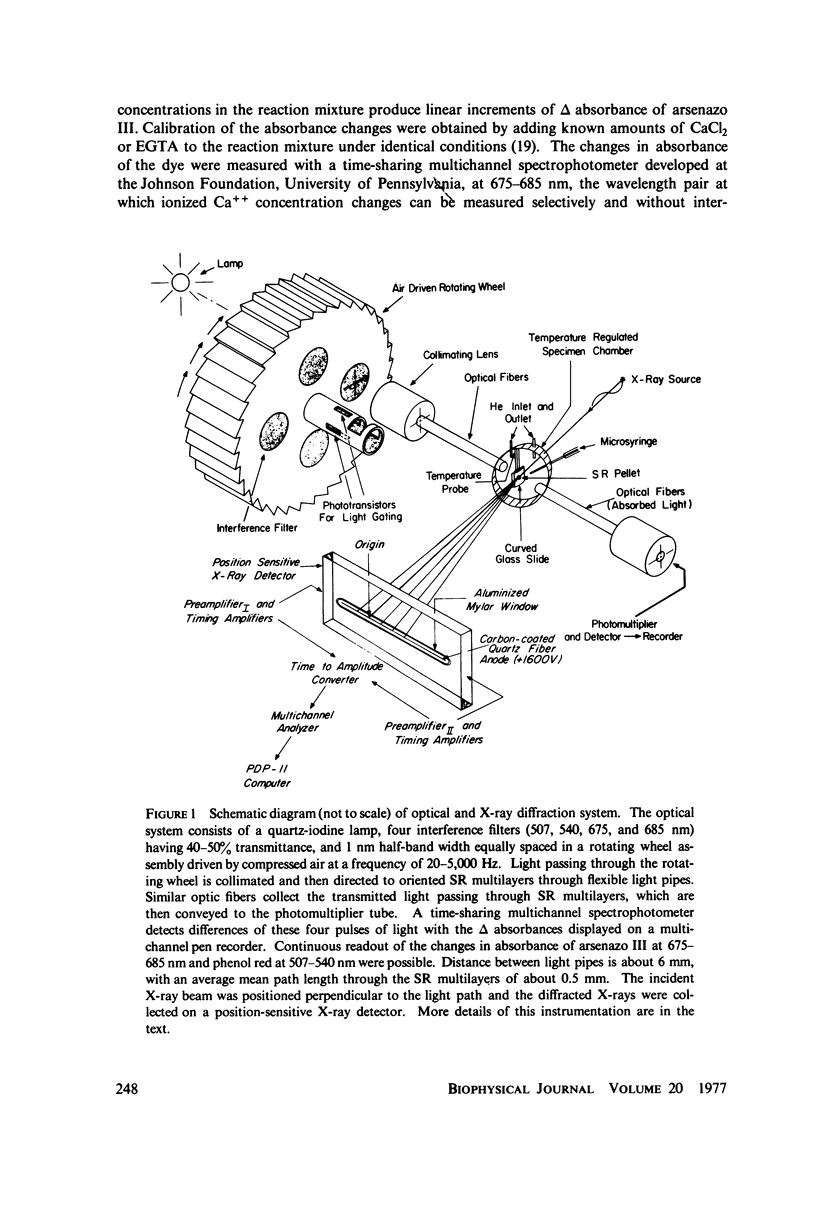
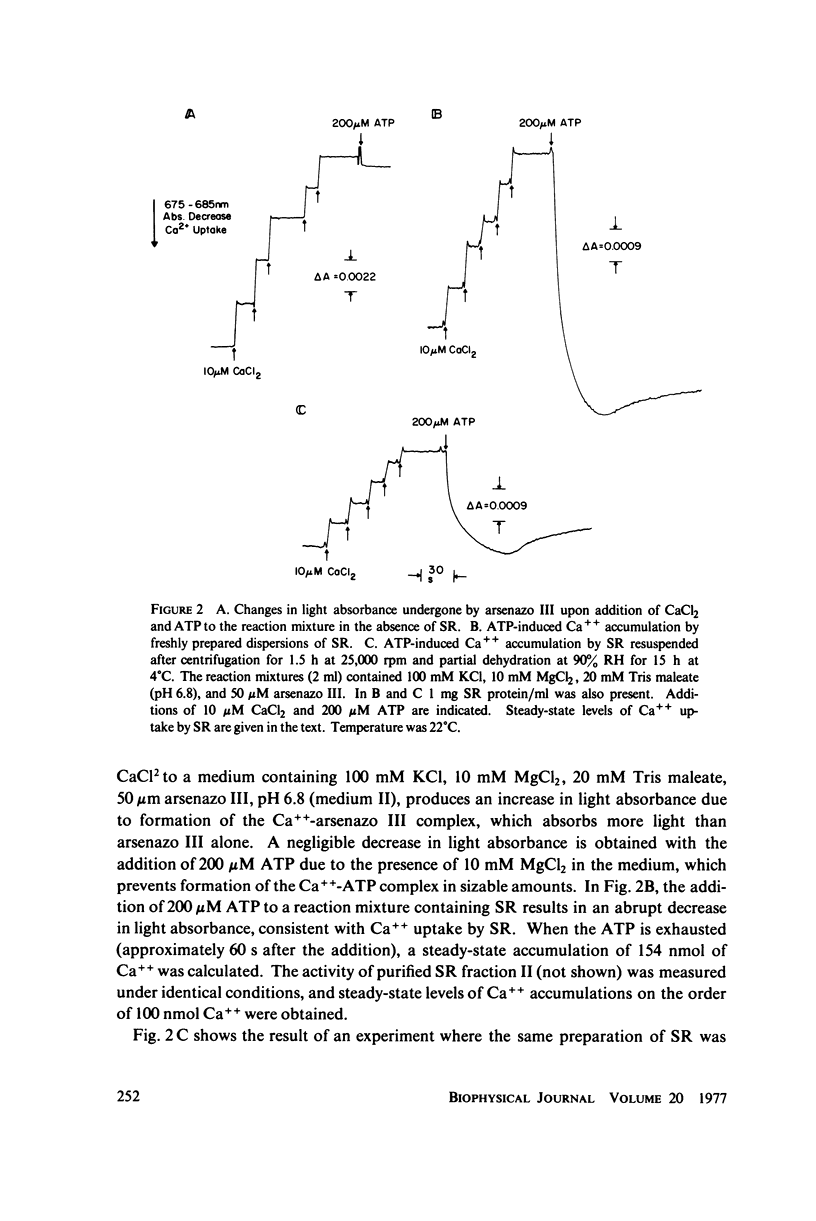
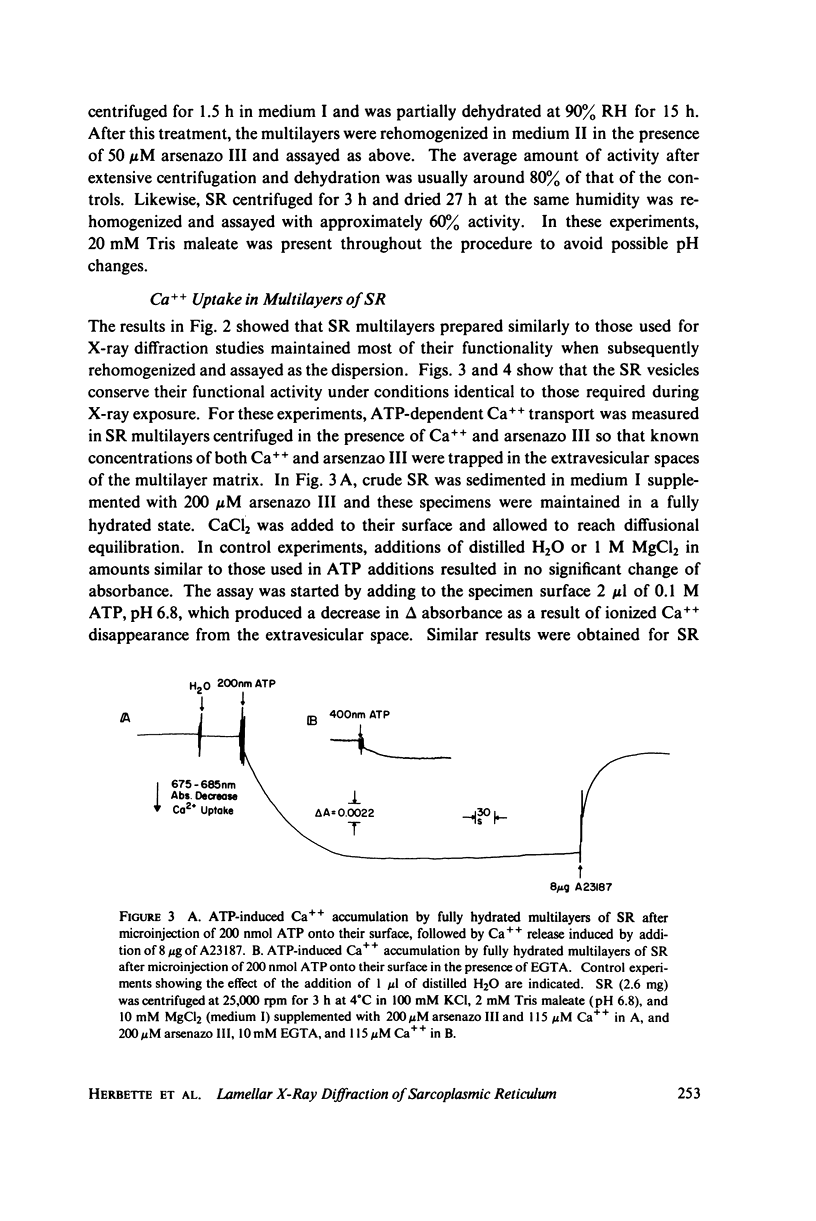
Abstract
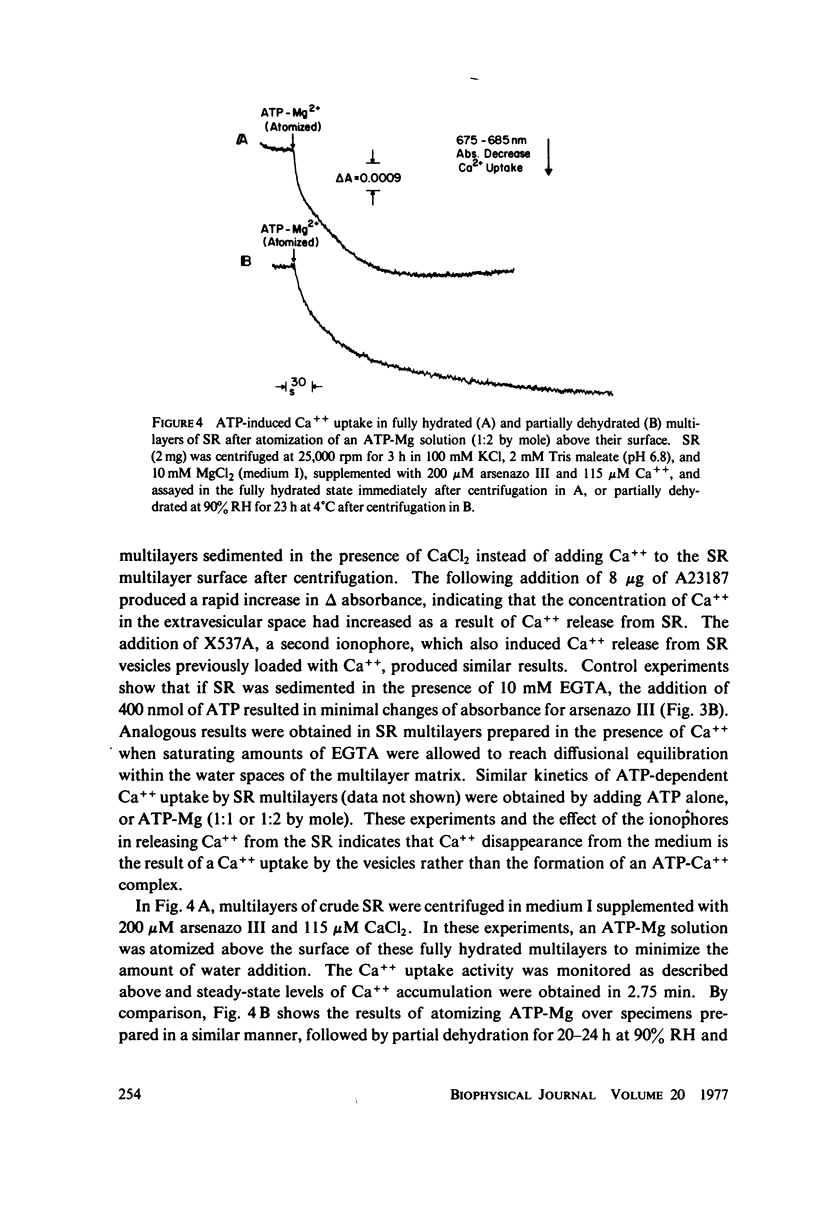
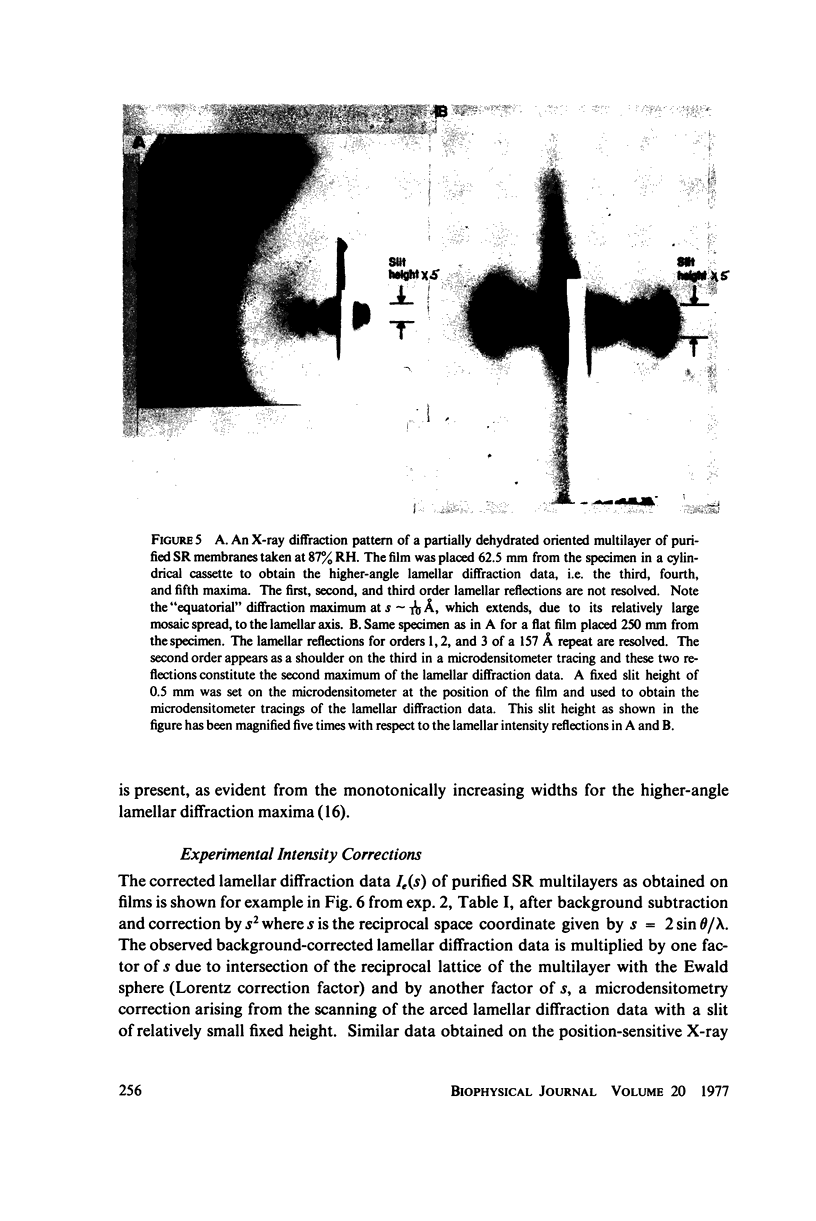
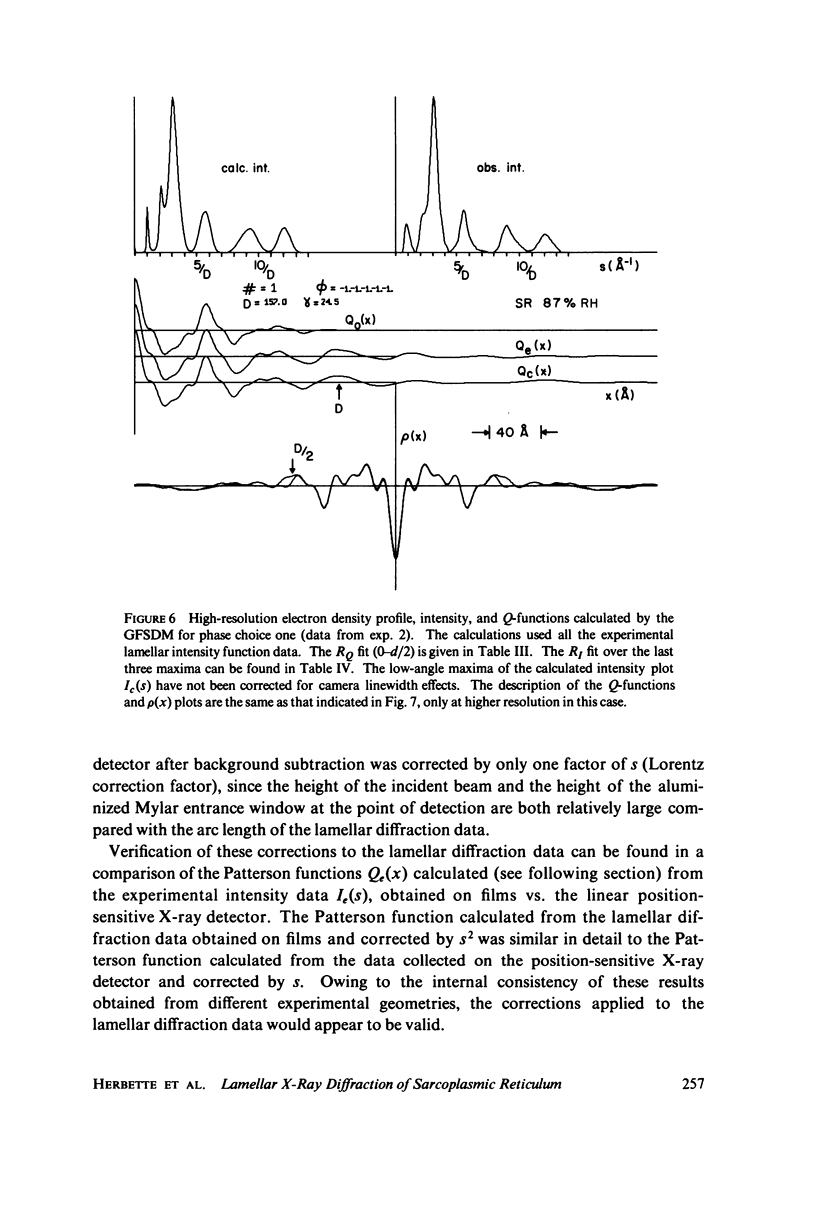
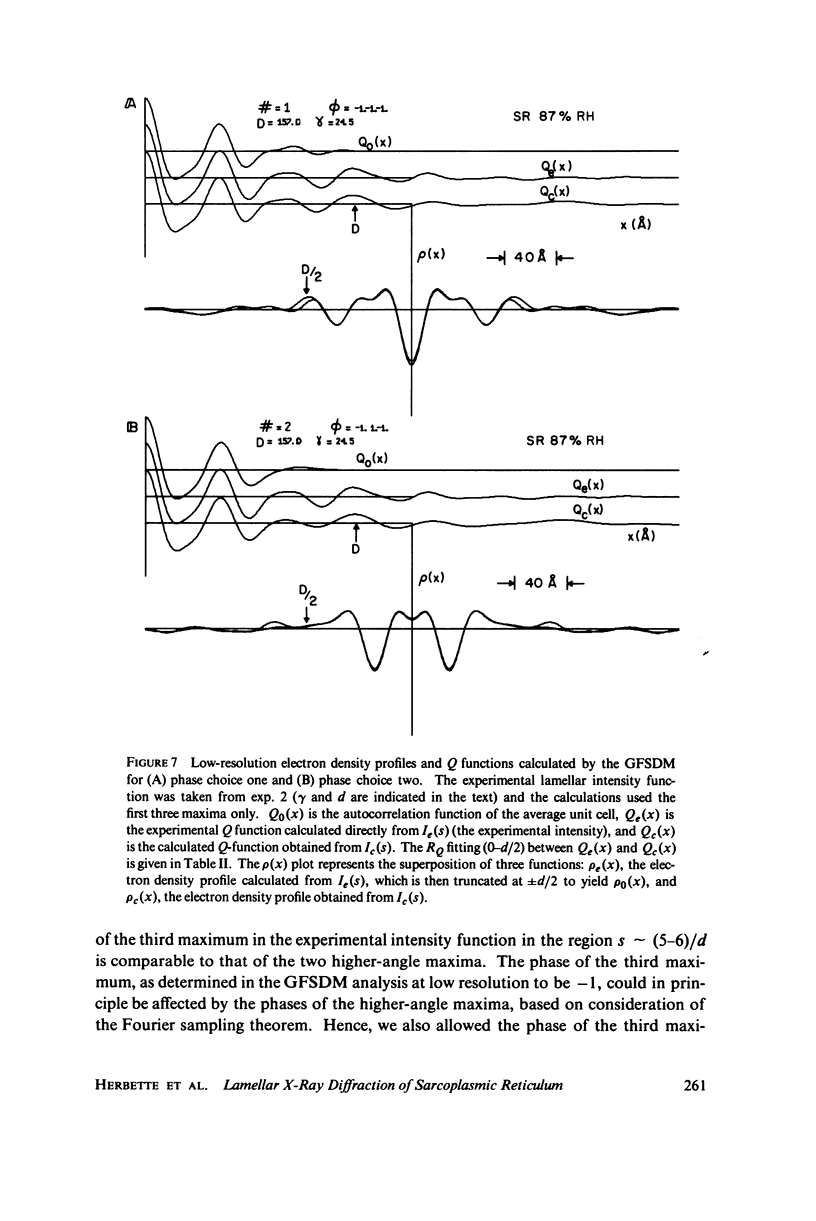
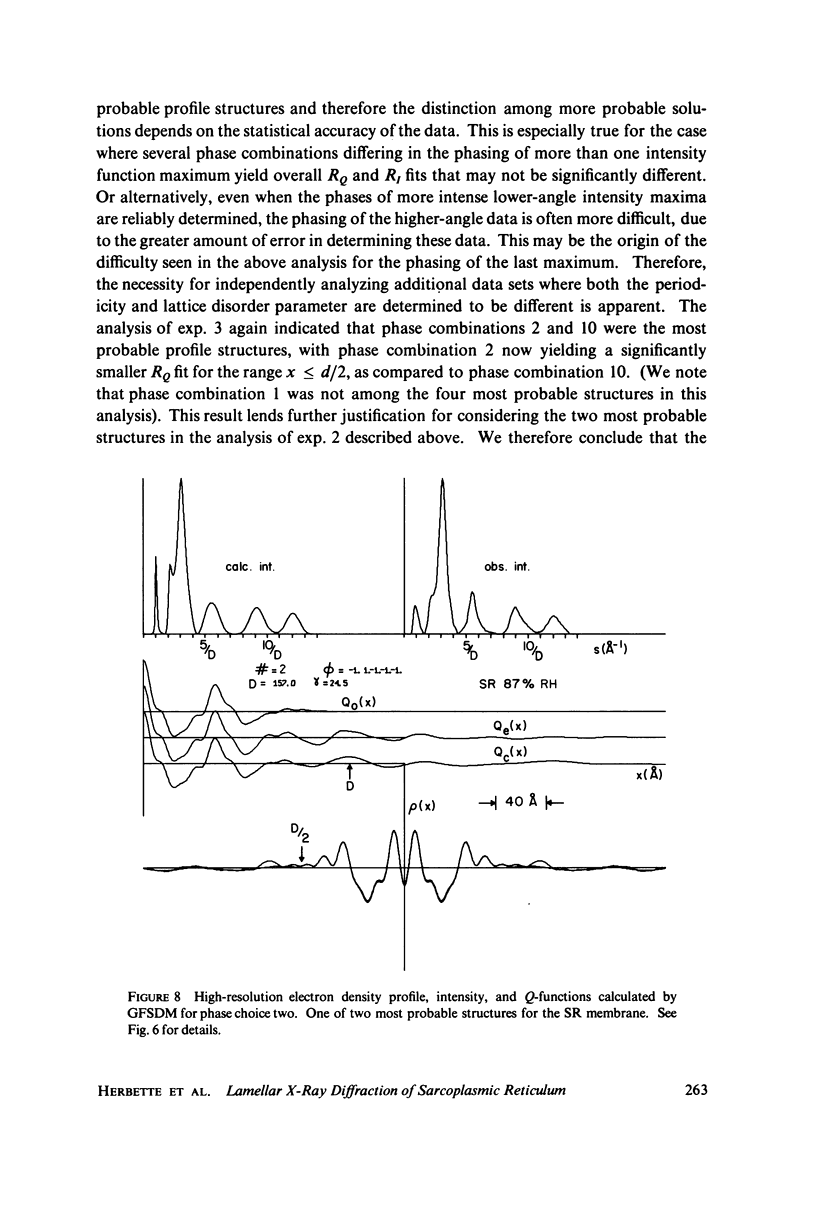
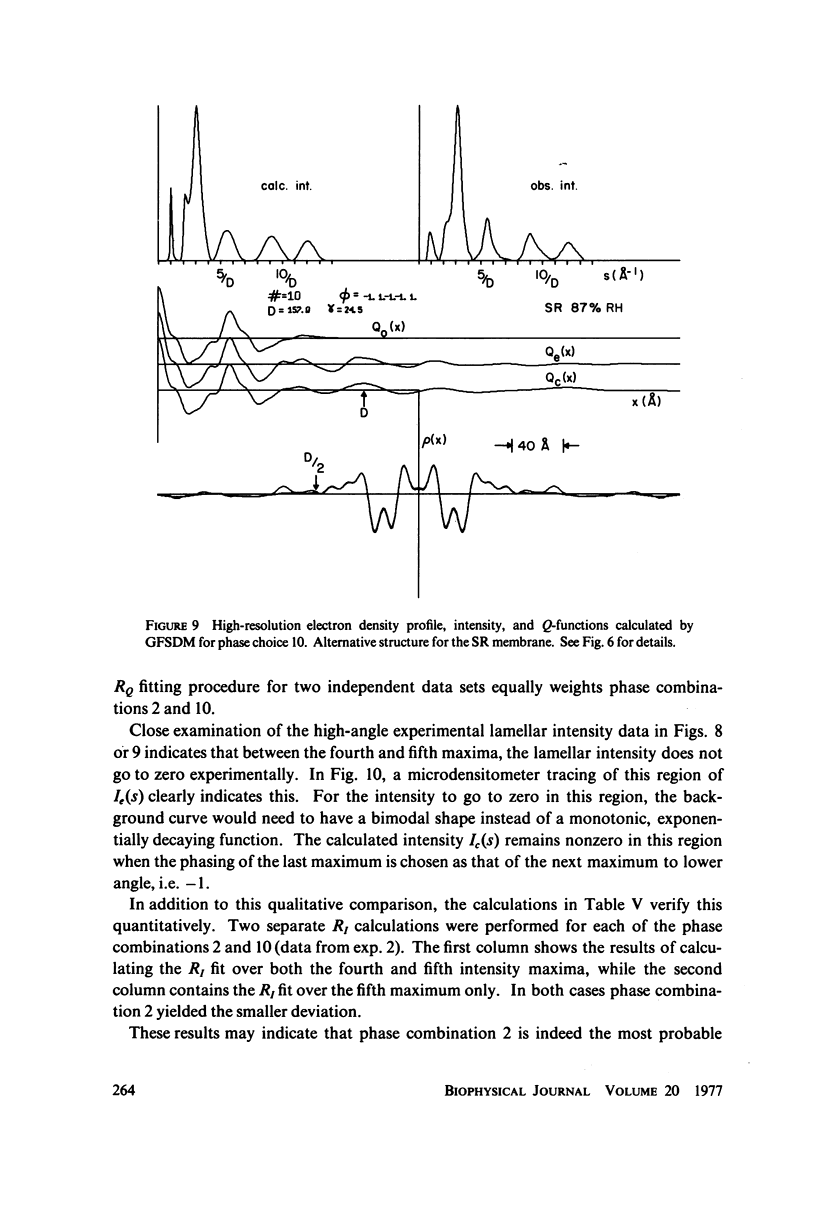
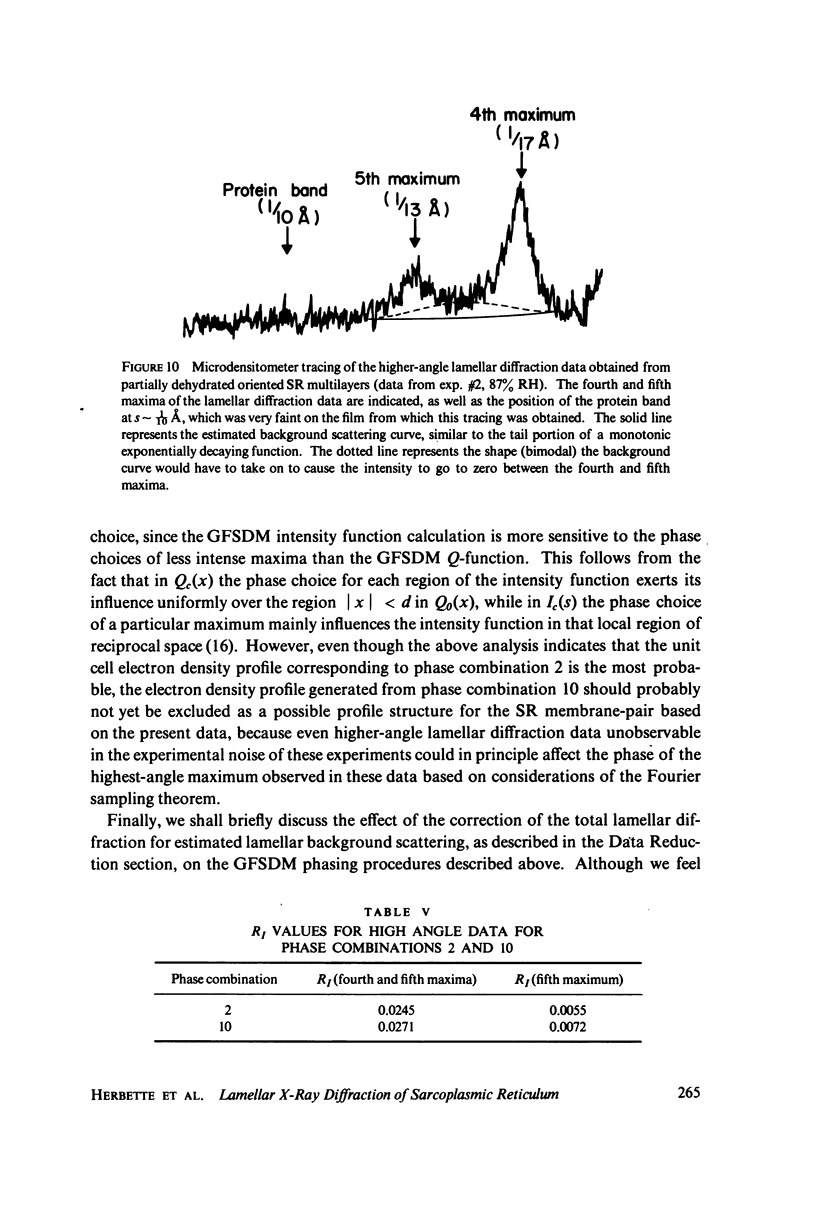
The profile structure of functional sarcoplasmic reticulum (SR) membranes was investigated by X-ray diffraction methods to a resolution of 10 A. The lamellar diffraction data from hydrated oriented multilayers of SR vesicles showed monotonically increasing widths for higher order lamellar reflections, indicative of simple lattice disorder within the multilayer. A generalized Patterson function analysis, previously developed for treating lamellar diffraction from lattice-disordered multilayers, was used to identify the autocorrelation function of the unit cell electron density profile. Subsequent deconvolution of this autocorrelation function provided the most probable unit cell electron density profile of the SR vesicle membrane pair. The resulting single membrane profile possesses marked asymmetry, suggesting that a major portion of the Ca++ -ATPase resides on the exterior of the vesicle. The electron density profile also suggests that the Ca++-dependent ATPase penetrates into the lipid hydrocarbon core of the SR membrane. Under conditions suitable for X-ray analysis, SR vesicles prepared as partially dehydrated oriented multilayers are shown to conserve most of their ATP-induced Ca++ uptake functionality, as monitored spectrophotometrically with the Ca++ indicator arsenazo III. This has been verified both in resuspensions of SR after centrifugation and slow partial dehydration, and directly in SR multilayers in a partially dehydrated state (20-30 percent water). Therefore, the profile structure of the SR membrane that we have determined may closely resemble that found in vivo.
Full text
PDF

















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blasie J. K., Worthington C. R. Molecular localization of frog retinal receptor photopigment by electron microscopy and low-angle X-ray diffraction. J Mol Biol. 1969 Feb 14;39(3):407–416. doi: 10.1016/0022-2836(69)90135-1. [DOI] [PubMed] [Google Scholar]
- Blaurock A. E. Bacteriorhodospin: a trans-membrane pump containing alpha-helix. J Mol Biol. 1975 Apr 5;93(2):139–158. doi: 10.1016/0022-2836(75)90124-2. [DOI] [PubMed] [Google Scholar]
- Duggan P. F., Martonosi A. Sarcoplasmic reticulum. IX. The permeability of sarcoplasmic reticulum membranes. J Gen Physiol. 1970 Aug;56(2):147–167. doi: 10.1085/jgp.56.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIOTT G. F., WORTHINGTON C. R. A SMALL-ANGLE OPTICALLY FOCUSING X-RAY DIFFRACTION CAMERA IN BIOLOGICAL RESEARCH. I. J Ultrastruct Res. 1963 Aug;49:166–170. doi: 10.1016/s0022-5320(63)80044-1. [DOI] [PubMed] [Google Scholar]
- Gabriel A., Dupont Y. A position sensitive proportional detector for x-ray crystallography. Rev Sci Instrum. 1972 Nov;43(11):1600–1602. doi: 10.1063/1.1685500. [DOI] [PubMed] [Google Scholar]
- Henderson R. The structure of the purple membrane from Halobacterium hallobium: analysis of the X-ray diffraction pattern. J Mol Biol. 1975 Apr 5;93(2):123–138. doi: 10.1016/0022-2836(75)90123-0. [DOI] [PubMed] [Google Scholar]
- Inesi G. Active transport of calcium ion in sarcoplasmic membranes. Annu Rev Biophys Bioeng. 1972;1:191–210. doi: 10.1146/annurev.bb.01.060172.001203. [DOI] [PubMed] [Google Scholar]
- Johnson R. G., Scarpa A. Ion permeability of isolated chromaffin granules. J Gen Physiol. 1976 Dec;68(6):601–631. doi: 10.1085/jgp.68.6.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTONOSI A., FERETOS R. SARCOPLASMIC RETICULUM. I. THE UPTAKE OF CA++ BY SARCOPLASMIC RETICULUM FRAGMENTS. J Biol Chem. 1964 Feb;239:648–658. [PubMed] [Google Scholar]
- MacLennan D. H. Purification and properties of an adenosine triphosphatase from sarcoplasmic reticulum. J Biol Chem. 1970 Sep 10;245(17):4508–4518. [PubMed] [Google Scholar]
- MacLennan D. H., Seeman P., Iles G. H., Yip C. C. Membrane formation by the adenosine triphosphatase of sarcoplasmic reticulum. J Biol Chem. 1971 Apr 25;246(8):2702–2710. [PubMed] [Google Scholar]
- McFarland B. H., Inesi G. Solubilization of sarcoplasmic reticulum with Triton X-100. Arch Biochem Biophys. 1971 Aug;145(2):456–464. doi: 10.1016/s0003-9861(71)80005-x. [DOI] [PubMed] [Google Scholar]
- Meissner G., Conner G. E., Fleischer S. Isolation of sarcoplasmic reticulum by zonal centrifugation and purification of Ca 2+ -pump and Ca 2+ -binding proteins. Biochim Biophys Acta. 1973 Mar 16;298(2):246–269. doi: 10.1016/0005-2736(73)90355-6. [DOI] [PubMed] [Google Scholar]
- Meissner G., Fleischer S. Dissociation and reconstitution of functional sarcoplasmic reticulum vesicles. J Biol Chem. 1974 Jan 10;249(1):302–309. [PubMed] [Google Scholar]
- OHNISHI T., EBASHI S. THE VELOCITY OF CALCIUM BINDING OF ISOLATED SARCOPLASMIC RETICULUM. J Biochem. 1964 Jun;55:599–603. doi: 10.1093/oxfordjournals.jbchem.a127932. [DOI] [PubMed] [Google Scholar]
- Scarpa A., Baldassare J., Inesi G. The effect of calcium ionophores on fragmented sarcoplasmic reticulum. J Gen Physiol. 1972 Dec;60(6):735–749. doi: 10.1085/jgp.60.6.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S., Cain J. E., Dratz E. A., Blasie J. K. An analysis of lamellar x-ray diffraction from disordered membrane multilayers with application to data from retinal rod outer segments. Biophys J. 1975 Dec;15(12):1201–1233. doi: 10.1016/S0006-3495(75)85895-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBER A., HERZ R., REISS I. On the mechanism of the relaxing effect of fragmented sarcoplasmic reticulum. J Gen Physiol. 1963 Mar;46:679–702. doi: 10.1085/jgp.46.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington C. R., Liu S. C. Structure of sarcoplasmic reticulum membranes at low resolution (17A). Arch Biochem Biophys. 1973 Aug;157(2):573–579. doi: 10.1016/0003-9861(73)90676-0. [DOI] [PubMed] [Google Scholar]