Abstract
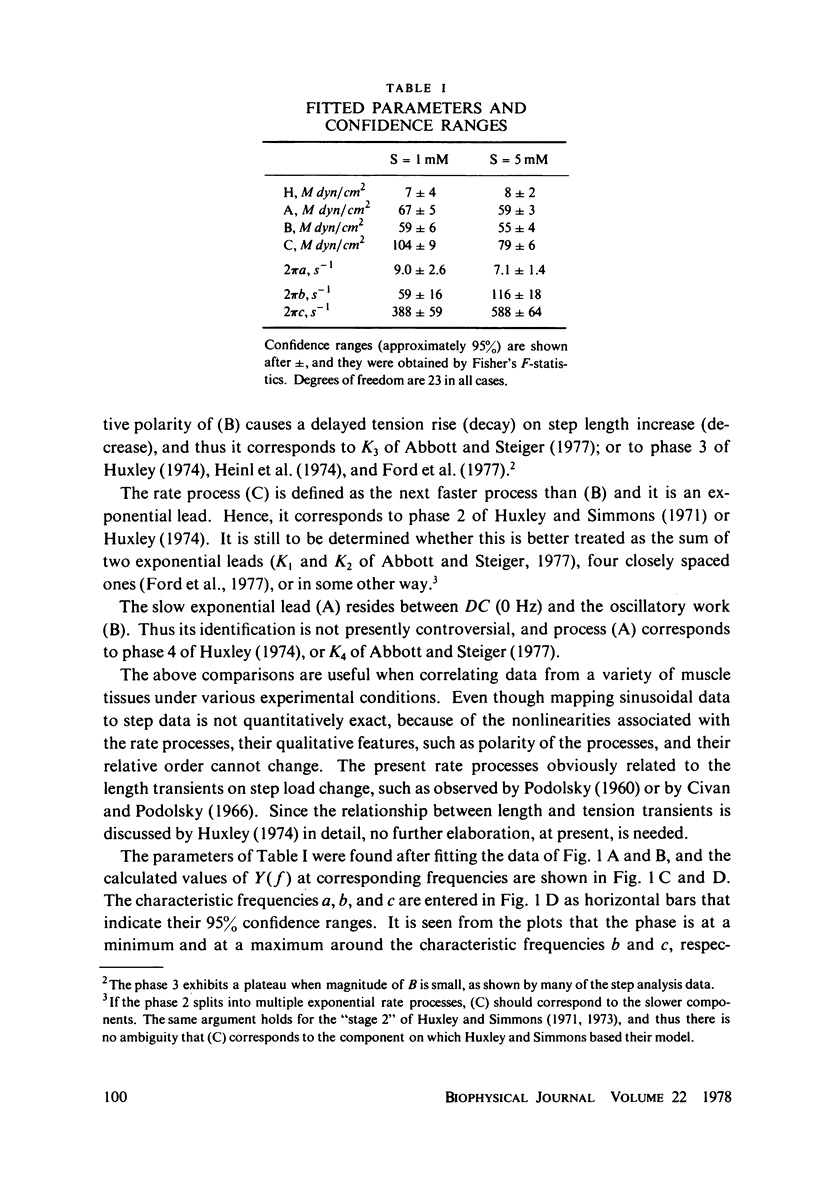
The mechanical response of fully activated muscle bundles (one to five fibers) to sinusoidal length perturbation (∼0.4% L0) was studied as a function of MgATP concentration. The frequency response (0.25-167 Hz; corresponding to 1 ms time resolution) of chemically skinned rabbit muscle fibers was resolved into three exponential rate processes, (A), (B), and (C). At 20°C, the apparent rate constants associated with the fast exponential lead (2πc = 388-588 s-1) and the oscillatory work (2πb = 59-116 s-1) both increase with increment of the MgATP concentration from 1 to 5 mM, and they both saturate for further increase. Over the whole range of MgATP concentrations the slow exponential lead (2πa = 9-7 s-1) remains constant. The effect of MgATP on processes (B) and (C) can be interpreted in the context of the biochemical evidence, in which MgATP enters the cross-bridge cycle after the desorption of the product, and the binding of MgATP to rigorlike cross-bridges promotes a rapid dissociation of actomyosin (Lymn and Taylor, 1971. Biochemistry. 10:4617-4624.). The effect is not predicted by a model for force generation in which head rotation dominates the fast component (“stage 2” of Huxley and Simmons, 1971. Nature (Lond.). 233:533-538. and 1973. Cold Spring Harbor Symp. Quant. Biol. 37:669-680.), and head dissociation dominates the slow component (“phase 4” of Huxley, 1974. J. Physiol. (Lond.). 243:1-43; Julian et al., 1974. Biophys. J. 14: 546-562.).
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chock S. P., Chock P. B., Eisenberg E. Pre-steady-state kinetic evidence for a cyclic interaction of myosin subfragment one with actin during the hydrolysis of adenosine 5'-triphosphate. Biochemistry. 1976 Jul 27;15(15):3244–3253. doi: 10.1021/bi00660a013. [DOI] [PubMed] [Google Scholar]
- Civan M. M., Podolsky R. J. Contraction kinetics of striated muscle fibres following quick changes in load. J Physiol. 1966 Jun;184(3):511–534. doi: 10.1113/jphysiol.1966.sp007929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol. 1977 Jul;269(2):441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinl P., Kuhn H. J., Rüegg J. C. Tension responses to quick length changes of glycerinated skeletal muscle fibres from the frog and tortoise. J Physiol. 1974 Mar;237(2):243–258. doi: 10.1113/jphysiol.1974.sp010480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley A. F. Muscular contraction. J Physiol. 1974 Nov;243(1):1–43. [PMC free article] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Julian F. J., Sollins K. R., Sollins M. R. A model for the transient and steady-state mechanical behavior of contracting muscle. Biophys J. 1974 Jul;14(7):546–562. doi: 10.1016/S0006-3495(74)85934-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M., Brandt P. W. Effectof MgATP on stiffness measured at two frequencies in Ca2+-activated muscle fibers. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4073–4075. doi: 10.1073/pnas.74.9.4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M., Brandt P., Orentlicher M. Dependence of energy transduction in intact skeletal muscles on the time in tension. Biophys J. 1977 May;18(2):161–172. doi: 10.1016/S0006-3495(77)85605-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M., Kuntz I. D. Optical diffraction studies of muscle fibers. Biophys J. 1973 Sep;13(9):857–876. doi: 10.1016/S0006-3495(73)86031-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymn R. W., Taylor E. W. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry. 1971 Dec 7;10(25):4617–4624. doi: 10.1021/bi00801a004. [DOI] [PubMed] [Google Scholar]
- PODOLSKY R. J. Kinetics of muscular contraction: the approach to the steady state. Nature. 1960 Nov 19;188:666–668. doi: 10.1038/188666a0. [DOI] [PubMed] [Google Scholar]
- Steiger G. J. Stretch activation and myogenic oscillation of isolated contractile structures of heart muscle. Pflugers Arch. 1971;330(4):347–361. doi: 10.1007/BF00588586. [DOI] [PubMed] [Google Scholar]
- White D. C., Thorson J. Phosphate starvation and the nonlinear dynamics of insect fibrillar flight muscle. J Gen Physiol. 1972 Sep;60(3):307–336. doi: 10.1085/jgp.60.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood D. S., Zollman J., Reuben J. P., Brandt P. W. Human skeletal muscle: properties of the "chemically skinned%" fiber. Science. 1975 Mar 21;187(4181):1075–1076. doi: 10.1126/science.187.4181.1075. [DOI] [PubMed] [Google Scholar]



