Abstract
Polypeptide drugs are generally short-lived species in circulation. In this study, we have covalently linked seven moieties of 2-sulfo-9-fluorenylmethoxycarbonyl (FMS) to the amino groups of human interferon-α2. The derivative thus obtained (FMS7–IFN-α2) has ≈4% the biological potency and 33 ± 4% the receptor binding capacity of the native cytokine. Upon incubation, FMS7–IFN-α2 undergoes time-dependent spontaneous hydrolysis, generating active interferon with t1/2 values of 24 ± 2 h at pH 8.5 and 98 ± 10 h at pH 7.4. When native IFN-α2 is intravenously administered to mice, circulating antiviral activity is maintained for a short duration and then declines with t1/2 = 4 ± 0.5 h, reaching undetectable values at ≈18 h after administration. With intravenously administered FMS7–IFN-α2, there is a lag period of 2 h, followed by a progressive elevation in circulating antiviral-active protein, which peaked at 20 h and declined with t1/2 = 35 ± 4 h. FMS7–IFN-α2 is resistant to α-chymotrypsin digest and to proteolytic inactivation by human serum proteases in vitro. We have thus introduced here an inactive IFN-α2 derivative, which is resistant to in situ inactivation and has the capability of slowly reverting to the native active protein at physiological conditions in vivo and in vitro. Having these attributes, FMS7–IFN-α2 maintains prolonged circulating antiviral activity in mice, exceeding 7–8 times the activity of intravenously administered native cytokine.
Keywords: protracting action, immune modulator, antiviral activity
Protein drugs of molecular mass lower than 50,000 daltons are in general short-lived species in vivo, having a circulatory half-life of about 5–20 min (1). Clearance of proteins occurs through several mechanisms, including glomerular infiltration in the kidney, receptor-mediated endocytosis, and degradation by peripheral tissues, and proteolysis at the tissue surfaces or by serum proteases (2). Considering also that protein drugs are not absorbed orally, prolonged maintenance of therapeutically active drugs in circulation is a desirable feature of primary clinical importance. This condition, however, is rarely achieved after a single administration of low molecular weight peptides and protein drugs.
Interferons are cytokines possessing a variety of antiviral (3), immunomodulation (4, 5), and antiproliferative effects (6). Human type I interferons include a large family of about 13 different IFN-α functional nonallelic genes, sharing about 70% homology (7). Interferons are produced and released from a variety of cell types in response to viral infections, before the appearance of humoral antibodies. Administration of interferon can prevent, but not cure, certain viral infections (3, 8). The action of interferon on viral infections is complex and may depend both on the immunomodulating and the antiviral activities of the protein. The biological action of type I interferons is initiated by IFN binding to two cellular receptors (ifnar1 and ifnar2), causing these receptors to associate through their binding to interferon (9). This binding initiates a cascade of cellular events, which ultimately arrest synthesis of viral proteins. A series of short 2′-5′-linked oligoadenylates, synthesized by an induced enzyme, activate a latent ribonuclease that degrades messenger RNAs (10). Interferon also induces a protein kinase that phosphorylates and inactivates E2F, a prerequisite factor for the initiation of protein synthesis (11).
IFN-α2 (Mr = 18 kDa, 165 amino acids) is a widely used, FDA-approved protein drug applied for the treatment of chronic hepatitis C (3), chronic hepatitis B (8), Kaposi's sarcoma in HIV-infected patients (12), and leukemia (13). IFN-α2 is administered intramuscularly, s.c., or intravenously, with each of these routes yielding a different pharmacokinetic pattern. A common feature for any of these administration modes, however, is rapid inactivation of IFN-α2 in body fluids and in various tissues (14), leading to the disappearance of the cytokine from the plasma within several hours after administration (15). Unlike many other administered protein drugs, the major route of IFN-α2 elimination in vivo takes place in the circulatory system through proteolysis and inactivation by serum proteases; only negligible amounts are excreted by the kidneys or removed through receptor-mediated endocytosis (1).
In this study, we designed and prepared an IFN-α2 derivative potentially capable of maintaining prolonged antiviral activity in situ, after i.v. administration to mice. Postulating that it is the native IFN-α2 conformation that is highly susceptible to proteolysis and inactivation by serum proteases, we presumed that its substantial alteration by several, covalently introduced FMS moieties should lead to proteolysis-resistant species. The resultant loss in antiviral activity, as expected upon massive modification of the protein, may be even advantageous, provided that FMS hydrolysis occurs with a satisfactory rate and kinetics, thereby generating immunomodulating and antiviral activity of the cytokine over a prolonged period in situ.
Materials and Methods
Preparation of Materials.
Nonglycosylated human IFN-α2 was prepared as described in detail by Piehler and Schreiber (16). Briefly, the gene coding for IFN-α2 was cloned into a two-cistron expression vector to yield the plasmid pT72Cα2. The codons for the first 23 amino acids were changed to improve the level of expression (17). The protein was made in TG1 cells containing the plasmid. IFN-α2 was found in inclusion bodies, which were dissolved in 8 M urea containing 5 mM DTT and refolded after 20-fold dilution. Purification was conducted by two consecutive procedures of ion-exchange chromatography (Q Sepharose and HiTrap Q, Amersham Pharmacia). The IFN-α2 thus prepared runs as a single band of 18 kDa on SDS/PAGE. Concentration was determined by using an extinction coefficient of ɛ280 = 18,070 (18). Fluorenylmethoxycarbonyl-N-hydroxysuccinimide (Fmoc-OSu) was purchased from Nova Biochem.
FMS-OSu (2-sulfo-9-fluorenylmethoxycarbonyl-N-hydroxysuccinimide) was prepared essentially by the procedure of Merrifield and Bach (19) with slight modifications. Fmoc-OSu (337.4 mg, 1 mmol) was dissolved in 4 ml of dichloromethane and cooled to 0°C. A solution of ClSO3H (60 μl, 0.9 mmol) in 2 ml of dichloromethane was added with constant stirring and cooled over a period of 15 min. The yellow-turning solution was allowed to warm to room temperature, and a white precipitate was formed within 1 h. At 2 h, cyclohexane (4 ml) was added to dissolve the unreacted Fmoc-OSu. The suspension was centrifuged and washed four times with 6 ml of 1:1 (vol/vol) cyclohexane/dichloromethane. The white solid thus formed was dried under P2O5 in vacuo for 24 h and had the following characteristics: yield 290 mg (86%), mp 140–146°C TLC (1-butanol/acetic acid/water, 8:1:1) Rf 0.31, and mass spectrum (ES−) m/z 416 (100%, M−1). FMS moieties, either free or covalently bound to proteins, absorb at the UV range with molar extinction coefficients ɛ280 = 21,200 and ɛ301 = 10,300. All other materials used in this study were of analytical grade.
Biological and Chemical Procedures.
Preparation of FMS7–IFN-α2.
To a stirred, cooled solution of IFN-α2 [0.95 mg (50 nmol) in 1.0 ml, 0.02 M NaHCO3], three 5-μl aliquots of a fresh solution of 33 mM FMS-OSu in DMSO were added at 0, 15, and 30 min. Altogether, 10 equivalents of FMS-OSu (500 nmol) over the protein were added. After 1 h, the reaction mixture was dialyzed overnight at 7°C against H2O and further purified by reverse-phase HPLC (spectra-physics SP8800), by using a prepacked column, C4-Vydac (250 × 6.4 mm), and applying a linear gradient between solutions A (0.1% trifluoroacetic acid in H2O) and B (0.1% trifluoroacetic acid in acetonitrile/H2O, ratio of 75:25). The main peak (emerged with retention time of 37.80 min) was collected and lyophilized. FMS7–IFN-α2 absorbs at 301 nm with a molar extinction coefficient ɛ301 = 76,200 and has the expected mass for IFN-α2 containing seven FMS moieties (Table 1).
Table 1.
Chemical features of FMS7–IFN-α2
| Characteristic | Numerical value |
|---|---|
| Moles FMS/mol IFN-α2* | 7.0 ± 0.2 |
| Absorbance at 280 nm† | ɛ280 = 166,500 |
| Absorbance at 301 nm‡ | ɛ301 = 76,200 |
| Mass spectra§ | |
| Calculated | 21,383 daltons |
| Found | 21,382 daltons |
| Solubility in aqueous buffer, pH 7.4 | >0.5 mg/ml |
| Retention time (analytical HPLC)¶ | 37.80 min |
| Reversion (%) to native IFN-α2 upon incubation at pH 8.5, 37°C, for the following durations (h)‖ | |
| 4 | 3 |
| 9 | 10 |
| 20 | 30 |
| 50 | 97 |
Determined by UV spectroscopy, measuring the absorbance at 280 and 301 nm. Derivative concentration was determined by acid hydrolysis of a 20-μl aliquot, followed by amino acid analysis; calculated according to aspartic acid (14 residues), alanine (9 residues), and isoleucine (8 residues).
Native IFN-α2 absorbs at 280 nm with ɛ280 = 18,070 (18).
Native IFN-α2 absorbs at 301 nm with ɛ301 = 4,100.
Mass spectra were determined by using the electrospray ionization technique.
IFN-α2 elutes under identical analytic HPLC procedure with retention time = 34 ± 0.2 min.
Determined by analytical HPLC procedure (increase in peak area corresponding to native IFN-α2).
Receptor binding affinities.
The interaction between recombinant ifnar2-EC and IFN-α2 was monitored under flow-through conditions by an optical probe called reflectometric interference spectroscopy (20). This method detects biomolecular interaction of ligands to transducer-bound proteins as a shift in the interference spectrum caused by change of the apparent optical thickness of the transducer chip and has been described in detail (16, 20, 21). A shift of 1 pm corresponds to approximately 1 pg/mm2 protein on the surface. The transducer surface was modified with a dextran layer and carboxylated by reaction with molten glutaric anhydride (Sigma) at 75°C for 2–8 h. On such surfaces, electrostatic preconcentration and covalent immobilization of proteins were carried out by standard BIAcore protocols. After this procedure, the extracellular part of IFN-α2 receptor (ifnar2-EC) was immobilized into a carboxylated dextran layer (16). All measurements were carried out in 50 mM Hepes, pH 7.4/150 mM NaCl/0.01% Triton X-100. A sample of 0.8 ml was injected for 80 s with a data acquisition rate of 1 Hz. Flow rates of 50 μl/s were applied. Under these conditions, the samples in the flow cell were exchanged within 1 s, allowing the analysis of processes within 5 s.
Antiviral activity.
Antiviral activity of IFN-α2 and its derivatives was determined by the capacity of the cytokine to protect human amnion WISH cells against vesicular stomatitis virus (VSV)-induced cytopathic effects (22). WISH cells (4.5 × 105 cells/ml) were seeded in a 96-well plate (100 μl/well) and incubated with 2-fold serial dilutions of IFN-α2 or its derivatives for 18 h at 37°C. WISH cell viability was determined by measuring the absorbance of crystal violet-stained cells in an ELISA plate. In this assay, native IFN-α2 shows 50% protection of VSV-induced WISH cells (ED50) at a concentration of 0.3 ± 0.04 pM. An IFN-α2 derivative exhibiting ED50 of 3 ± 0.3 pM in this assay was considered as having 10% of the native antiviral potency.
Results
Progressive Modification of Amino Acid Moieties of IFN-α2 with FMS-OSu.
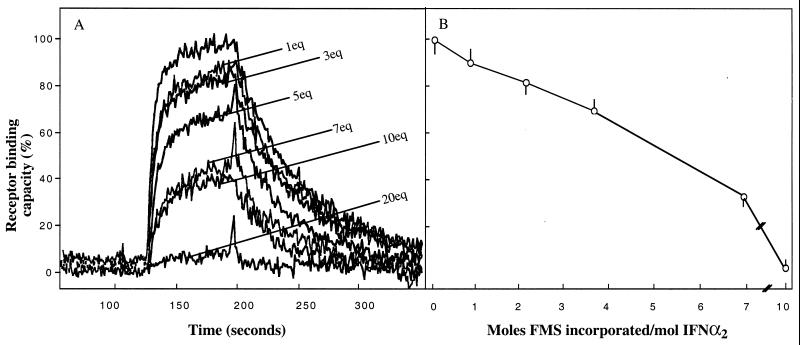
Proteins can incur a varying degree of covalent modification of “surface” amino acid moieties before being inactivated, provided that the modification is not directed toward an unusually reactive moiety, often involved in substrate binding and/or in catalysis (23). Fig. 1A summarizes a set of experiments in which samples of IFN-α2 were modified with increasing concentrations of FMS-OSu. Upon reacting IFN-α2 with 1, 3, 5, 10, and 20 molar excess of FMS-OSu, the respective receptor binding affinities were 90 ± 3, 82 ± 4, 69 ± 3, 33 ± 3, and 3 ± 0.3% of the native cytokine binding potency (Fig. 1A). Measuring UV absorption of the modified cytokine at 301 nm and applying mass spectroscopy, we found that for each such treatment the number of FMS moieties incorporated into IFN-α2 was 0.7, 2.0, 3.5, 7.0, and 10.0 mol FMS/IFN-α2, respectively. Thus, the receptor binding affinity gradually decreases as a function of the number of FMS moieties incorporated into the cytokine (Fig. 1B), although substantial binding affinity remains even when 7 mol of FMS are covalently introduced into the protein. Notably, three of the 13 amino side-chain moieties of IFN-α2 are not accessible to derivatization by FMS-OSu (20-fold excess, Fig. 1B). The derivative FMS7–IFN-α2 was selected for further characterization.
Figure 1.
Progressive modification of the amino acid moieties of IFN-α2 with FMS-OSu; loss of receptor binding capacity as a function of FMS moieties incorporated into IFN-α2. Human IFN-α2 was modified at pH 8.5 with increasing concentrations of FMS-OSu, ranging from 1 equivalent up to 20 molar equivalents of FMS-OSu. For each treatment, receptor binding capacity (A) and moles FMS introduced covalently into IFN-α2 (B) were determined. Receptor binding capacity toward immobilized ifnar2-EC was assessed by the reflectometric interference spectroscopy procedure. The rising and declining curves represent, respectively, ligand association and ligand dissociation from ifnar2-EC. Moles of FMS/moles of IFN-α2 were determined by UV absorption at 301 nm after dialysis and by mass spectroscopy (see Materials and Methods).
Chemical Characterization of FMS7–IFN-α2.
Table 1 summarizes the main chemical features of HPLC-purified FMS7–IFN-α2. This derivative contains 7 mol of FMS/mol protein as deduced by its absorption spectra at 280 nm (ɛ280 = 166,500) and at 301 nm (ɛ301 = 76,200), values that correspond to the absorption of 7 mol of FMS plus that of the native protein. Mass spectrum analyses revealed the corrected mass of 21,383 daltons, as calculated for IFN-α2 containing seven moieties of FMS (calculated mass = 21,382 daltons, Table 1). The derivative is soluble in aqueous solutions (pH 7.4). It migrates as a single peak on HPLC with a retention time value of 37.80 min. FMS7–IFN-α2 almost fully reverted to the native protein upon incubation at pH 8.5 (37°C) for 2 days, as judged by analytic HPLC procedure (Table 1).
FMS7–IFN-α2 Undergoes Hydrolysis at Physiological pH and Temperature with the Generation of the Cytokine Antiviral Potency.
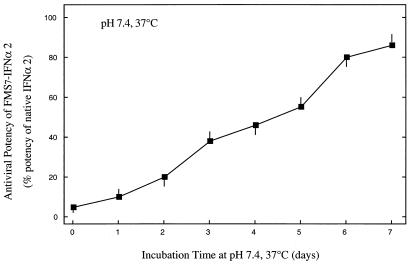
Fig. 2 displays the recovery of the antiviral potency of FMS7–IFN-α2 after incubation in PBS buffer (pH 7.4) at 37°C. Aliquots were withdrawn daily, and antiviral activity was assessed in VSV-induced WISH cells. FMS7–IFN-α2 has generated antiviral potency in a slow and homogeneous fashion with a t1/2 value of 4.0 ± 0.4 days (Fig. 2). After 7 days of incubation, FMS7–IFN-α2 regained 87 ± 4% of the native cytokine antiviral potency. FMS10–IFN-α2, a derivative in which 10 of the 13 amino side-chain moieties of IFN-α2 were modified with FMS, has regained only little antiviral potency upon prolonged incubation at physiological pH and temperature, most likely as a result of irreversible structural alteration (not shown).
Figure 2.
Time course of reactivation of FMS7–IFN-α2 upon incubation at 37°C, pH 7.4. FMS7–IFN-α2 (1 mg/ml in PBS buffer, pH 7.4) was incubated at 37°C. Aliquots were withdrawn daily and analyzed for their antiviral potency to inhibit VSV-induced cytopathic effects in human WISH cells (see Materials and Methods). Results are expressed as percent of antiviral potency of the native cytokine. Half-maximal inhibition of cytopathic effect was obtained at 0.3 ± 0.03 pM IFN-α2. IFN-α2 derivative exhibiting half-maximal inhibition at a concentration of 30 ± 3 pM was considered as having 1% of the native antiviral potency.
FMS7–IFN-α2 Is Resistant to Proteolysis.
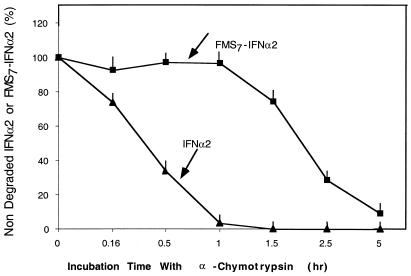
Fig. 3 describes the treatment of native IFN-α2 and FMS7–IFN-α2 with 1% α-chymotrypsin in PBS buffer (pH 7.4) at 37°C. Aliquots were withdrawn during proteolysis, then acidified and analyzed by RP-HPLC for evaluating the extent of degradation. Under these conditions, native IFN-α2 and FMS7–IFN-α2 were proteolyzed by α-chymotrypsin with t1/2 values of 20 ± 3 min and 120 ± 10 min, respectively. Thus, FMS7–IFN-α2 is fairly resistant to proteolysis by α-chymotrypsin relative to the native cytokine.
Figure 3.
Susceptibility of FMS7–IFN-α2 toward enzymatic degradation by α-chymotrypsin. FMS7–IFN-α2 and native IFN-α2 (1 mg/ml each in PBS buffer, pH 7.4) were incubated with α-chymotrypsin (1% wt/wt). At the indicated time points, aliquots were subjected to analytical HPLC. The quantity of the cytokine (peak area) at t = 0 was assigned 100%.
FMS7–IFN-α2 Is Resistant to Inactivation in Human Serum.
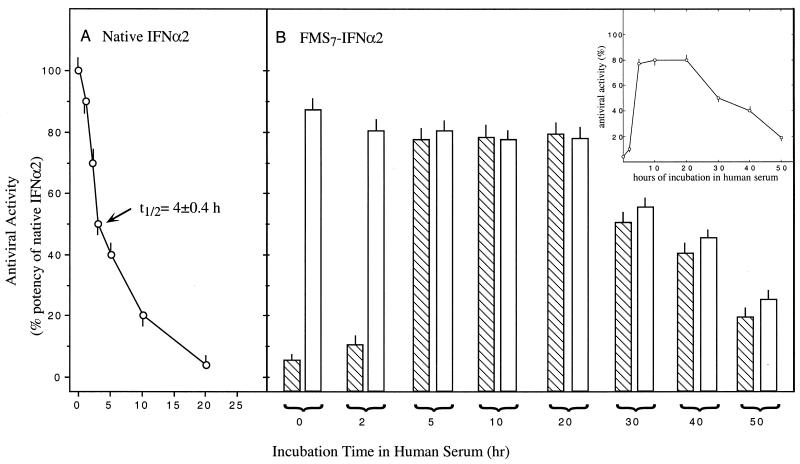
Native IFN-α2 is a short-lived species in vivo, predominantly because of inactivation by serum proteases (see Introduction). Hence, the inactivation profile of IFN-α2 incubated in serum in vitro at 37°C is expected to be rather similar in many aspects to the pharmacokinetic profile of the native cytokine after i.v. administration in rodents. Fig. 4 shows a set of experiments in which either IFN-α2 or FMS7–IFN-α2 were incubated in human serum at 37°C for various lengths of time, and their antiviral potencies were determined. With serum-incubated FMS7–IFN-α2, antiviral activity was determined in samples taken before and after an additional period of incubation for 18 h, at pH 8.5, 37°C, to ensure full FMS hydrolysis.
Figure 4.
Resistance of FMS7–IFN-α2 to inactivation in human serum. FMS7–IFN-α2 and native IFN-α2 were incubated in human serum at 37°C. At the indicated time points, aliquots were tested for antiviral potency to inhibit VSV-induced cytopathic effects in human WISH cells (see Materials and Methods). (A) Antiviral activity of native IFN-α2. (B) Aliquots of FMS7–IFN-α2 incubated in serum were either introduced directly to WISH cells (dashed columns) or pretreated for 18 h at pH 8.5, 37°C (open columns).
The antiviral activity of native IFN-α2 declined under these physiological conditions with t1/2 = 4.0 ± 0.4 h, reaching undetectable levels 20 h after incubation (Fig. 4A). With FMS7–IFN-α2, little antiviral activity was found within the first 2 h of incubation (Fig. 4B, hatched columns). Activity increased steeply, peaking at 5 h, then maintained at a high plateau over a period of 15 h before declining with t1/2 of 25 ± 2 h (Fig. 4B and Inset). A high level of antiviral potency was found in the serum aliquot withdrawn at 2 h of incubation after FMS7–IFN-α2 deprotection (Fig. 4B, open columns).
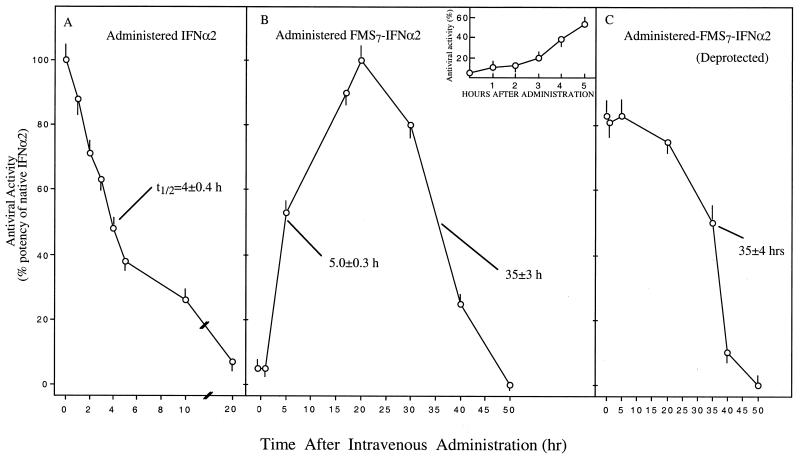
Intravenously Administered FMS7–IFN-α2 in Mice Facilitates Antiviral Activity Over a Prolonged Period.
In the experiments summarized in Fig. 5, either IFN-α2 or FMS7–IFN-α2 was administered intravenously to groups of mice (n = 5 in each group). At various time points, blood aliquots were withdrawn and analyzed for antiviral activity in WISH cells against VSV-induced cytopathic effects (see Materials and Methods). In mice receiving FMS7–IFN-α2, each blood aliquot was measured for its antiviral activity before (Fig. 5B) and after (Fig. 5C) an additional period of incubation for 18 h at pH 8.5, 37°C, to effect FMS hydrolysis and reactivation of the FMS-modified protein that has not been reactivated in situ. After administration of the native cytokine, circulating antiviral activity declined with a t1/2 value of 4 ± 0.3 h, reaching undetectable levels at ≈20 h after administration (Fig. 5A). Fig. 5B shows the kinetic behavior of intravenously administered FMS7–IFN-α2 in mice. Only after a lag period of 2 h (Fig. 5B, Inset), there was a noticeable elevation in antiviral activity. Circulating antiviral activity was then steeply increased, reaching 50% the native potency at 5 h (Fig. 5B). Antiviral potency is further elevated to maintain a “wide plateau” between 17 and 30 h after administration, before declining with a t1/2 value of 35 ± 3 h (Fig. 5B).
Figure 5.
Intravenously administered FMS7–IFN-α in mice facilitates prolonged circulating antiviral activity. Groups of mice (n = 5 for each group) received intravenously (10 μg/mouse) native-IFN-α2 (A) or FMS7–IFN-α2 (B and C). Blood aliquots were withdrawn at the indicated time points. Circulatory antiviral activities in aliquots were determined in human WISH cells before (B) and after (C) an additional period of incubation for 18 h (pH 8.5, 37°C).
Fig. 5C shows the antiviral potencies of the same aliquots after hydrolysis of the FMS moieties by an additional incubation period. A high level of antiviral potency was obtained at earlier time points after administration and maintained over a period of 20 h. Antiviral activity was then decreased with a t1/2 value of 35 ± 3 h (Fig. 5C). Thus, starting 2 h after i.v. administration of FMS7–IFN-α2 into mice, there is a prolonged high level of circulating antiviral activity, which lasts over a period of 40 h.
Preliminary studies were performed aiming to determine whether FMS7–IFN-α2 was antigenic. No antibodies to either FMS7–IFN-α2 or to the native cytokine could be detected after treating mice with FMS7–IFN-α2 in complete Freund's adjuvant (Y.S. and M.F., unpublished work).
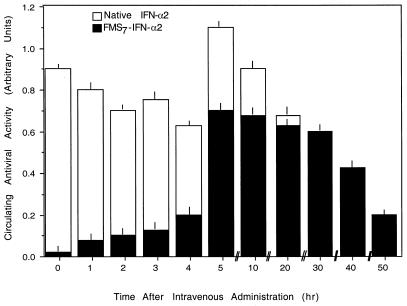
Because circulating antiviral activity in FMS7–IFN-α2-administered mice emerged after a 2-h lag period (Fig. 5B), we next coadministered the native cytokine together with FMS7–IFN-α2 (10 μg each per mouse), as summarized in Fig. 6. As expected, under these experimental conditions, no lag period was found. A high level of antiviral activity has been initiated shortly after administration and maintained over a prolonged period, before declining with a t1/2 value of 37 ± 2 h (Fig. 6).
Figure 6.
Intravenous coadministration of native IFN-α2 and FMS–IFN-α2 facilitates prolonged circulating antiviral activity from the time of administration. Groups of mice (n = 3 for each group) received intravenously native IFN-α2 (10 μg/mouse) or FMS7–IFN-α2 (10 μg/mouse) or both (10 μg of each). Blood aliquots were withdrawn at the indicated time points and analyzed in human WISH cells for antiviral activity.
FMS7–IFN-α2 administered to mice s.c. or intraperitoneally has maintained prolonged circulating antiviral activities as well, exceeding several times that of the native cytokine administered under identical experimental conditions (data not shown).
Discussion
A new conceptual approach to prolonging the half-life of protein drugs in vivo is a scientific challenge with potentially broad clinical implications. In previous studies, we introduced an acidic sulfonic moiety into the flurene ring of Fmoc-OSu and covalently linked FMS moieties to the amino groups of several proteins. We found that in aqueous neutral solutions, FMS moieties undergo slow, spontaneous hydrolysis with the generation of the native proteins (24). We next prepared an insulin derivative having three FMS moieties. FMS3-insulin had little receptor binding affinity and biological potency of the native hormone. After administration to streptozocin rats, FMS3-insulin lowers circulating glucose level over a prolonged period (t1/2 = 30 h). Because receptor-mediated endocytosis is the major degradative pathway for insulin (25), we hypothesized that the protracted action of FMS3-insulin in vivo predominantly reflected the escape of FMS3-insulin from receptor-mediated degradation, before the hydrolysis of the FMS moieties and reactivation of this derivative (24). A key question that remained unanswered is whether this approach is also applicable to prolonging half-lives of short-lived proteins in vivo, either because of clearance by glomerular infiltration in the kidney or as a result of proteolytic inactivation in the circulatory system.
After the modification of IFN-α2 with increasing concentrations of FMS-OSu, we first discovered that progressive derivatization is linearly correlated with a decrease in antiviral potency, but as many as seven FMS moieties could be introduced into IFN-α2 before the antiviral potency was substantially lost (Figs. 1 and 2). Notably, FMS7–IFN-α2 has regained its full antiviral potency after 5 h of incubation in human serum (Fig. 4). Yet, stability against proteolysis by serum proteases has been maintained for as long as 30 h thereafter (Figs. 4 and 5). It is therefore conceivable that FMS moieties located at or near the binding site hydrolyze at a faster rate than those linked to nonactive regions of the protein. According to this, the latter moieties preserve the already reactivated conjugate from being readily proteolyzed. This view is also supported by our real-time binding measurements presented in Fig. 1A. An increase in the FMS/IFN-α2 ratio decreased the number of protein molecules that participate in binding (lower signal), with no reduction in binding affinities of conjugates toward ifnar2 (Fig. 1A).
HPLC-purified FMS7–IFN-α2 was selected for further studies and chemically characterized. Reversion of FMS7–IFN-α2 to the native protein upon incubation was confirmed by HPLC (Table 1) and by regaining the antiviral potency of FMS7–IFN-α2 upon incubation at 37°C, pH 7.4 (Fig. 2). We next examined whether FMS7–IFN-α2 was resistant to proteolysis by using α-chymotrypsin. Derivatization of IFN-α2 with FMS moieties significantly protected the protein against general proteolysis. The antiviral potency of FMS7–IFN-α2 upon incubating the derivative in human serum at 37°C was then monitored. Under these conditions, antiviral activity appeared at 2 h, peaked at 5 h, and was maintained elevated for a period of 40 h (Fig. 4B, Inset).
We next compared the pharmacokinetic profile of FMS7–IFN-α2 vs. the native protein after i.v. administration in mice. The native cytokine and its derivative showed a remarkably similar pharmacokinetic profile to the one obtained in vitro in human serum (Fig. 4 vs. Fig. 5). Thus, as previously suggested, IFN-α2 is a short-lived protein in vivo, predominantly because of inactivation in the serum. We conclude that our technology is also applicable toward protein drugs that are excluded in situ by this mechanism.
Our experiments provide an explanation to the prolonged antiviral activity of FMS7–IFN-α2 in vivo. This feature appears to be the outcome of two processes acting in harmony, namely, resistance of FMS7–IFN-α2 to inactivation in serum before the hydrolysis of FMS moieties from the protein (Figs. 4 and 5) and reactivation of the derivative's antiviral potency after a small fraction of the FMS moieties have already been hydrolyzed (Fig. 1). The coordinated relationships of these two ongoing events appear to preserve antiviral potency in vivo over a period of 40 h, starting ≈2 h after i.v. administration of this derivative (Fig. 5).
In one parameter, however, the behavior of FMS7–IFN-α 2 in vitro differs from its pharmacokinetic pattern in vivo and deserves further discussion. The rate of reactivation of FMS7–IFN-α2 in vivo (or in human serum) exceeds 10–20 times that found in PBS buffer at pH 7.4, 37°C (Fig. 2 vs. Figs. 4 and 5). We postulate that the nucleophilic capacity of the serum considerably accelerates FMS hydrolysis and reactivation of the IFN-α2 derivative. Regarding the resistance of FMS7–IFN-α2 to proteolytic inactivation in serum, we have recently found that FMS protein conjugates associate with albumins, an event that protects conjugates from being proteolyzed (Y.S. and M.F., unpublished work).
Acknowledgments
We thank Elana Friedman for typing the manuscript, Yigal Avivi for editing it, Dr. Qian Sun for technical assistance, and Prof. Menachem Rubinstein (Department of Virology, The Weizmann Institute) for assistance and consultation. M.F. is the Lester Pearson Professor of Protein Chemistry; Y.S. is the incumbent of C.H. Hollenberg Chair in Metabolic and Diabetes Research, established by the friends and associates of Dr. C.H. Hollenberg of Toronto, Canada.
Abbreviations
- FMS
(2-sulfo)-9-fluorenylmethoxycarbonyl
- OSu
N-hydroxysuccinimide
- Fmoc
fluorenylmethoxycarbonyl
- VSV
vesicular stomatitis virus
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Goodman L, Gilman A E. In: Goodman & Gilman: Handbook of Pharmacology. Gilman A G, Rall T W, Nies A S, Taylor P, editors. Oxford: Pergamon; 1990. [Google Scholar]
- 2.Olson T S, Dice J F. Curr Opin Cell Biol. 1989;1:1194–2000. doi: 10.1016/s0955-0674(89)80071-7. [DOI] [PubMed] [Google Scholar]
- 3.Barnes E, Webster G, Jacobs R, Dusheiko G. J Hepatol. 1999;31:244–249. doi: 10.1016/s0168-8278(99)80410-3. [DOI] [PubMed] [Google Scholar]
- 4.Lundgren E, Hedman H, Landberg G, Chiang F, Roos G, Sanders R. Eur J Cancer. 1991;27:S82–S84. doi: 10.1016/0277-5379(91)90584-z. [DOI] [PubMed] [Google Scholar]
- 5.Holan V, Nakamura S, Minowada J. Immunology. 1992;75:176–181. [PMC free article] [PubMed] [Google Scholar]
- 6.Fiorani C, Tonelli S, Casolari B, Sacchi S. Curr Pharm Des. 1999;5:987–1013. [PubMed] [Google Scholar]
- 7.Weissmann C, Weber H. Prog Nucleic Acid Res Mol Biol. 1986;33:251–300. doi: 10.1016/s0079-6603(08)60026-4. [DOI] [PubMed] [Google Scholar]
- 8.Janssen H L, Gerken G, Carreno V, Marcellin P, Naoumov N V, Craxi A, Ring-Larsen H, Kitis G, van Hattum J, de Vries R A, et al. Hepatology. 1999;30:238–243. doi: 10.1002/hep.510300113. [DOI] [PubMed] [Google Scholar]
- 9.Novick D, Cohen B, Rubinstein M. Cell. 1994;77:391–400. doi: 10.1016/0092-8674(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 10.Baglioni C, Maroney P A. J Biol Chem. 1980;255:8390–8393. [PubMed] [Google Scholar]
- 11.Iwase S, Furukawa Y, Kikuchi J, Nagai M, Terui Y, Nakamura M, Yamada H. J Biol Chem. 1997;272:12406–12414. doi: 10.1074/jbc.272.19.12406. [DOI] [PubMed] [Google Scholar]
- 12.Krown S E. Hematol Oncol Clin North Am. 1991;5:311–322. [PubMed] [Google Scholar]
- 13.Zinzani P L, Lauria F, Salvucci M, Rondelli D, Raspadori D, Bendandi M, Magagnoli M, Tura S. Haematologica. 1997;82:152–155. [PubMed] [Google Scholar]
- 14.O'Kelly P, Thomsen L, Tilles J G, Cesario T. Proc Soc Exp Biol Med. 1985;178:407–411. doi: 10.3181/00379727-178-42024. [DOI] [PubMed] [Google Scholar]
- 15.Rostaing L, Chatelut E, Payen J L, Izopet J, Thalamas C, Ton-That H, Pascal J P, Durand D, Canal P. J Am Soc Nephrol. 1998;9:2344–2348. doi: 10.1681/ASN.V9122344. [DOI] [PubMed] [Google Scholar]
- 16.Piehler J, Schreiber G. J Mol Biol. 1999;289:57–67. doi: 10.1006/jmbi.1999.2726. [DOI] [PubMed] [Google Scholar]
- 17.Schoner B E, Belagaje R M, Schoner R G. Methods Enzymol. 1990;185:94–103. doi: 10.1016/0076-6879(90)85010-l. [DOI] [PubMed] [Google Scholar]
- 18.Gill S C, von Hippel P H. Anal Biochem. 1989;182:319–326. doi: 10.1016/0003-2697(89)90602-7. , and erratum (1990) 189, 283. [DOI] [PubMed] [Google Scholar]
- 19.Merrifield R B, Bach A E. J Org Chem. 1978;43:4808–4816. [Google Scholar]
- 20.Striebel C, Brecht A, Gauglitz G. Biosens Bioelectron. 1994;9:139–146. doi: 10.1016/0956-5663(94)80105-3. [DOI] [PubMed] [Google Scholar]
- 21.Piehler J, Brecht A, Geckeler K E, Gauglitz G. Biosens Bioelectron. 1996;11:579–590. doi: 10.1016/0956-5663(96)83293-3. [DOI] [PubMed] [Google Scholar]
- 22.Rubinstein S, Familletti P C, Pestka S. J Virol. 1981;37:755–758. doi: 10.1128/jvi.37.2.755-758.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Means G A, Feeney R E. Chemical Modification of Proteins. San Francisco: Holden–Day; 1971. [Google Scholar]
- 24.Gershonov E, Goldwasser I, Fridkin M, Shechter Y. J Med Chem. 2000;43:2530–2537. doi: 10.1021/jm990533m. [DOI] [PubMed] [Google Scholar]
- 25.Duckworth W C. Endocr Rev. 1988;9:319–345. doi: 10.1210/edrv-9-3-319. [DOI] [PubMed] [Google Scholar]