Abstract
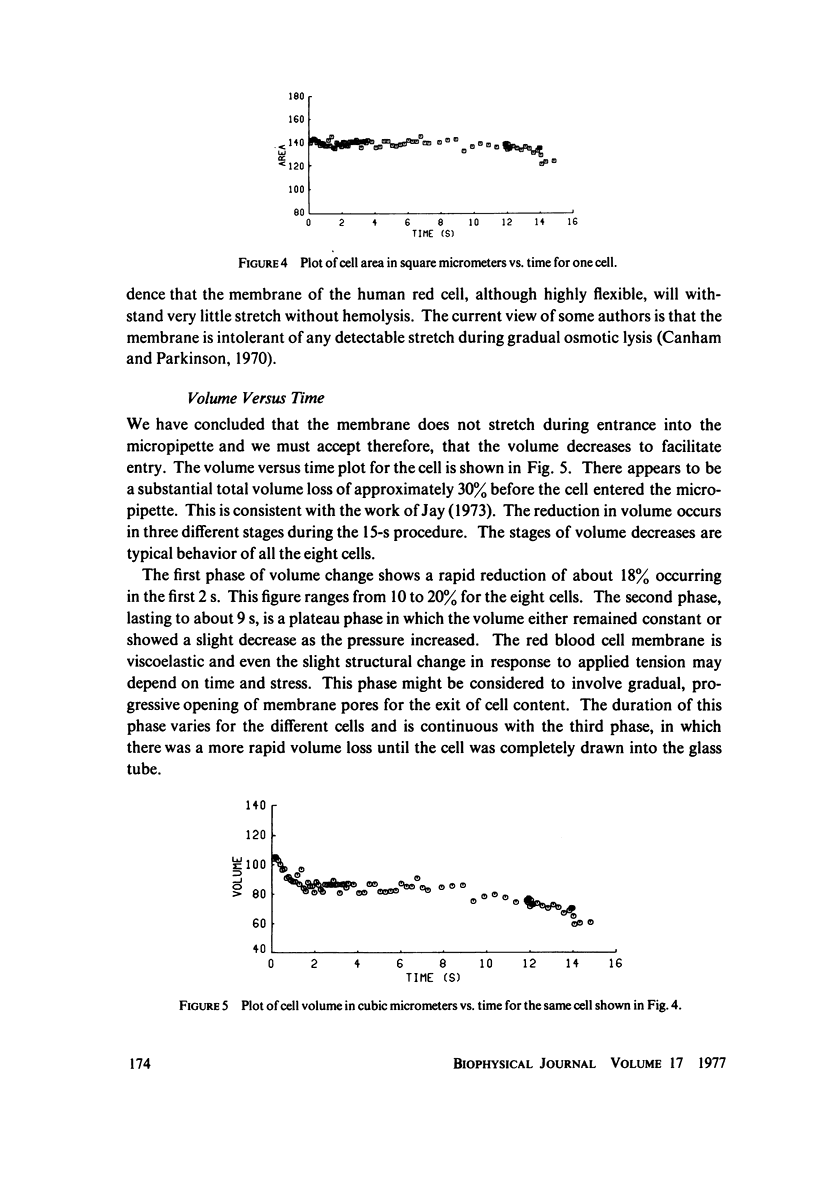
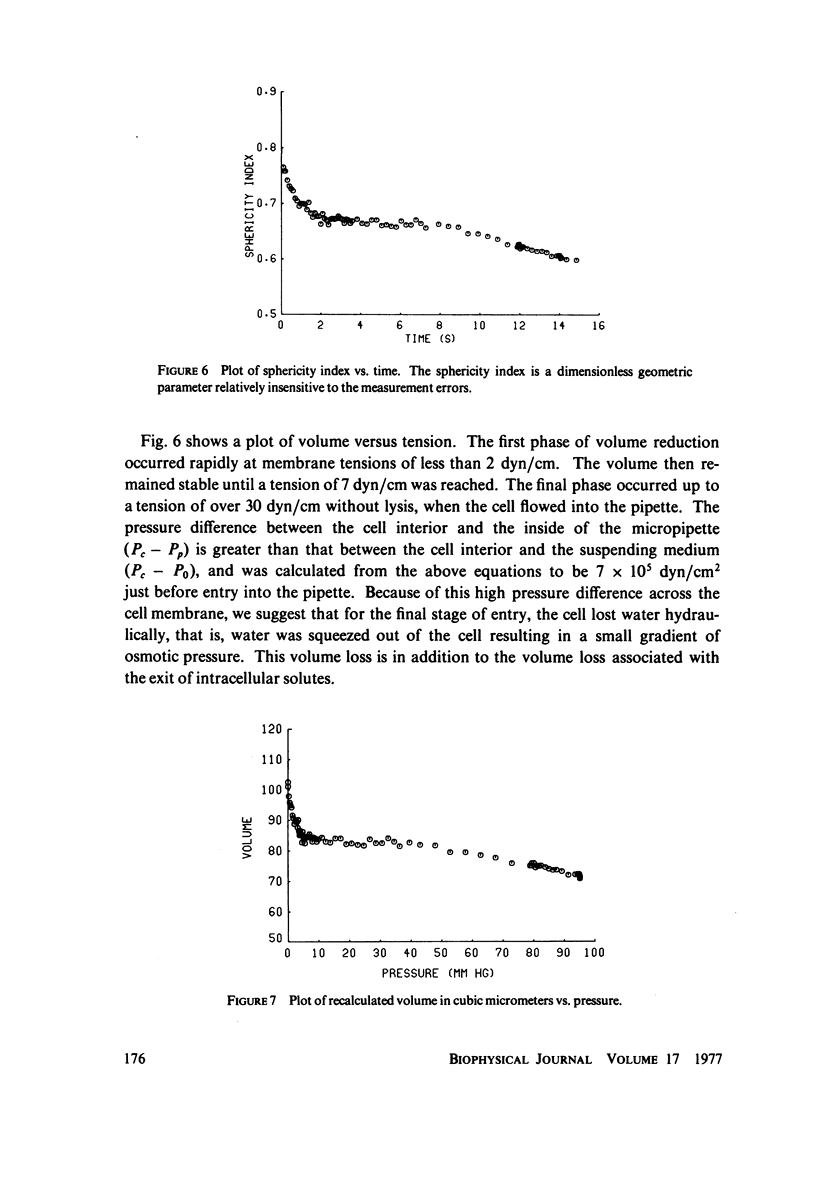
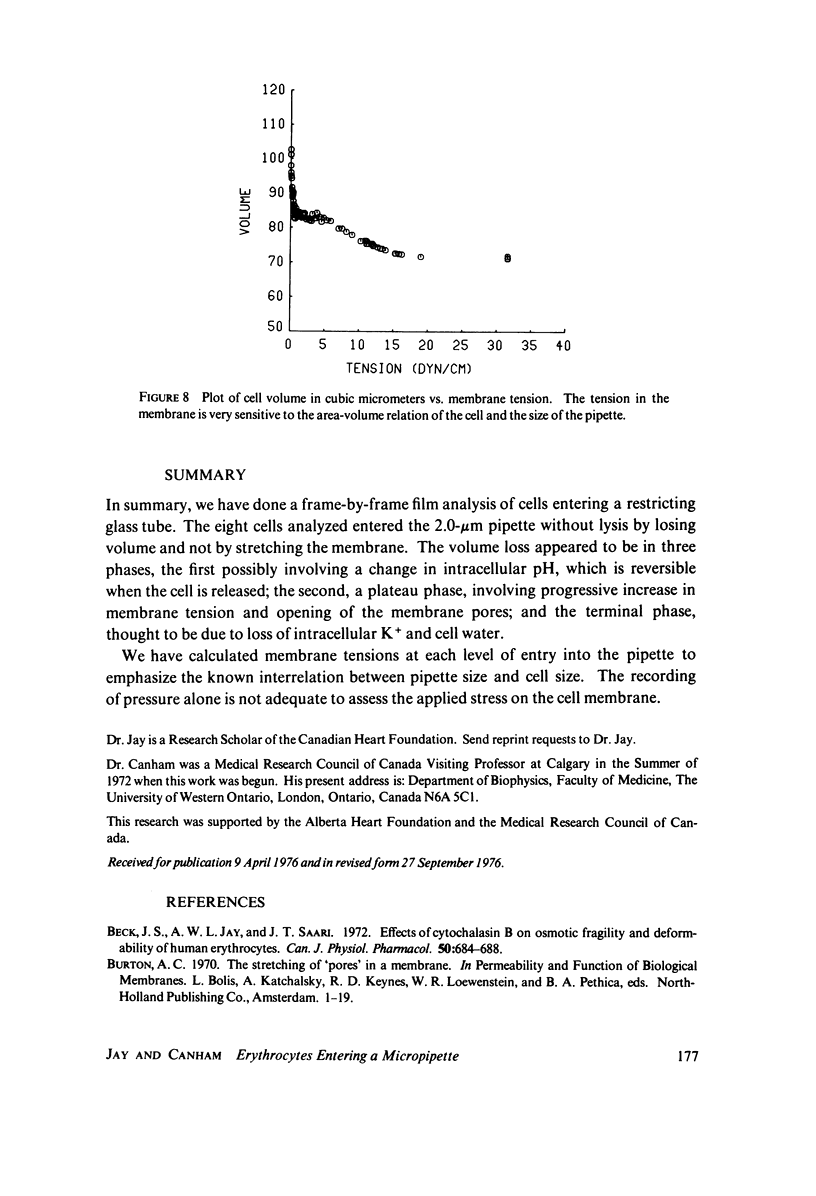
Previous work demonstrated that human red cells can be drawn into cylindrical glass micropipettes of internal diameter approximately 2.0 mum without lysing. For pipettes of less than approximately 2.9 mum inside diameter, the red cell must become less spherical, that is, reduce its volume-to-area ratio. In this work measurements were made from 16-mm film records that allowed the determination of the cellular area and volume of individual erythrocytes as they were drawn into a 2.0-mum pipette with negative pressures. The results showed that the total surface area of the membrane remains constant and that the cell endures the passage into the pipette by losing volume. The volume loss was interpreted to be due to cell water and solute loss when the membrane is under stress. The loss of cell volume, rather than the stretching of the membrane, adds confirmation that although it is very deformable, the membrane is very resistant to two-dimensional strain.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck J. S., Jay A. W., Saari J. T. Effects of cytochalasin B on osmotic fragility and deformability of human erythrocytes. Can J Physiol Pharmacol. 1972 Jul;50(7):684–688. doi: 10.1139/y72-099. [DOI] [PubMed] [Google Scholar]
- Canham P. B., Burton A. C. Distribution of size and shape in populations of normal human red cells. Circ Res. 1968 Mar;22(3):405–422. doi: 10.1161/01.res.22.3.405. [DOI] [PubMed] [Google Scholar]
- Canham P. B., Parkinson D. R. The area and volume of single human erythrocytes during gradual osmotic swelling to hemolysis. Can J Physiol Pharmacol. 1970 Jun;48(6):369–376. doi: 10.1139/y70-059. [DOI] [PubMed] [Google Scholar]
- Chien S., Luse S. A., Bryant C. A. Hemolysis during filtration through micropores: a scanning electron microscopic and hemorheologic correlation. Microvasc Res. 1971 Apr;3(2):183–203. doi: 10.1016/0026-2862(71)90022-7. [DOI] [PubMed] [Google Scholar]
- Evans E. A., Hochmuth R. M. Membrane viscoelasticity. Biophys J. 1976 Jan;16(1):1–11. doi: 10.1016/S0006-3495(76)85658-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A., La Celle P. L. Intrinsic material properties of the erythrocyte membrane indicated by mechanical analysis of deformation. Blood. 1975 Jan;45(1):29–43. [PubMed] [Google Scholar]
- Evans E. A. New membrane concept applied to the analysis of fluid shear- and micropipette-deformed red blood cells. Biophys J. 1973 Sep;13(9):941–954. doi: 10.1016/S0006-3495(73)86036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregersen M. I., Bryant C. A., Hammerle W. E., Usami S., Chien S. Flow Characteristics of Human Erythrocytes through Polycarbonate Sieves. Science. 1967 Aug 18;157(3790):825–827. doi: 10.1126/science.157.3790.825. [DOI] [PubMed] [Google Scholar]
- Jay A. W. Geometry of the human erythrocyte. I. Effect of albumin on cell geometry. Biophys J. 1975 Mar;15(3):205–222. doi: 10.1016/S0006-3495(75)85812-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay A. W., Rowlands S. The stages of osmotic haemolysis. J Physiol. 1975 Nov;252(3):817–832. doi: 10.1113/jphysiol.1975.sp011172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay A. W. Viscoelastic properties of the human red blood cell membrane. I. Deformation, volume loss, and rupture of red cells in micropipettes. Biophys J. 1973 Nov;13(11):1166–1182. doi: 10.1016/S0006-3495(73)86053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaCelle P. L. Effect of sphering on erythrocyte deformability. Biorheology. 1972 Jun;9(2):51–59. doi: 10.3233/bir-1972-9202. [DOI] [PubMed] [Google Scholar]
- MacGregor R. D., 2nd, Tobias C. A. Molecular sieving of red cell membranes during gradual osmotic hemolysis. J Membr Biol. 1972 Dec 29;10(3):345–356. doi: 10.1007/BF01867865. [DOI] [PubMed] [Google Scholar]
- RAND R. P., BURTON A. C. MECHANICAL PROPERTIES OF THE RED CELL MEMBRANE. I. MEMBRANE STIFFNESS AND INTRACELLULAR PRESSURE. Biophys J. 1964 Mar;4:115–135. doi: 10.1016/s0006-3495(64)86773-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAND R. P. MECHANICAL PROPERTIES OF THE RED CELL MEMBRANE. II. VISCOELASTIC BREAKDOWN OF THE MEMBRANE. Biophys J. 1964 Jul;4:303–316. doi: 10.1016/s0006-3495(64)86784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P., Sauks T., Argent W., Kwant W. O. The effect of membrane-strain rate and of temperature on erythrocyte fragility and critical hemolytic volume. Biochim Biophys Acta. 1969;183(3):476–489. doi: 10.1016/0005-2736(69)90162-x. [DOI] [PubMed] [Google Scholar]
- Seeman P. Transient holes in the erythrocyte membrane during hypotonic hemolysis and stable holes in the membrane after lysis by saponin and lysolecithin. J Cell Biol. 1967 Jan;32(1):55–70. doi: 10.1083/jcb.32.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed R. I., LaCelle P. L., Merrill E. W. Metabolic dependence of red cell deformability. J Clin Invest. 1969 May;48(5):795–809. doi: 10.1172/JCI106038. [DOI] [PMC free article] [PubMed] [Google Scholar]