Abstract
We have analyzed the decrease in synthesis of individual size classes of heterogeneous nuclear RNA (hnRNA) in ultraviolet (UV)-irradiated Merwin plasmacytoma (MPC-11) cells at various times of postirradiation incubation. HnRNA from nonirradiated control cells is distributed over a wide range from approximately 60S to 5S, with 42S RNA carrying more label than any other size class. HnRNA from UV-irradiated cells shows a dose-dependent shift in size distribution toward lower molecular weight. The size distribution of hnRNA synthesized after prolonged times of postirradiation incubation is restored toward normal, i.e., synthesis of long RNA molecules increases relative to the synthesis of short ones. Analysis of the total number of hnRNA chains synthesized during a 20-min [3H]uridine pulse shows a considerable reduction in their number with increasing UV dose. Murine cell lines are excision-repair-deficient but capable of post replication repair inhibited by caffeine. HnRNA transcripts of cells incubated in its presence were studied. The caffeine, which has no effect on hnRNA size in control cells, inhibits to a considerable extent the restoration of full-length transcripts during postirradiation incubation. The lack of excision repair in MPC-11 was confirmed by the analysis of pyrimidine dimers in trichloracetic acid-insoluble and soluble fractions within 8 h of postirradiation incubation.
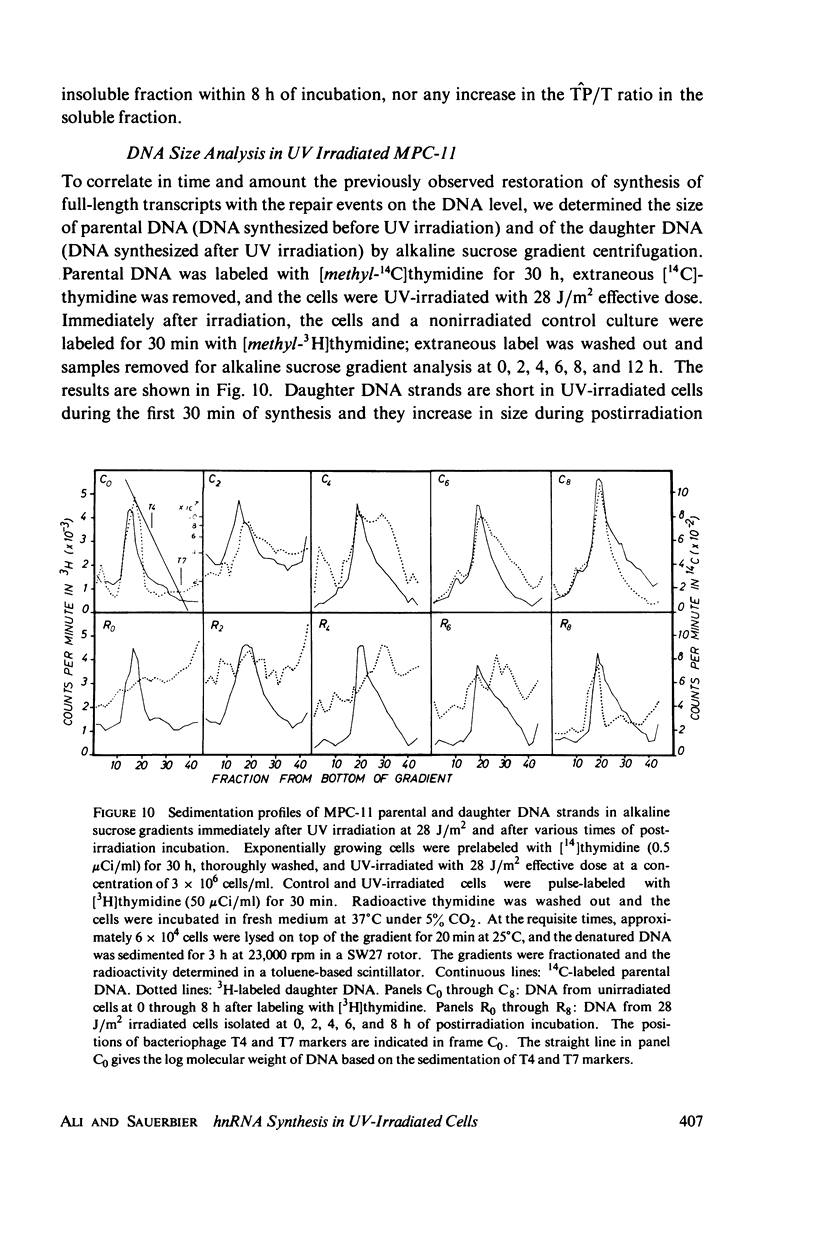
The size of parental and daughter strand DNA in UV-irradiated cells was correlated with RNA transcript size. The parental DNA in these experiments does not change its size as a consequence of UV exposure and postirradiation incubation. In contrast, daughter DNA strands are short in UV-irradiated cells and they increase in size during postirradiation incubation to reach the size of parental strands after 8 h.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali R., Millette R., Sauerbier W. End group of naturally terminated and UV lesion terminated T7 in vitro RNA. Nucleic Acids Res. 1976 Dec;3(12):3359–3367. doi: 10.1093/nar/3.12.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoshechkin A. G. Chromosomal damage in Chinese hamster cells grown in U.V.-irradiated medium. Photochem Photobiol. 1970 Jan;11(1):49–52. doi: 10.1111/j.1751-1097.1970.tb05716.x. [DOI] [PubMed] [Google Scholar]
- Buhl S. N., Regan J. D. Effect of caffeine on postreplication repair in human cells. Biophys J. 1974 Jul;14(7):519–527. doi: 10.1016/S0006-3495(74)85932-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl S. N., Setlow R. B., Regan J. D. Recovery of the ability to synthesize DNA in segments of normal size at long times after ultraviolet irradiation of human cells. Biophys J. 1973 Dec;13(12):1265–1275. doi: 10.1016/S0006-3495(73)86061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl S. N., Setlow R. B., Regan J. D. Steps in DNA chain elongation and joining after ultra-violet irradiation of human cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1972 Nov;22(5):417–424. doi: 10.1080/09553007214551301. [DOI] [PubMed] [Google Scholar]
- Buhl S. N., Stillman R. M., Setlow R. B., Regan J. D. DNA chain elongation and joining in normal human and xeroderma pigmentosum cells after ultraviolet irradiation. Biophys J. 1972 Sep;12(9):1183–1191. doi: 10.1016/S0006-3495(72)86154-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J. M., Hewitt R. R. Significance of dimers to the size of newly synthesized DNA in UV-irradiated Chinese hamster ovary cells. Biophys J. 1976 Oct;16(10):1155–1164. doi: 10.1016/S0006-3495(76)85764-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver J. E. Repair replication of mammalian cell DNA: effects of compounds that inhibit DNA synthesis or dark repair. Radiat Res. 1969 Feb;37(2):334–348. [PubMed] [Google Scholar]
- Cleaver J. E., Thomas G. H. Single strand interruptions in DNA and the effects of caffeine in Chinese hamster cells irradiated with ultraviolet light. Biochem Biophys Res Commun. 1969 Jul 23;36(2):203–208. doi: 10.1016/0006-291x(69)90315-5. [DOI] [PubMed] [Google Scholar]
- Edenberg H. J., Hanawalt P. C. The timecourse of DNA repair replication in ultraviolet-irradiated HeLa cells. Biochim Biophys Acta. 1973 Oct 12;324(2):206–217. doi: 10.1016/0005-2787(73)90138-x. [DOI] [PubMed] [Google Scholar]
- Giorno R., Sauerbier W. A radiological analysis of the transcription units for heterogeneous nuclear RNA in cultured murine cells. Cell. 1976 Dec;9(4 Pt 2):775–783. doi: 10.1016/0092-8674(76)90140-9. [DOI] [PubMed] [Google Scholar]
- Hackett P. B., Sauerbier W. Radiological mapping of the ribosomal RNA transcription unit in E. coli. Nature. 1974 Oct 18;251(5476):639–641. doi: 10.1038/251639a0. [DOI] [PubMed] [Google Scholar]
- Hackett P. B., Sauerbier W. The transcriptional organization of the ribosomal RNA genes in mouse L cells. J Mol Biol. 1975 Jan 25;91(3):235–256. doi: 10.1016/0022-2836(75)90378-2. [DOI] [PubMed] [Google Scholar]
- Hercules K., Jovanovich S., Sauerbrier W. Early gene expression in bacteriophage T7. I. In vivo synthesis, inactivation, and translational utilization of early mRNA's. J Virol. 1976 Feb;17(2):642–658. doi: 10.1128/jvi.17.2.642-658.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klímek M. Thymine dimerization in L-strain mammalian cells after irradiation with ultraviolet light and the search for repair mechanisms. Photochem Photobiol. 1966 Aug;5(8):603–607. doi: 10.1111/j.1751-1097.1966.tb05806.x. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R. Post-replication repair of DNA in ultraviolet-irradiated mammalian cells. No gaps in DNA synthesized late after ultraviolet irradiation. Eur J Biochem. 1972 Dec 18;31(3):438–445. doi: 10.1111/j.1432-1033.1972.tb02550.x. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R. Postreplication repair of DNA in ultraviolet-irradiated mammalian cells. J Mol Biol. 1972 May 28;66(3):319–337. doi: 10.1016/0022-2836(72)90418-4. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath R. A., Williams R. W. Reconstruction in vivo of irradiated Escherichia coli deoxyribonucleic acid; the rejoining of broken pieces. Nature. 1966 Oct 29;212(5061):534–535. doi: 10.1038/212534a0. [DOI] [PubMed] [Google Scholar]
- Meneghini R. Gaps in DNA synthesized by ultraviolet light-irradiated WI38 human cells. Biochim Biophys Acta. 1976 Apr 2;425(4):419–427. doi: 10.1016/0005-2787(76)90006-x. [DOI] [PubMed] [Google Scholar]
- Meneghini R., Hanawalt P. T4-endonuclease V-sensitive sites in DNA from ultraviolet-irradiated human cells. Biochim Biophys Acta. 1976 Apr 2;425(4):428–437. doi: 10.1016/0005-2787(76)90007-1. [DOI] [PubMed] [Google Scholar]
- Meyn R. E., Hewitt R. R., Thomas L. F., Humphrey R. M. Effects of ultraviolet irradiation on the rate and sequence of DNA replication in synchronized Chinese hamster cells. Biophys J. 1976 May;16(5):517–525. doi: 10.1016/S0006-3495(76)85706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyn R. E., Humphrey R. M. Deoxyribonucleic acid synthesis in ultraviolet-light-irradiated Chinese hamster cells. Biophys J. 1971 Mar;11(3):295–301. doi: 10.1016/S0006-3495(71)86215-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalke H., Bremer H. RNA synthesis in Escherichia coli after irradiation with ultraviolet light. J Mol Biol. 1969 Apr 14;41(1):1–23. doi: 10.1016/0022-2836(69)90122-3. [DOI] [PubMed] [Google Scholar]
- Nilsson K., Lehmann A. R. The effect of methylated oxypurines on the size of newly-synthesized DNA and on the production of chromosome aberrations after UV irradiation in Chinese hamster cells. Mutat Res. 1975 Nov;30(2):255–266. [PubMed] [Google Scholar]
- Nocentini S. Inhibition and recovery of ribosomal RNA synthesis in ultraviolet-irradiation mammalian cells. Biochim Biophys Acta. 1976 Nov 12;454(1):114–128. doi: 10.1016/0005-2787(76)90359-2. [DOI] [PubMed] [Google Scholar]
- Okazaki R., Okazaki T., Sakabe K., Sugimoto K., Sugino A. Mechanism of DNA chain growth. I. Possible discontinuity and unusual secondary structure of newly synthesized chains. Proc Natl Acad Sci U S A. 1968 Feb;59(2):598–605. doi: 10.1073/pnas.59.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Inhibition of RNA synthesis by actinomycin D: characteristic dose-response of different RNA species. J Cell Physiol. 1970 Oct;76(2):127–139. doi: 10.1002/jcp.1040760202. [DOI] [PubMed] [Google Scholar]
- Povirk L. F., Painter R. B. Rate of DNA chain elongation in ultraviolet light-irradiated mammalian cells as estimated by a bromodeoxyuridine photolysis method. Biophys J. 1976 Aug;16(8):883–889. doi: 10.1016/S0006-3495(76)85738-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen R. E., Painter R. B. Radiation-stimulated DNA synthesis in cultured mammalian cells. J Cell Biol. 1966 Apr;29(1):11–19. doi: 10.1083/jcb.29.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan J. D., Trosko J. E., Carrier W. L. Evidence for excision of ultraviolet-induced pyrimidine dimers from the DNA of human cells in vitro. Biophys J. 1968 Mar;8(3):319–325. doi: 10.1016/S0006-3495(68)86490-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerbier W., Bräutigam A. R. A simple method for isolating RNA from bacteria. Biochim Biophys Acta. 1970 Jan 21;199(1):36–40. doi: 10.1016/0005-2787(70)90692-1. [DOI] [PubMed] [Google Scholar]
- Sauerbier W. Inhibition of host cell reactivation in phage T1 by caffeine. Biochem Biophys Res Commun. 1964;14:340–346. doi: 10.1016/s0006-291x(64)80007-3. [DOI] [PubMed] [Google Scholar]
- Sauerbier W., Millette R. L., Hackett P. B., Jr The effects of ultraviolet irradiation on the transcription of T4 DNA. Biochim Biophys Acta. 1970;209(2):368–386. doi: 10.1016/0005-2787(70)90735-5. [DOI] [PubMed] [Google Scholar]
- WULFF D. L. THE ROLE OF THYMINE DIMER IN THE PHOTO-INACTIVATION OF THE BACTERIOPHAGE T4V1. J Mol Biol. 1963 Oct;7:431–441. doi: 10.1016/s0022-2836(63)80035-2. [DOI] [PubMed] [Google Scholar]
- Walker I. G., Sridhar R. The formation and repair of single-strand breaks in DNA of cultured mammalian cells treated with UV-light, methylating agents or 4-nitroquinoline-1-oxide. Chem Biol Interact. 1976 Mar;12(3-4):229–239. doi: 10.1016/0009-2797(76)90039-9. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


