Abstract
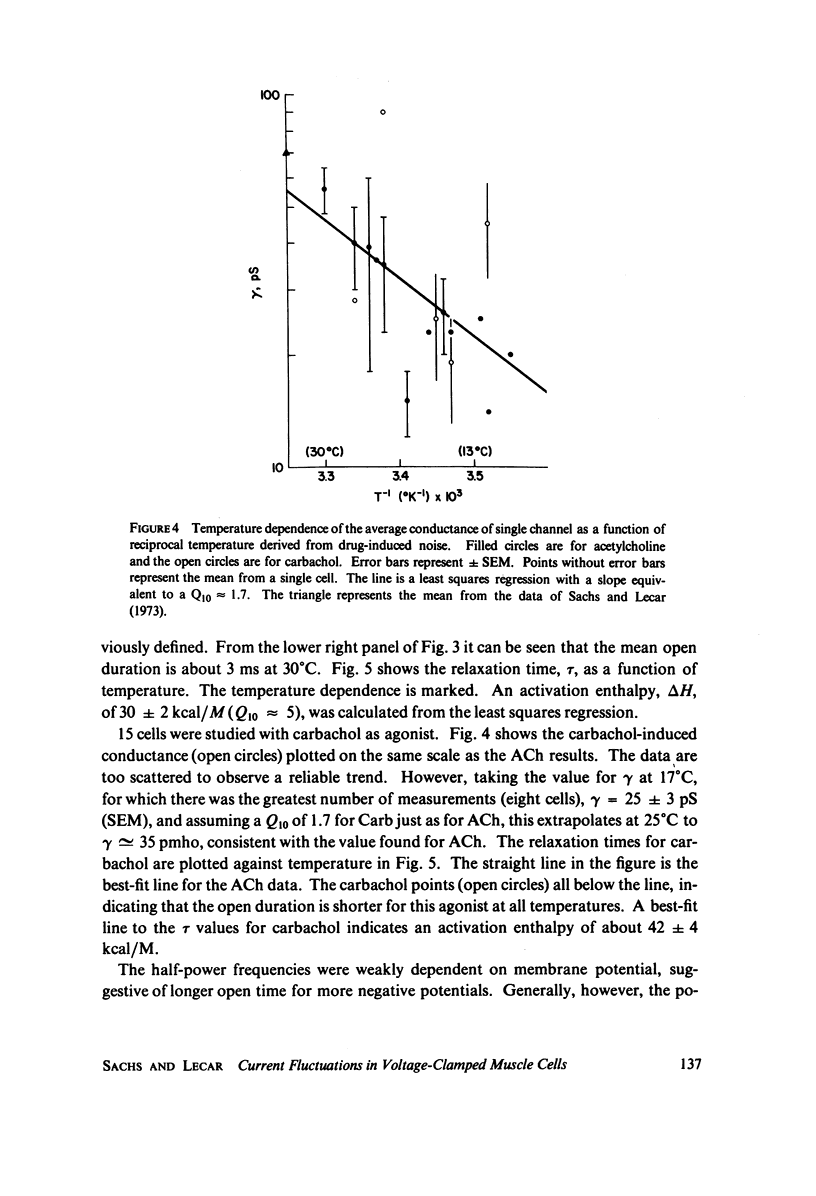
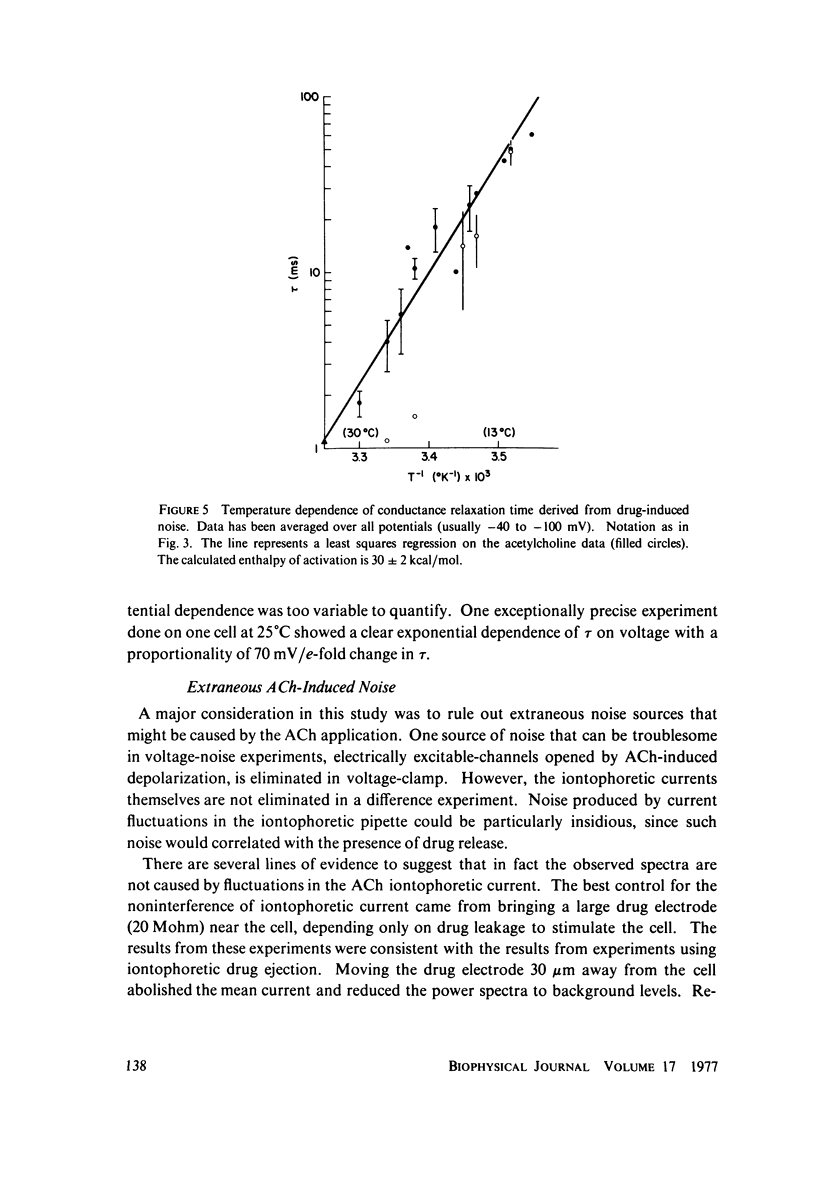
Acetylcholine applied ionophoretically to chick skeletal muscle cells grown in tissue culture produces membrane current fluctuations. Cells treated with vinblastine are transformed to a roughly spherical shape. Such transformed cells can be voltage-clamped with microelectrodes. The frequency spectrum of the current fluctuations at fixed voltage obeys a relation of the Lorentz form. From analysis of the current noise, the conductance of a single ionic channel is estimated to be 39 pmho at a temperature of 28 degrees C, and increases with increasing temperature, exhibiting a Q10 of 1.7. The relaxation time for the channel conductance is more sharply temperature dependent, showing a Q10 of approximately 5. These results are in agreement with the picture of acetylcholine-activated ionic channels determined from experiments on frog end plate (Anderson and Stevens, 1973). The relaxation time for carbachol activation is shorter than for acetylcholine, and appears to be more temperature sensitive.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. R., Cull-Candy S. G., Miledi R. Glutamate and quisqualate noise in voltage-clamped locust muscle fibres. Nature. 1976 May 13;261(5556):151–153. doi: 10.1038/261151a0. [DOI] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. N., Graubard K. Fluorescent staining of cat motoneurons in vivo with beveled micropipettes. Brain Res. 1970 Mar 17;18(3):565–568. doi: 10.1016/0006-8993(70)90143-5. [DOI] [PubMed] [Google Scholar]
- Bean R. C., Shepherd W. C., Chan H., Eichner J. Discrete conductance fluctuations in lipid bilayer protein membranes. J Gen Physiol. 1969 Jun;53(6):741–757. doi: 10.1085/jgp.53.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A. Sodium transport by the acetylcholine receptor of cultured muscle cells. J Biol Chem. 1975 Mar 10;250(5):1776–1781. [PubMed] [Google Scholar]
- Colburn T. R., Schwartz E. A. Linear voltage control of current passed through a micropipette with variable resistance. Med Biol Eng. 1972 Jul;10(4):504–509. doi: 10.1007/BF02474198. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Dionne V. E., Steinbach J. H., Stevens C. F. Conductance of channels opened by acetylcholine-like drugs in muscle end-plate. Nature. 1975 Jan 17;253(5488):204–206. doi: 10.1038/253204a0. [DOI] [PubMed] [Google Scholar]
- Conti F., De Felice L. J., Wanke E. Potassium and sodium ion current noise in the membrane of the squid giant axon. J Physiol. 1975 Jun;248(1):45–82. doi: 10.1113/jphysiol.1975.sp010962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelice L. J., Firth D. R. Spontaneous voltage fluctuations in glass microelectrodes. IEEE Trans Biomed Eng. 1971 Sep;18(5):339–351. doi: 10.1109/tbme.1971.4502865. [DOI] [PubMed] [Google Scholar]
- Ehrenstein G., Lecar H., Nossal R. The nature of the negative resistance in bimolecular lipid membranes containing excitability-inducing material. J Gen Physiol. 1970 Jan;55(1):119–133. doi: 10.1085/jgp.55.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda J., Fischbach G. D., Smith T. G., Jr A voltage clamp study of the sodium, calcium and chloride spikes of chick skeletal muscle cells grown in tissue culture. Dev Biol. 1976 Apr;49(2):412–424. doi: 10.1016/0012-1606(76)90184-6. [DOI] [PubMed] [Google Scholar]
- Fukuda J., Henkart M. P., Fischbach G. D., Smith T. G., Jr Physiological and structural properties of colchicine-treated chick skeletal muscle cells grown in tissue culture. Dev Biol. 1976 Apr;49(2):395–411. doi: 10.1016/0012-1606(76)90183-4. [DOI] [PubMed] [Google Scholar]
- HUXLEY A. F. Ion movements during nerve activity. Ann N Y Acad Sci. 1959 Aug 28;81:221–246. doi: 10.1111/j.1749-6632.1959.tb49311.x. [DOI] [PubMed] [Google Scholar]
- Hagins W. A. Electrical signs of information flow in photoreceptors. Cold Spring Harb Symp Quant Biol. 1965;30:403–418. doi: 10.1101/sqb.1965.030.01.040. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The characteristics of 'end-plate noise' produced by different depolarizing drugs. J Physiol. 1973 May;230(3):707–717. doi: 10.1113/jphysiol.1973.sp010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lass Y., Fischbach G. D. A discontinuous relationship between the acetylcholine-activated channel conductance and temperature. Nature. 1976 Sep 9;263(5573):150–151. doi: 10.1038/263150a0. [DOI] [PubMed] [Google Scholar]
- Latorre R., Alvarez O., Verdugo P. Temperature characterization of the conductance of the excitability inducing material channel in oxidized cholesterol membranes. Biochim Biophys Acta. 1974 Nov 15;367(3):361–365. doi: 10.1016/0005-2736(74)90092-3. [DOI] [PubMed] [Google Scholar]
- Lecar H., Ehrenstein G., Latorre R. Mechanism for channel gating in excitable bilayers. Ann N Y Acad Sci. 1975 Dec 30;264:304–313. doi: 10.1111/j.1749-6632.1975.tb31491.x. [DOI] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 1976 Apr 29;260(5554):799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Voltage-dependence of drug-induced conductance in frog neuromuscular junction. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2140–2144. doi: 10.1073/pnas.72.6.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson P. G. Nerve and muscle cells in culture. Physiol Rev. 1975 Jan;55(1):1–61. doi: 10.1152/physrev.1975.55.1.1. [DOI] [PubMed] [Google Scholar]
- Ritchie A. K., Fambrough D. M. Ionic properties of the acetylcholine receptor in cultured rat myotubes. J Gen Physiol. 1975 Jun;65(6):751–767. doi: 10.1085/jgp.65.6.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs F., Lecar H. Acetylcholine noise in tissue culture muscle cells. Nat New Biol. 1973 Dec 19;246(155):214–216. doi: 10.1038/newbio246214a0. [DOI] [PubMed] [Google Scholar]
- Sytkowski A. J., Vogel Z., Nirenberg M. W. Development of acetylcholine receptor clusters on cultured muscle cells. Proc Natl Acad Sci U S A. 1973 Jan;70(1):270–274. doi: 10.1073/pnas.70.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]



