Abstract
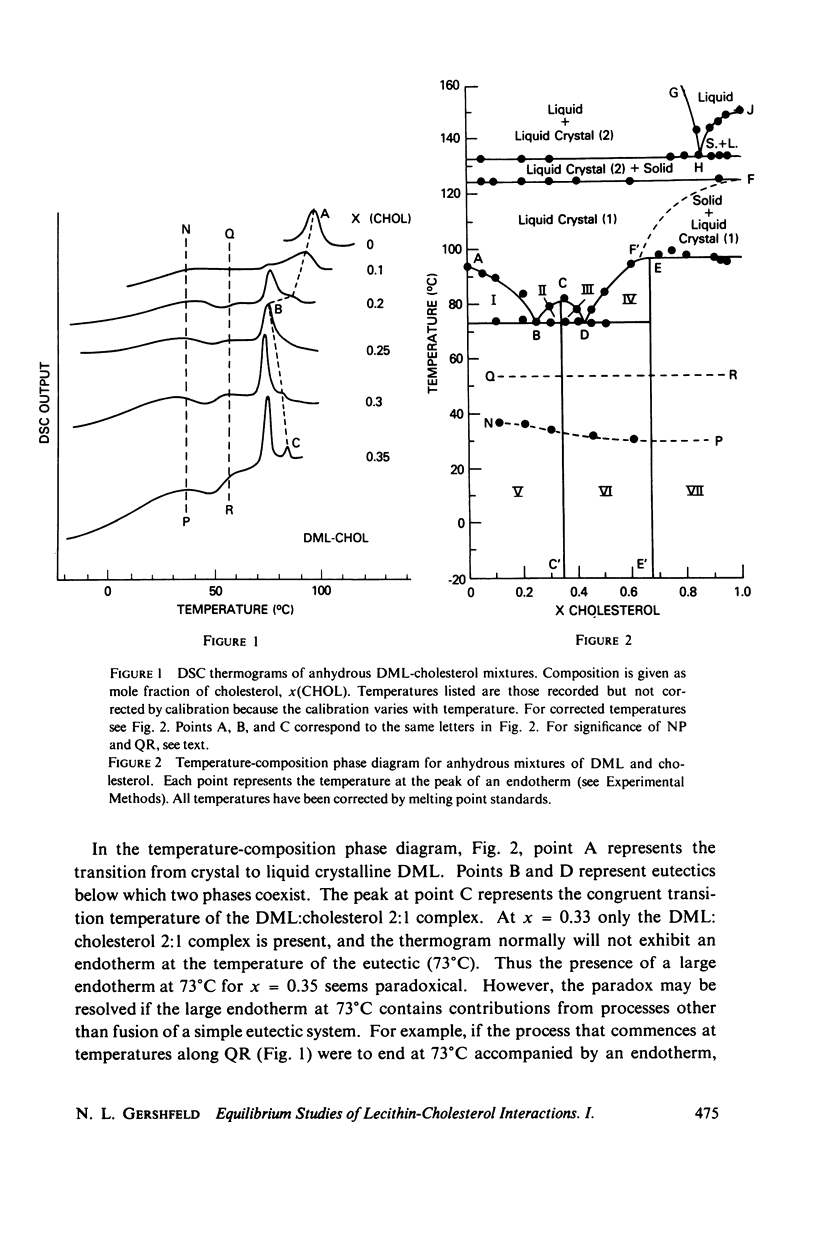
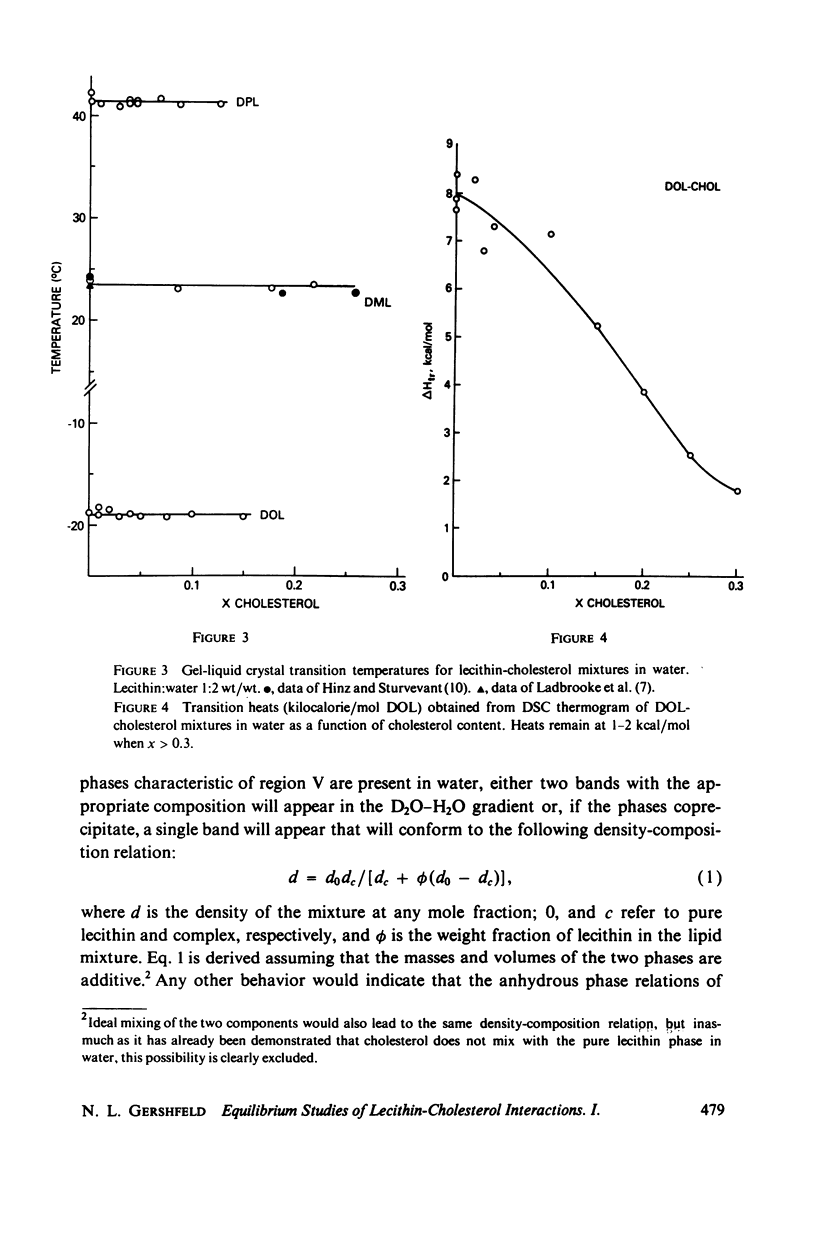
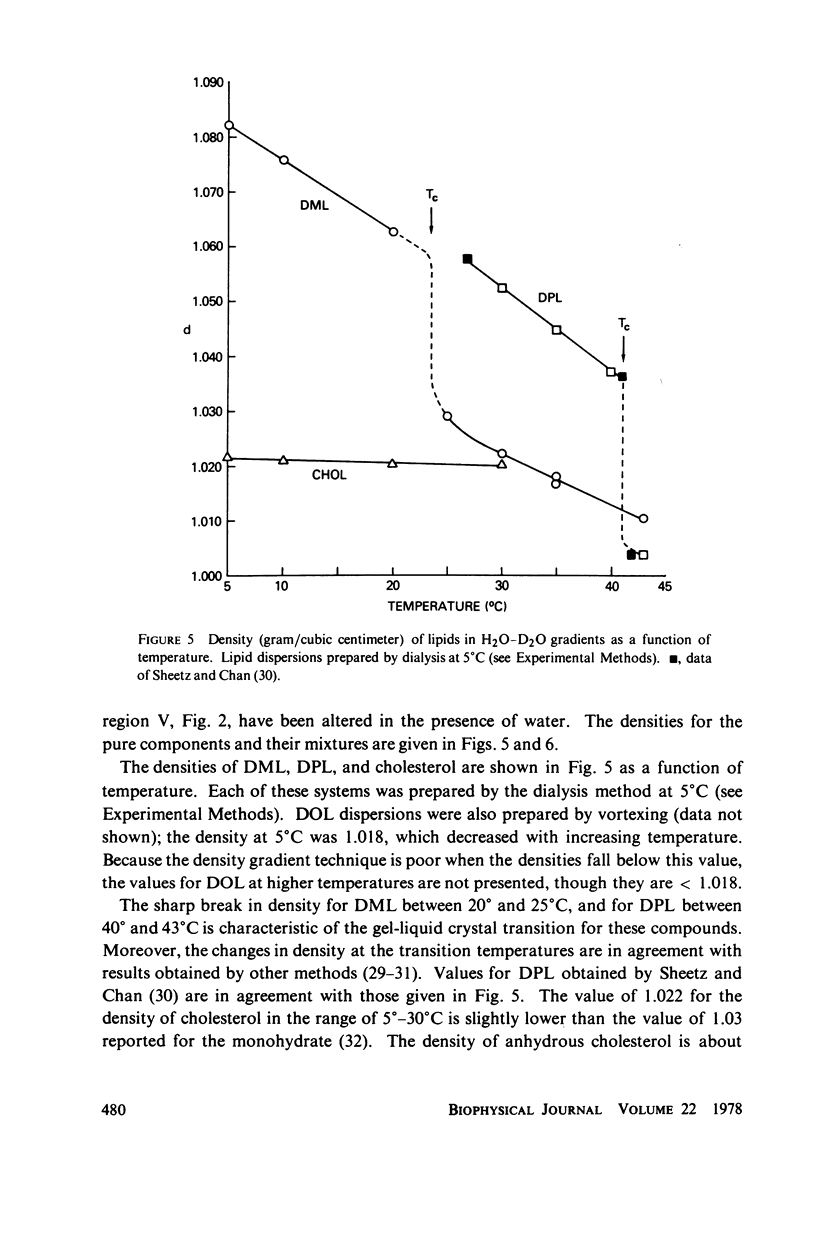
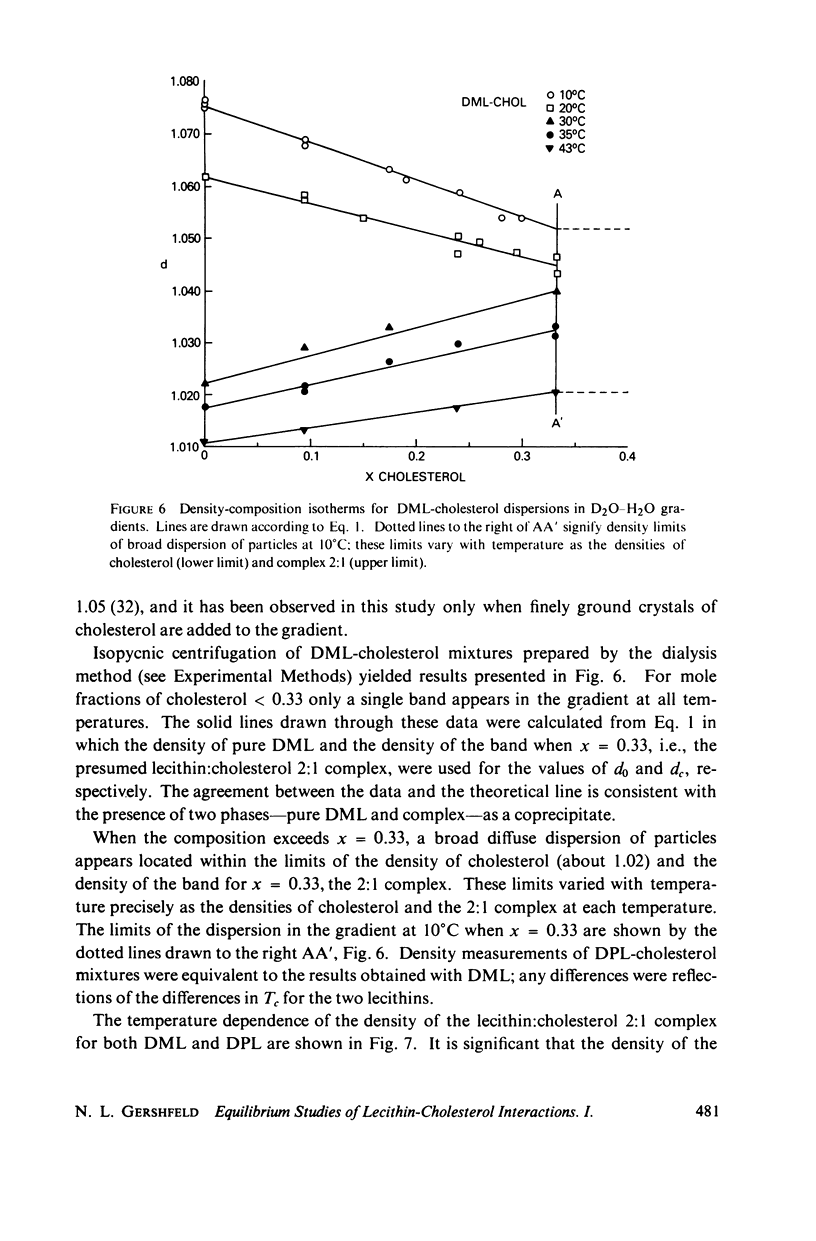
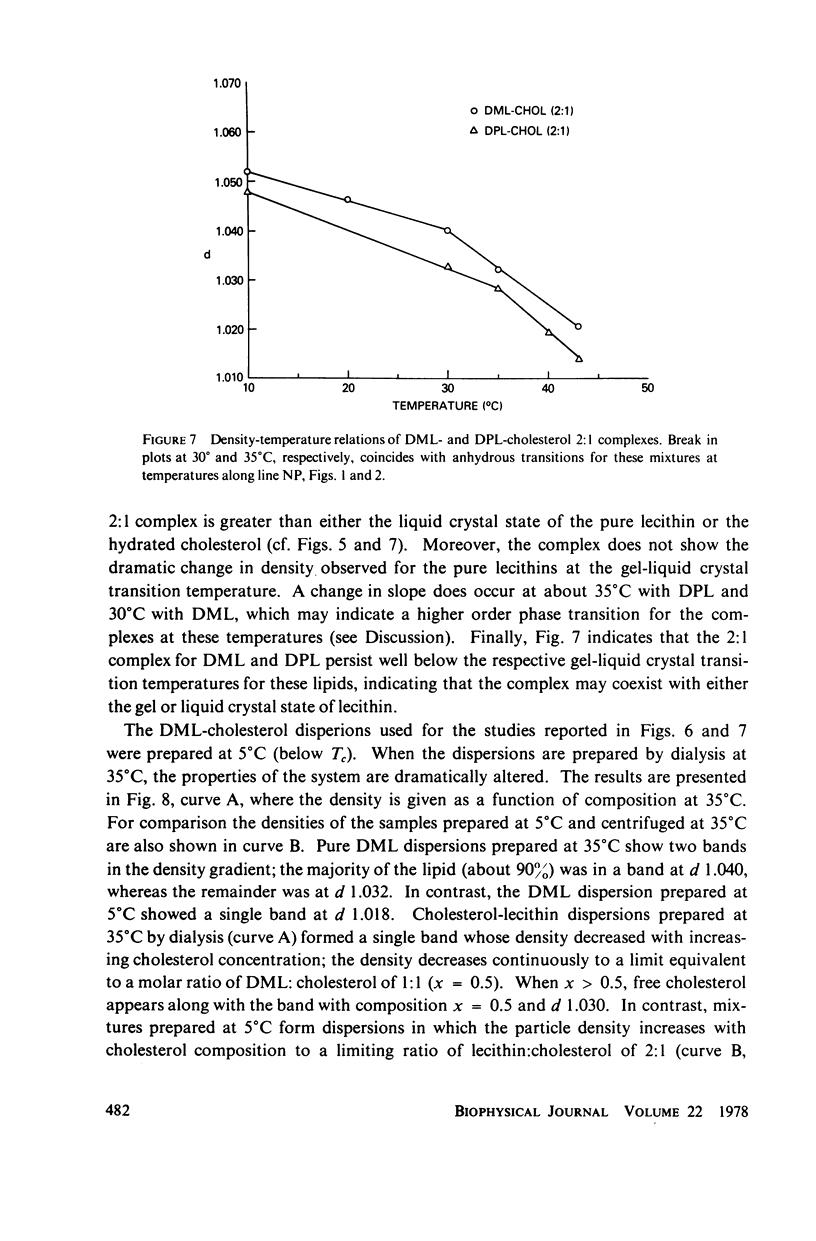
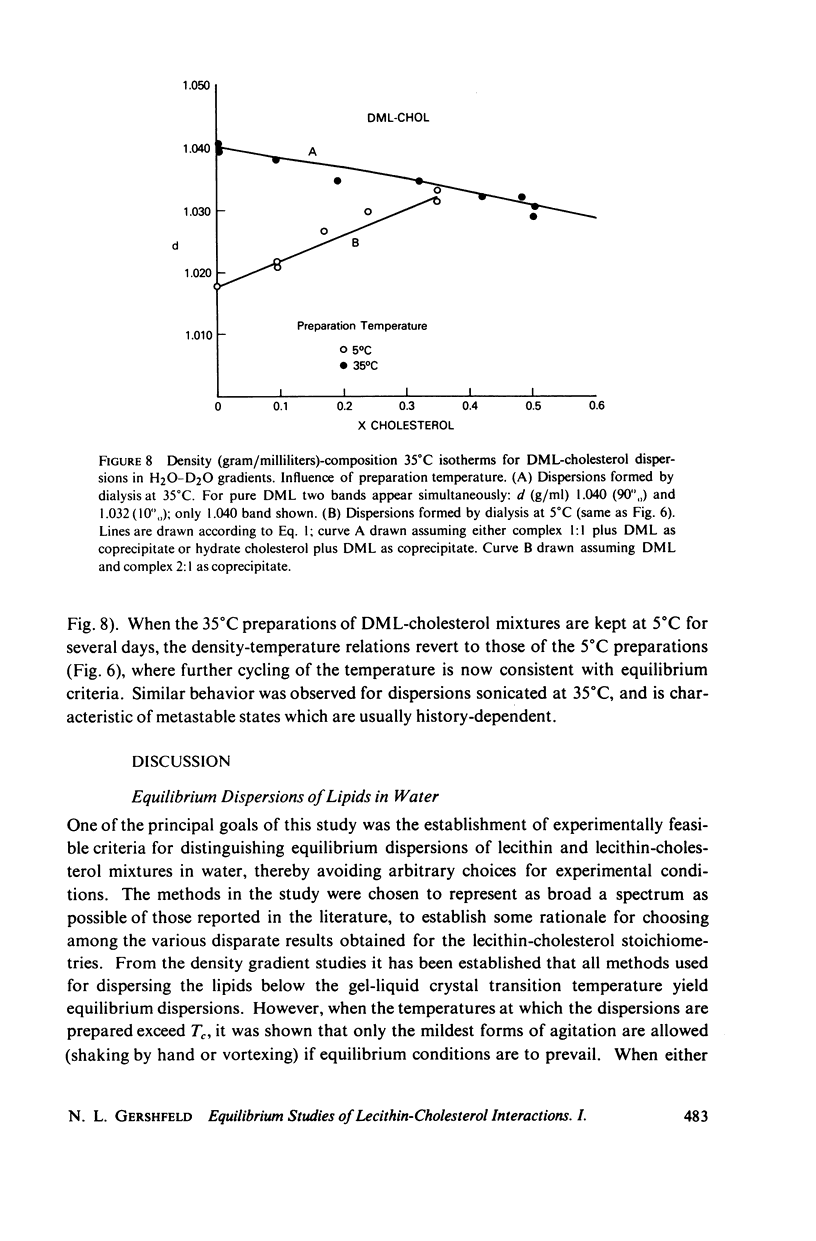
The maximum molar ratio of lecithin:cholesterol in aqueous dispersions has been reported to be 2:1, 1:1, or 1:2. The source of the desparate results has been examined in this study by analyzing (a) the phase relations in anhydrous mixtures (from which most dispersions are prepared) and (b) various methods of preparing aqueous dispersions, with the purpose of avoiding the formation of metastable states that may be responsible for the variability of the lecithin-cholesterol stoichiometry. Temperature-composition phase diagrams for anhydrous mixtures of cholesterol (CHOL) with dimyristoyl (DML) and with dipalmitoyl (DPL) lecithin were obtained by differential scanning calorimetry (DSC). Complexes form with molar ratios for lecithin:CHOL of 2:1 and 1:2; they are stable up to 70°C. When x(CHOL) < 0.33, two phases coexist: complex (2:1) plus pure lecithin; when 0.33 < x(CHOL) < 0.67 complexes (2:1) and (1:2) coexist as separate phases. The corresponding phase diagram in water for these mixtures was determined by DSC and isopycnic centrifugation in D2O-H2O gradients. Aqueous dispersions were prepared by various methods (vortexing, dialysis, sonication) yielding identical results except as noted below. The data presented supports the following phase relations. When x(CHOL) < 0.33, two lipid phases coexist: pure lecithin plus complex (2:1) where the properties of the lecithin phase are determined by whether the temperature is below or above Tc, the gel-liquid crystal transition temperature. Therefore, complex (2:1) will coexist with gel state below Tc and with liquid crystal above Tc. The densities follow in the order gel > complex (2:1) > liquid crystal. The density of complex (2:1) is less sensitive to temperature in the range 5°-45°C compared to the temperature dependence for DML and DPL where large changes in density occur at Tc. When x(CHOL) > 0.33, CHOL phase coexists with complex (2:1); anhydrous complex (1:2) is apparently not stable in H2O. The results are independent of the method and temperature used for preparing the lipid dispersions. However, when dispersions are prepared by sonication or with solvents at T > Tc, an apparent 1:1 complex is formed. Evidence suggests the 1:1 complex is metastable.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batzri S., Korn E. D. Single bilayer liposomes prepared without sonication. Biochim Biophys Acta. 1973 Apr 16;298(4):1015–1019. doi: 10.1016/0005-2736(73)90408-2. [DOI] [PubMed] [Google Scholar]
- Bruckdorfer K. R., Graham J. M., Green C. The incorporation of steroid molecules into lecithin sols, beta-lipoproteins and cellular membranes. Eur J Biochem. 1968 May;4(4):512–518. doi: 10.1111/j.1432-1033.1968.tb00242.x. [DOI] [PubMed] [Google Scholar]
- Bunow M. R., Levin I. W. Vibrational Raman spectra of lipid systems containing amphotericin B. Biochim Biophys Acta. 1977 Jan 4;464(1):202–216. doi: 10.1016/0005-2736(77)90382-0. [DOI] [PubMed] [Google Scholar]
- Chapman D., Collin D. T. Differential thermal analysis of phospholipids. Nature. 1965 Apr 10;206(980):189–189. doi: 10.1038/206189a0. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Gelmann E. P. Physical properties of membrane lipids: biological relevance and regulation. Bacteriol Rev. 1975 Sep;39(3):232–256. doi: 10.1128/br.39.3.232-256.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darke A., Finer E. G., Flook A. G., Phillips M. C. Complex and cluster formation in mixed lecithincholesterol bilayers. Cooperativity of motion in lipid systems. FEBS Lett. 1971 Nov 1;18(2):326–330. doi: 10.1016/0014-5793(71)80478-7. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Rothman J. E. The planar organization of lecithin-cholesterol bilayers. J Biol Chem. 1972 Jun 10;247(11):3694–3697. [PubMed] [Google Scholar]
- Finean J. B. Cholesterol: lecithin association at molecular ratios of up to 2 : 1. Chem Phys Lipids. 1975 Aug;14(4):313–320. doi: 10.1016/0009-3084(75)90067-5. [DOI] [PubMed] [Google Scholar]
- Hinz H. J., Sturtevant J. M. Calorimetric investigation of the influence of cholesterol on the transition properties of bilayers formed from synthetic L- -lecithins in aqueous suspension. J Biol Chem. 1972 Jun 10;247(11):3697–3700. [PubMed] [Google Scholar]
- Hinz H. J., Sturtevant J. M. Calorimetric studies of dilute aqueous suspensions of bilayers formed from synthetic L- -lecithins. J Biol Chem. 1972 Oct 10;247(19):6071–6075. [PubMed] [Google Scholar]
- Horwitz C., Krut L., Kaminsky L. Cholesterol uptake by egg-yolk phosphatidylcholine. Biochim Biophys Acta. 1971 Jul 13;239(2):329–336. doi: 10.1016/0005-2760(71)90178-0. [DOI] [PubMed] [Google Scholar]
- Huang C. Studies on phosphatidylcholine vesicles. Formation and physical characteristics. Biochemistry. 1969 Jan;8(1):344–352. doi: 10.1021/bi00829a048. [DOI] [PubMed] [Google Scholar]
- Kellaway I. W., Saunders L. The solubilization of some steroids by phosphatidyl choline and lysophosphatidyl choline. Biochim Biophys Acta. 1967 Aug 8;144(1):145–148. doi: 10.1016/0005-2760(67)90087-2. [DOI] [PubMed] [Google Scholar]
- Kroon P. A., Kainosho M., Chan S. I. State of molecular motion of cholesterol in lecithin bilayers. Nature. 1975 Aug 14;256(5518):582–584. doi: 10.1038/256582a0. [DOI] [PubMed] [Google Scholar]
- Ladbrooke B. D., Williams R. M., Chapman D. Studies on lecithin-cholesterol-water interactions by differential scanning calorimetry and X-ray diffraction. Biochim Biophys Acta. 1968 Apr 29;150(3):333–340. doi: 10.1016/0005-2736(68)90132-6. [DOI] [PubMed] [Google Scholar]
- Lecuyer H., Dervichian D. G. Structure of aqueous mixtures of lecithin and cholesterol. J Mol Biol. 1969 Oct 14;45(1):39–57. doi: 10.1016/0022-2836(69)90208-3. [DOI] [PubMed] [Google Scholar]
- Lippert J. L., Peticolas W. L. Laser Raman investigation of the effect of cholesterol on conformational changes in dipalmitoyl lecithin multilayers. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1572–1576. doi: 10.1073/pnas.68.7.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabrey S., Sturtevant J. M. Investigation of phase transitions of lipids and lipid mixtures by sensitivity differential scanning calorimetry. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3862–3866. doi: 10.1073/pnas.73.11.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior D. L., Morowitz H. J. Dilatometry of dilute suspensions of synthetic lecithin aggregates. Biochemistry. 1972 Nov 21;11(24):4558–4562. doi: 10.1021/bi00774a020. [DOI] [PubMed] [Google Scholar]
- Nagle J. F. Lipid bilayer phase transition: density measurements and theory. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3443–3444. doi: 10.1073/pnas.70.12.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield E., Chapman D. Dynamics of lipids in membranes: Heterogeneity and the role of cholesterol. FEBS Lett. 1972 Jul 1;23(3):285–297. doi: 10.1016/0014-5793(72)80300-4. [DOI] [PubMed] [Google Scholar]
- Opella S. J., Yesinowski J. P., Waugh J. S. Nuclear magnetic resonance description of molecular motion and phase separations of cholesterol in lecithin dispersions. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3812–3815. doi: 10.1073/pnas.73.11.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. C., Finer E. G. The stoichiometry and dynamics of lecithin-cholesterol clusters in bilayer membranes. Biochim Biophys Acta. 1974 Jul 31;356(2):199–206. doi: 10.1016/0005-2736(74)90283-1. [DOI] [PubMed] [Google Scholar]
- Phillips M. C., Hauser H., Paltauf F. The inter- and intra-molecular mixing of hydrocarbon chains in lecithin-water systems. Chem Phys Lipids. 1972 Mar;8(2):127–133. doi: 10.1016/0009-3084(72)90024-2. [DOI] [PubMed] [Google Scholar]
- Prestegard J. H., Fellmeth B. Fusion of dimyristoyllecithin vesicles as studied by proton magnetic resonance spectroscopy. Biochemistry. 1974 Mar 12;13(6):1122–1126. doi: 10.1021/bi00703a011. [DOI] [PubMed] [Google Scholar]
- Rouser G., Fkeischer S., Yamamoto A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970 May;5(5):494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Chan S. I. Effect of sonication on the structure of lecithin bilayers. Biochemistry. 1972 Nov 21;11(24):4573–4581. doi: 10.1021/bi00774a024. [DOI] [PubMed] [Google Scholar]
- Shimshick E. J., McConnell H. M. Lateral phase separations in binary mixtures of cholesterol and phospholipids. Biochem Biophys Res Commun. 1973 Jul 17;53(2):446–451. doi: 10.1016/0006-291x(73)90682-7. [DOI] [PubMed] [Google Scholar]
- Small D. M. Phase equilibria and structure of dry and hydrated egg lecithin. J Lipid Res. 1967 Nov;8(6):551–557. [PubMed] [Google Scholar]
- Tajima K., Gershfeld N. L. Equilibrium studies of lecithin-cholesterol interactions. II. Phase relations in surface films: analysis of the "condensing" effect of cholesterol. Biophys J. 1978 Jun;22(3):489–500. doi: 10.1016/S0006-3495(78)85501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeagle P. L., Hutton W. C., Huang C., Martin R. B. Phospholipid head-group conformations; intermolecular interactions and cholesterol effects. Biochemistry. 1977 Oct 4;16(20):4344–4349. doi: 10.1021/bi00639a003. [DOI] [PubMed] [Google Scholar]
- Zlatkis A., Zak B. Study of a new cholesterol reagent. Anal Biochem. 1969 Apr 11;29(1):143–148. doi: 10.1016/0003-2697(69)90017-7. [DOI] [PubMed] [Google Scholar]
- Zull J. E., Greanoff S., Adam H. K. Interaction of egg lecithin with cholesterol in the solid state. Biochemistry. 1968 Dec;7(12):4172–4176. doi: 10.1021/bi00852a005. [DOI] [PubMed] [Google Scholar]



