Abstract
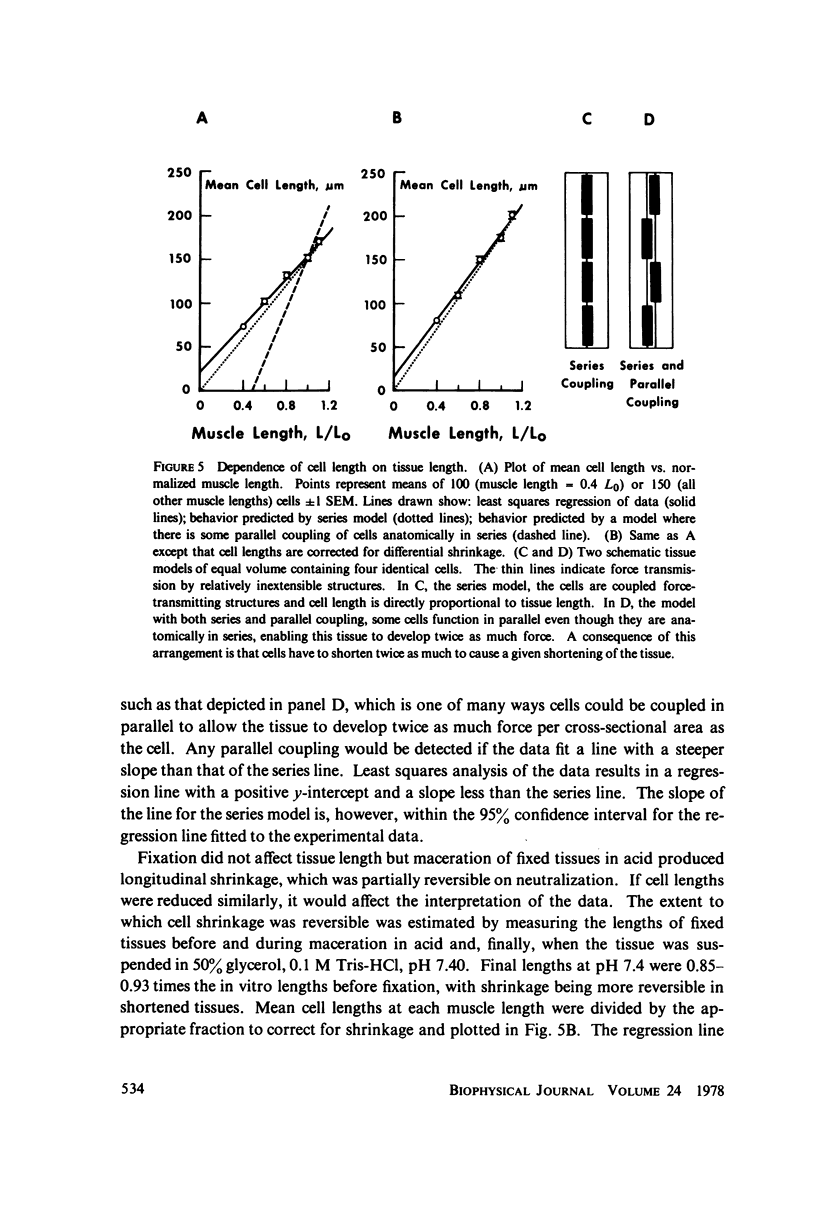
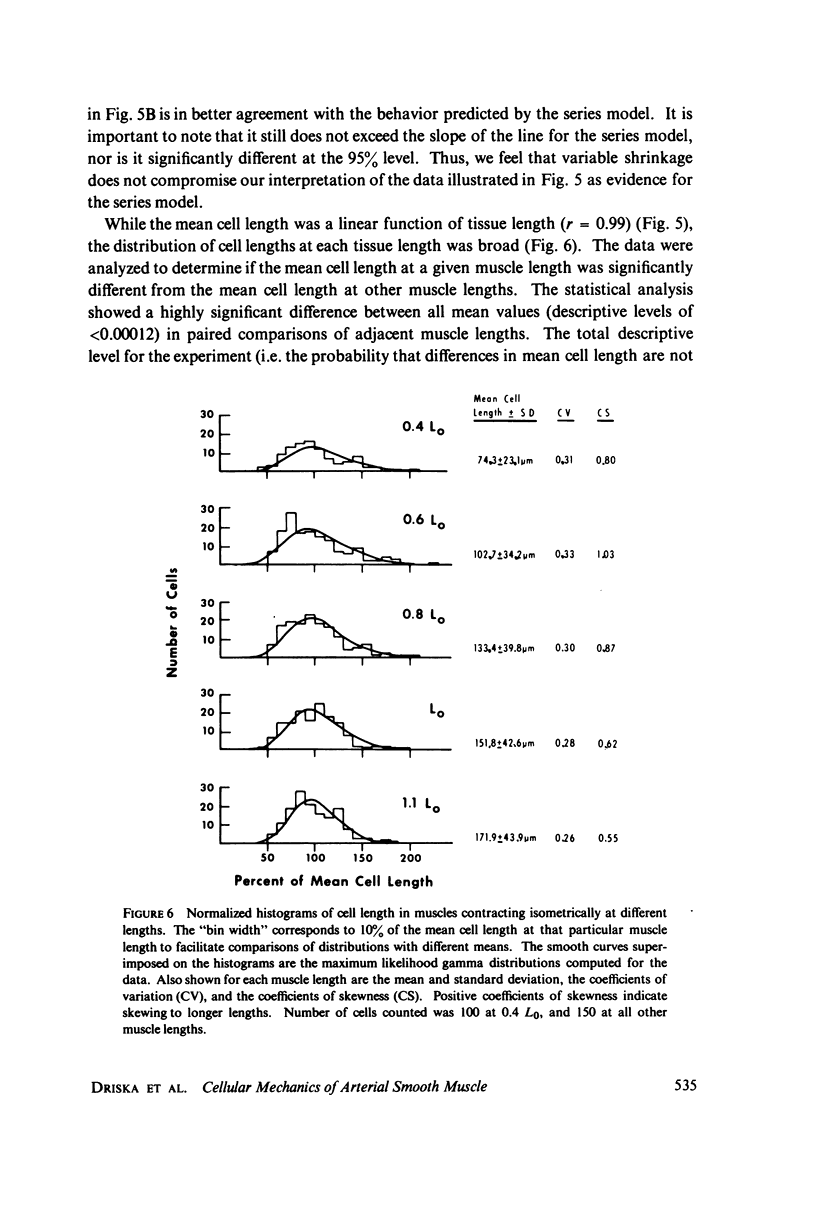
Estimates of force generation or shortening obtained from smooth muscle tissues are valid for individual cells only if each cell is contracting homogeneously and if cells anatomically arranged in series are mechanically coupled. These two assumptions were tested and shown to be valid for the pig carotid media under certain conditions. Homogeneity of cellular responses in carotid strips was estimated from the motion of markers on the tissue during K+ -induced isometric contractions. When tissues were stretched to L0 (the optimum length for force generation), there was little marker movement on stimulation. However, considerable marker movement was observed on stimulation at shorter muscle lengths, reflecting localized shortening or stretching. The mechanical coupling of the very small cells in the media was determined by measuring the dependence of cell length on tissue length. Tissues were fixed with glutaraldehyde during isometric contractions at various tissue lengths (0.4--1.1 x L0). The fixed tissues were macerated with acid and the lengths of the dispersed cells were measured. Cell lengths were broadly distributed at all muscle lengths. However, the direct proportionality between mean cell length and muscle length (as a fraction of L0) indicated that cells which are anatomically in series are coupled force-transmitting structures. We conclude that valid estimates of cellular mechanical function in this preparation can be obtained from tissue measurements at lengths greater than about 0.9L0.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arborgh B., Bell P., Brunk U., Collins V. P. The osmotic effect of glutaraldehyde during fixation. A transmission electron microscopy, scanning electron microscopy and cytochemical study. J Ultrastruct Res. 1976 Sep;56(3):339–350. doi: 10.1016/s0022-5320(76)90009-5. [DOI] [PubMed] [Google Scholar]
- Ashton F. T., Somlyo A. V., Somlyo A. P. The contractile apparatus of vascular smooth muscle: intermediate high voltage stereo electron microscopy. J Mol Biol. 1975 Oct 15;98(1):17–29. doi: 10.1016/s0022-2836(75)80098-2. [DOI] [PubMed] [Google Scholar]
- BURTON A. C. Relation of structure to function of the tissues of the wall of blood vessels. Physiol Rev. 1954 Oct;34(4):619–642. doi: 10.1152/physrev.1954.34.4.619. [DOI] [PubMed] [Google Scholar]
- Bagby R. M. Time course of isotonic contraction in single cells and muscle strips from Bufo marinus stomach. Am J Physiol. 1974 Oct;227(4):789–793. doi: 10.1152/ajplegacy.1974.227.4.789. [DOI] [PubMed] [Google Scholar]
- Close R. I. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972 Jan;52(1):129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- Cooke P. H., Fay F. S. Correlation between fiber length, ultrastructure, and the length-tension relationship of mammalian smooth muscle. J Cell Biol. 1972 Jan;52(1):105–116. doi: 10.1083/jcb.52.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driska S. P., Murphy R. A. Estimate of cellular force generation in an arterial smooth muscle with a high actin: myosin ratio. Blood Vessels. 1978;15(1-3):26–32. doi: 10.1159/000158150. [DOI] [PubMed] [Google Scholar]
- Fay F. S., Singer J. J. Characteristics of response of isolated smooth muscle cells to cholinergic drugs. Am J Physiol. 1977 Mar;232(3):C144–C154. doi: 10.1152/ajpcell.1977.232.3.C144. [DOI] [PubMed] [Google Scholar]
- Gabella G. The force generated by a visceral smooth muscle. J Physiol. 1976 Dec;263(2):199–213. doi: 10.1113/jphysiol.1976.sp011628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Halpern W., Mulvany M. J., Warshaw D. M. Mechanical properties of smooth muscle cells in the walls of arterial resistance vessels. J Physiol. 1978 Feb;275:85–101. doi: 10.1113/jphysiol.1978.sp012179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlihy J. T., Murphy R. A. Force-velocity and series elastic characteristics of smooth muscle from the hog carotid artery. Circ Res. 1974 Apr;34(4):461–466. doi: 10.1161/01.res.34.4.461. [DOI] [PubMed] [Google Scholar]
- Herlihy J. T., Murphy R. A. Length-tension relationship of smooth muscle of the hog carotid artery. Circ Res. 1973 Sep;33(3):275–283. doi: 10.1161/01.res.33.3.275. [DOI] [PubMed] [Google Scholar]
- JEWELL B. R., WILKIE D. R. An analysis of the mechanical components in frog's striated muscle. J Physiol. 1958 Oct 31;143(3):515–540. doi: 10.1113/jphysiol.1958.sp006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiss R. A. Dynamic stiffness of rabbit mesotubarium smooth muscle: effect of isometric length. Am J Physiol. 1978 Jan;234(1):C14–C26. doi: 10.1152/ajpcell.1978.234.1.C14. [DOI] [PubMed] [Google Scholar]
- Murphy R. A. Contractile system function in mammalian smooth muscle. Blood Vessels. 1976;13(1-2):1–23. doi: 10.1159/000158076. [DOI] [PubMed] [Google Scholar]
- Murphy R. A., Herlihy J. T., Megerman J. Force-generating capacity and contractile protein content of arterial smooth muscle. J Gen Physiol. 1974 Dec;64(6):691–705. doi: 10.1085/jgp.64.6.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSENBLUTH J. SMOOTH MUSCLE: AN ULTRASTRUCTURAL BASIS FOR THE DYNAMIC OF ITS CONTRACTION. Science. 1965 Jun 4;148(3675):1337–1339. doi: 10.1126/science.148.3675.1337. [DOI] [PubMed] [Google Scholar]
- Small J. V. Studies on isolated smooth muscle cells: The contractile apparatus. J Cell Sci. 1977 Apr;24:327–349. doi: 10.1242/jcs.24.1.327. [DOI] [PubMed] [Google Scholar]
- Uvelius B. Isometric and isotonic length-tension relations and variaitonsin cell length in longitudinal smooth muscel from rabbit urinary bladder. Acta Physiol Scand. 1976 Mar;97(1):1–12. doi: 10.1111/j.1748-1716.1976.tb10230.x. [DOI] [PubMed] [Google Scholar]



