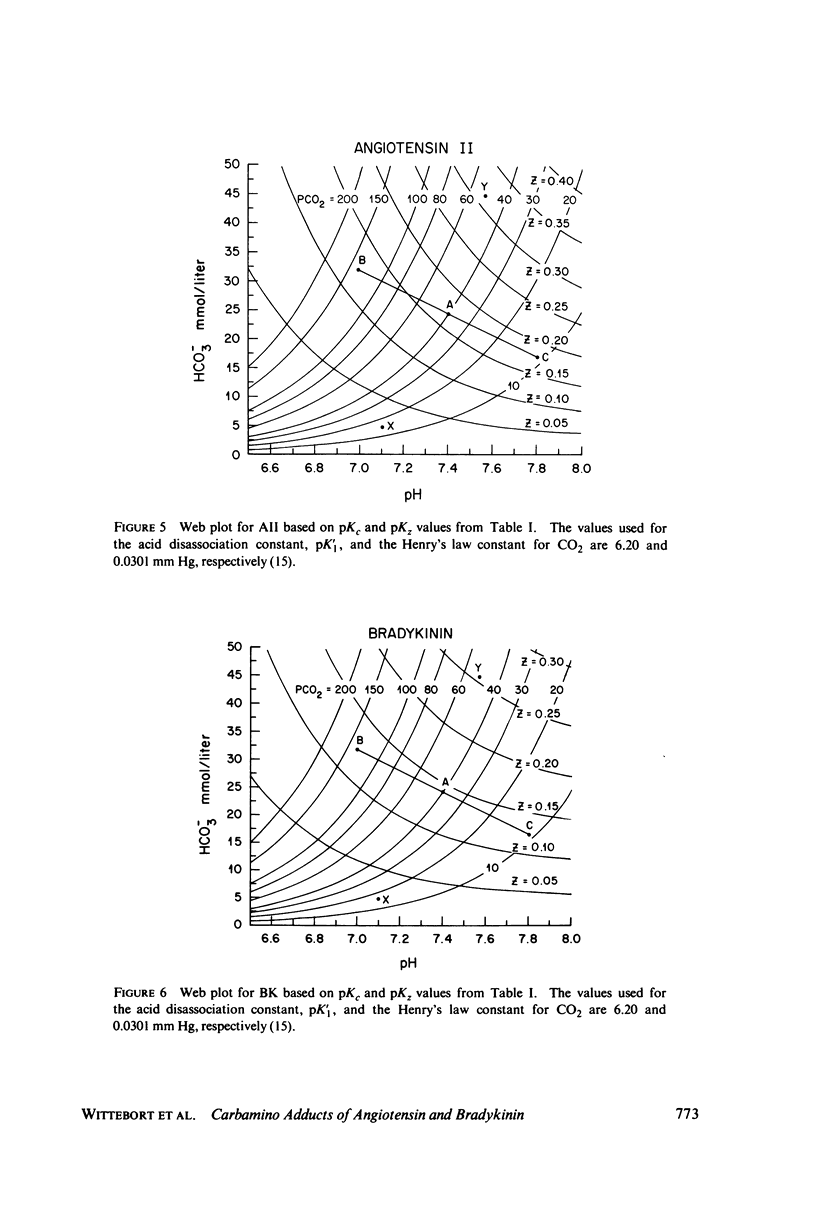
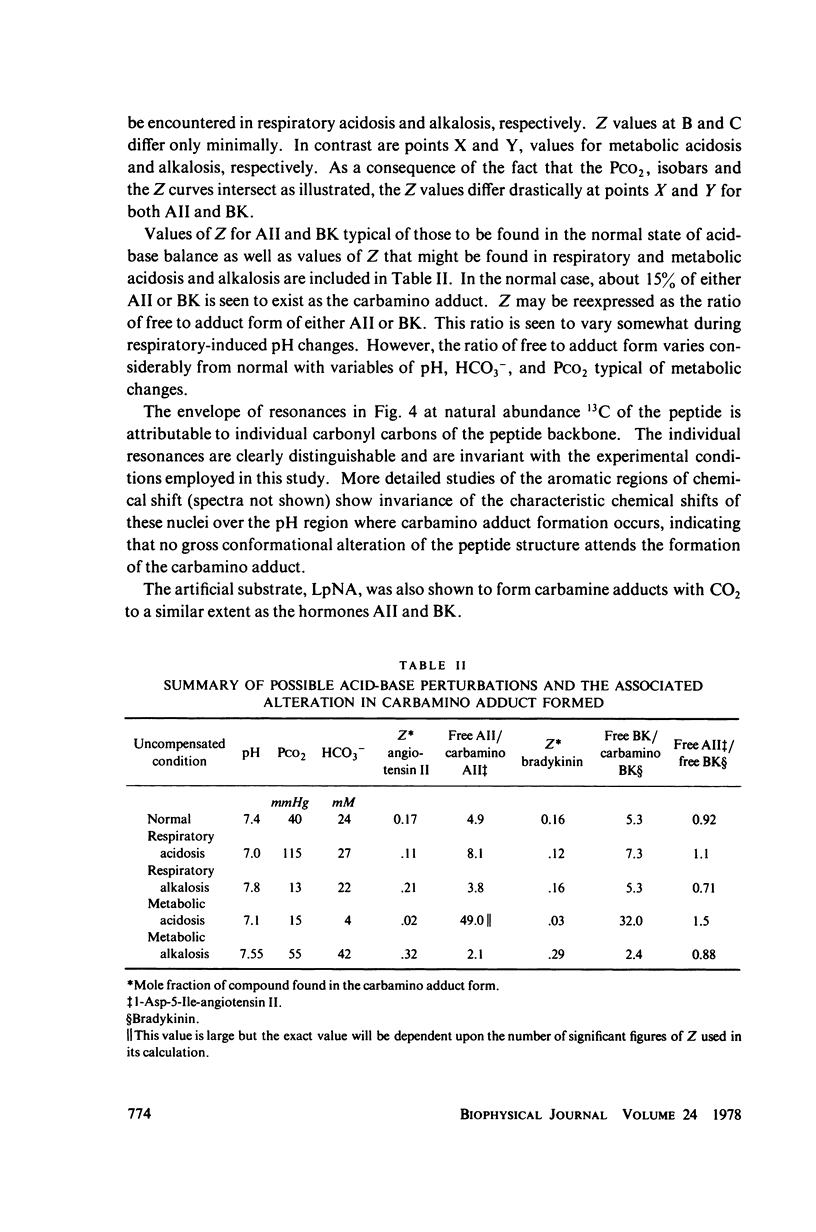
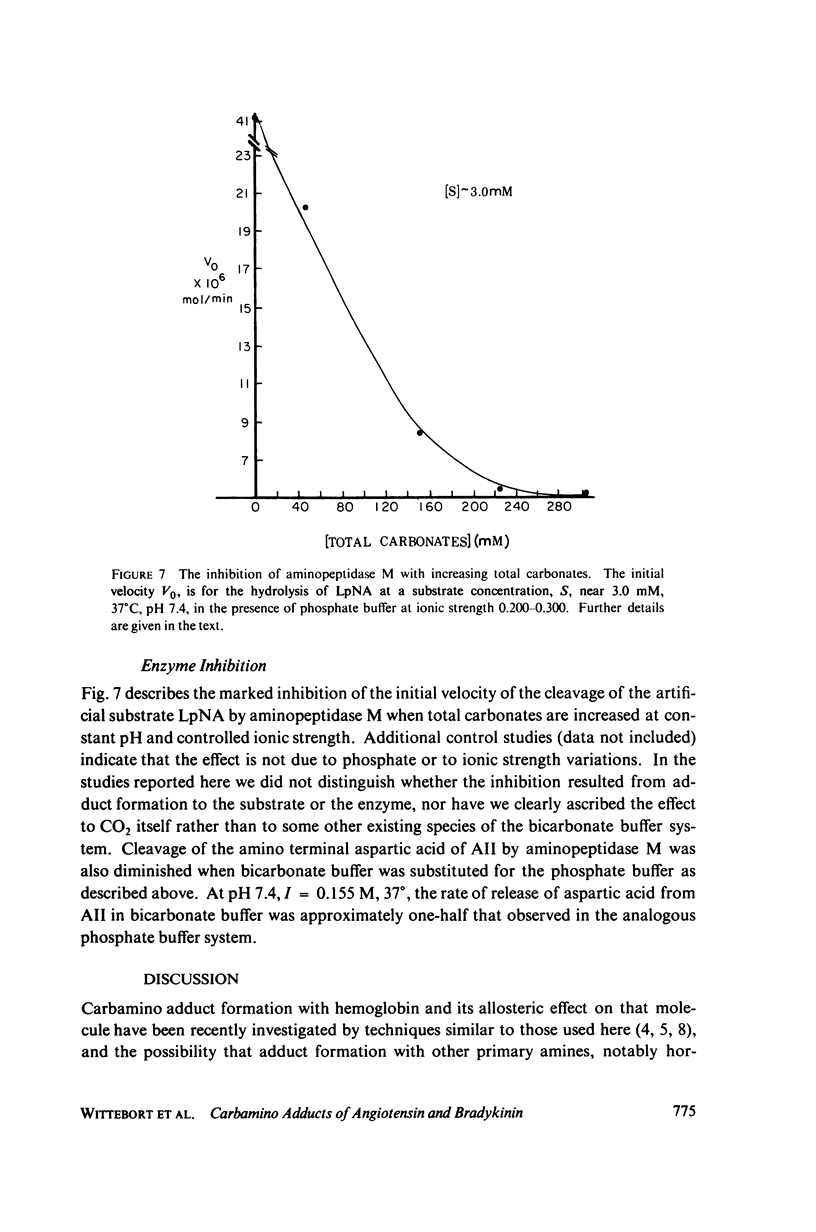
Abstract
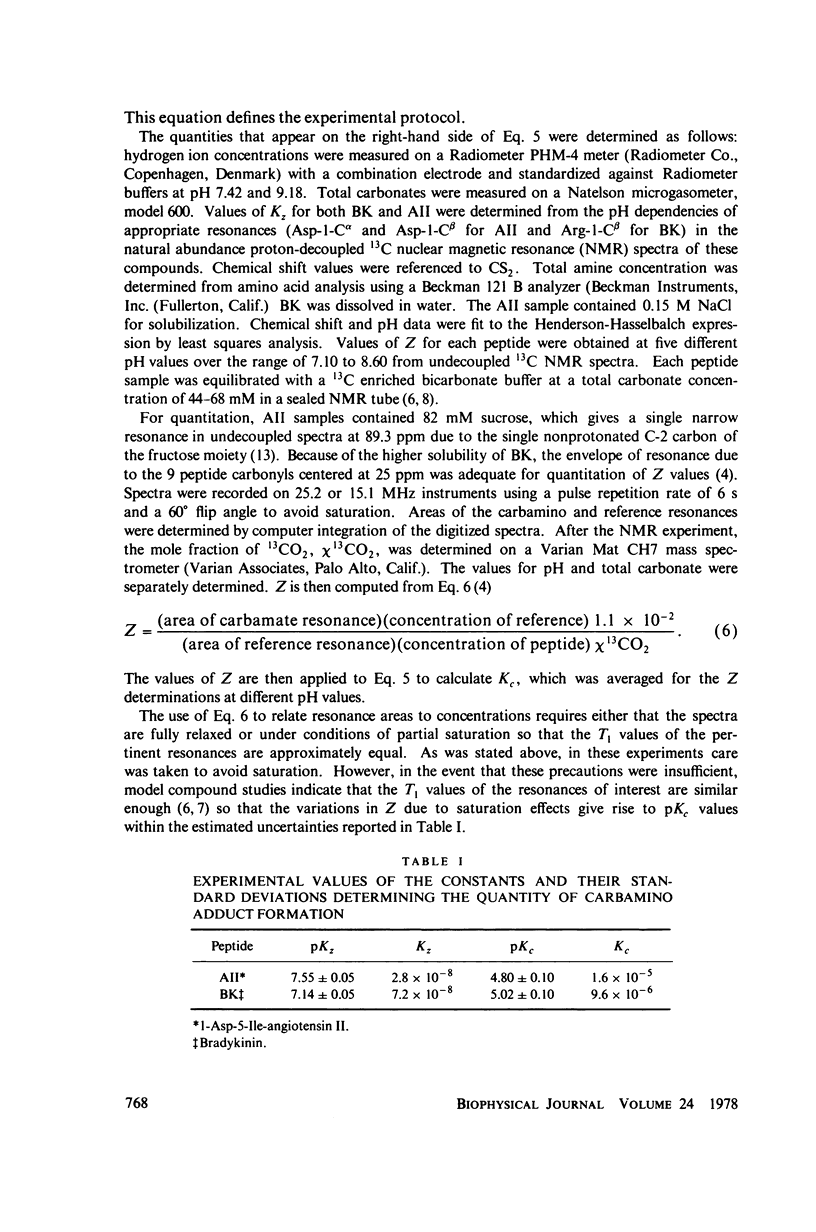
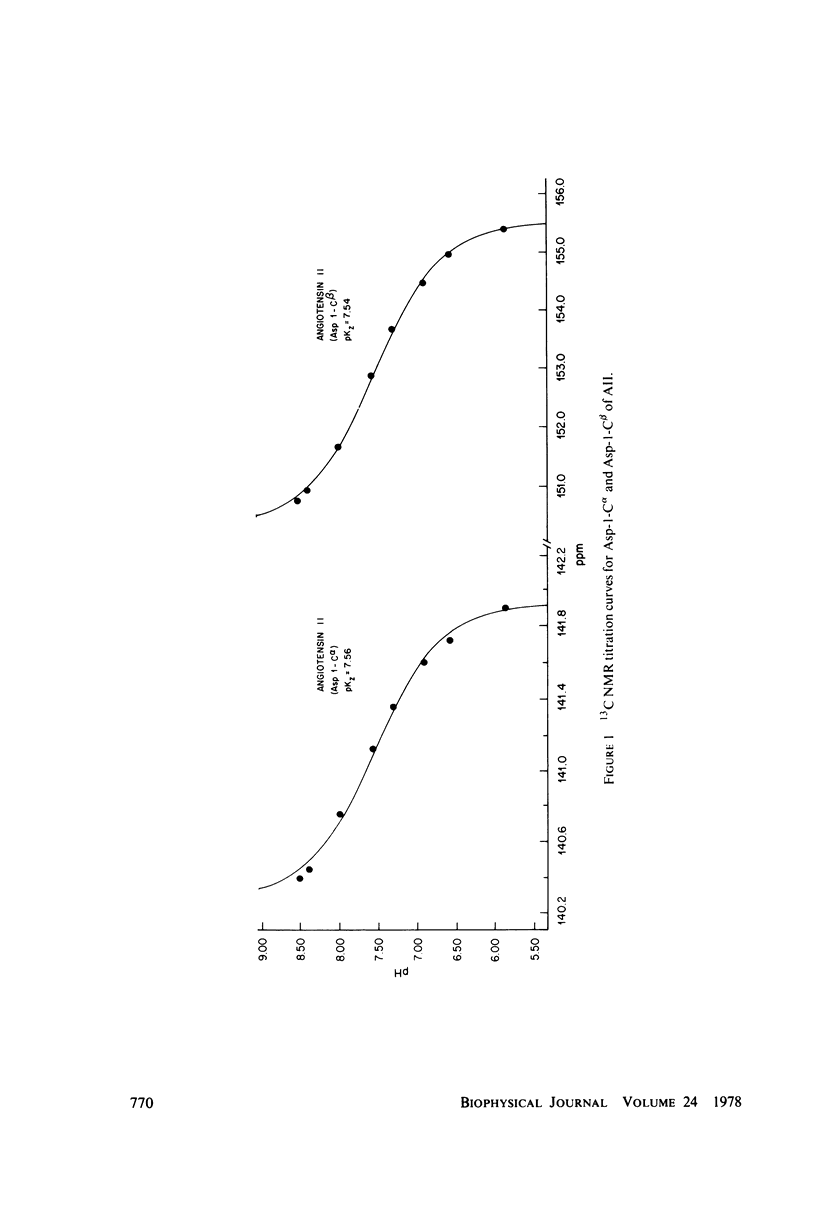
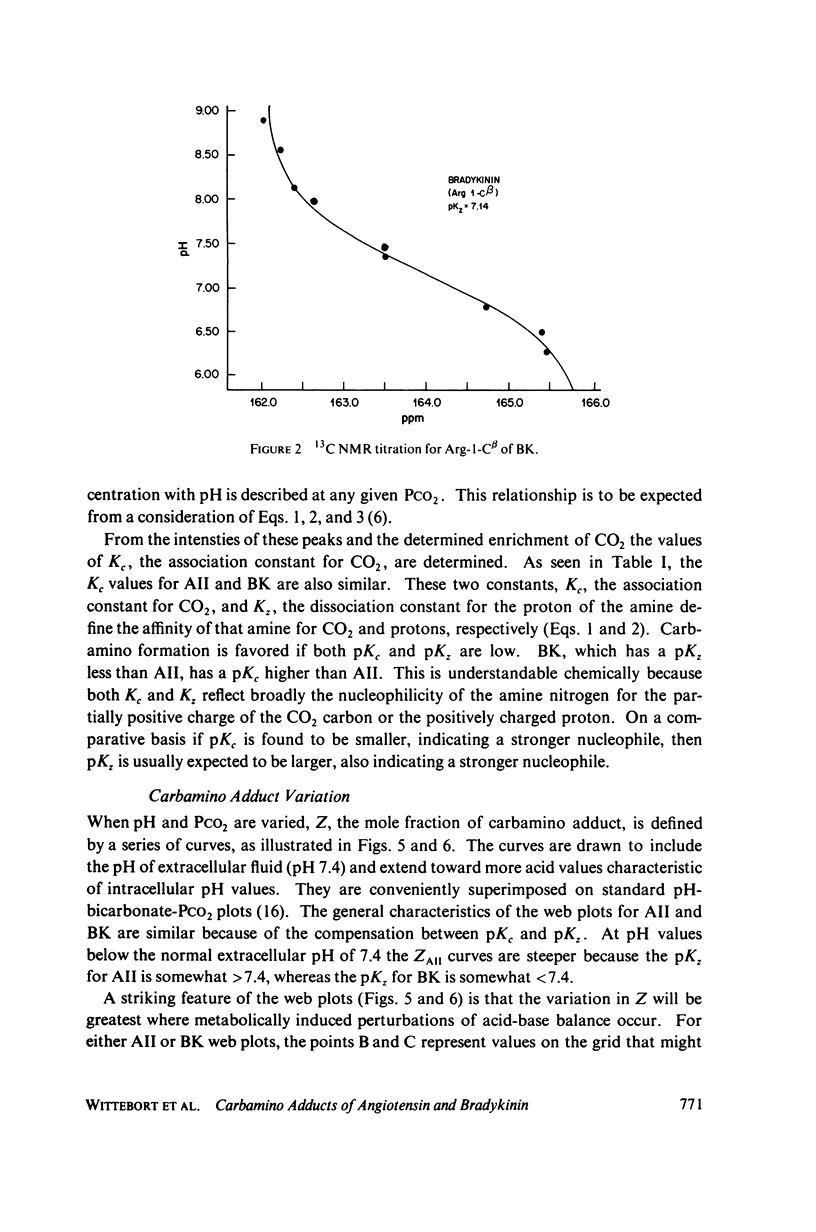
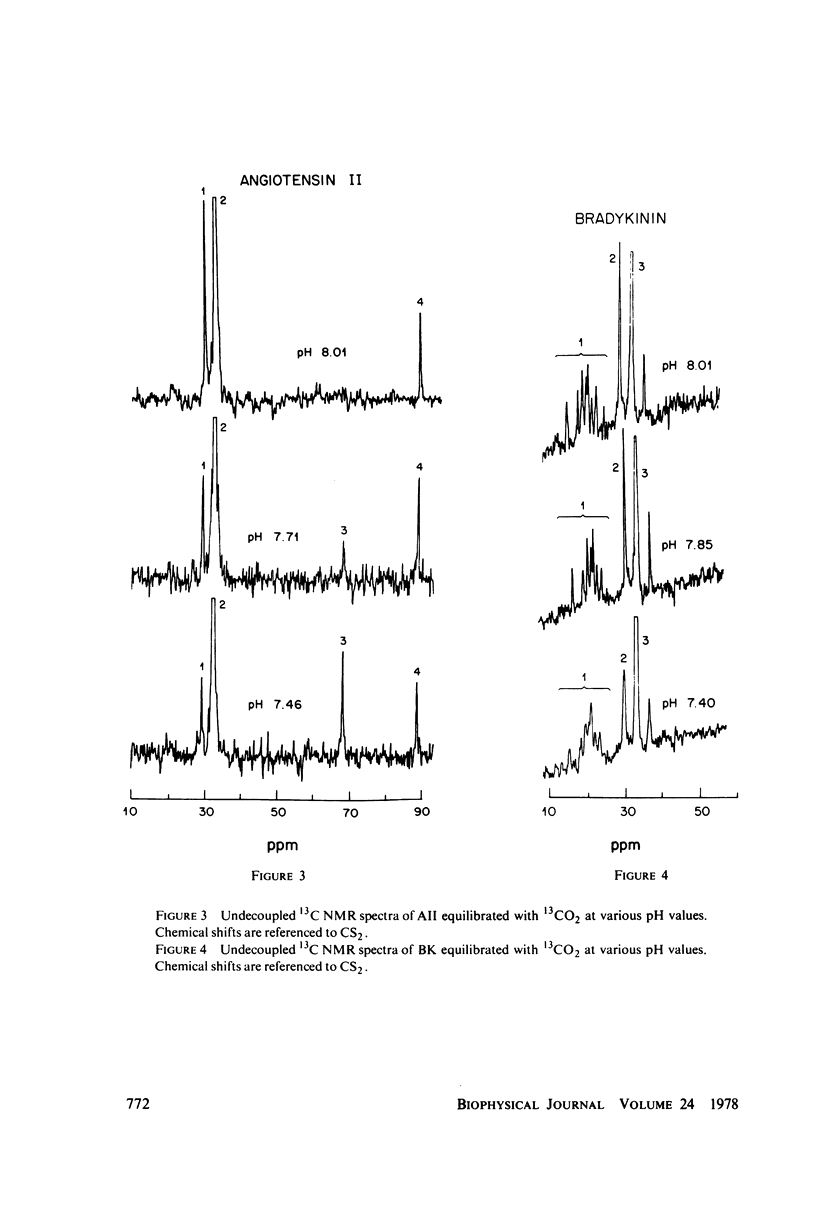
The two equilibrium constants that define the extent of carbamino adduct formation with amines for all values of pH and PCO2 are determined for the alpha-amino groups of the peptide hormones angiotensin II(AII) and bradykinin (BK) by nuclear magnetic resonance techniques. From these constants the variation of carbamino adduct formation has been calculated over the pH range 6.60--8.00 with variable PCO2, and the results are superimposed upon standard pH-bicarbonate diagrams. PCO2, and the results are superimposed upon standard pH-bicarbonate diagrams. The mole fraction, Z, of carbamino adduct form of AII or BK shows a maximum variation in going from metabolic alkalosis, Z congruent to 0.30, to metabolic acidosis, Z congruent to 0.02, with Z near 0.2 for normal acid-base conditions. Adduct formation to hormone may alter the biological effect of the hormone (a) by limiting proteolysis, particularly at the amino-terminal, (b) by altering hormone binding affinity to specific receptors, or (c) by converting the hormone to an antagonist which binds to receptor but does not activate subsequent metabolic events. The requirements for any of these mechanisms to operate are examined in terms of simple equilibrium considerations, and experimental evidence of inhibition of an aminopeptidase model system is presented. These results are consistent with the hypothesis that regulation of some physiological processes through formation of carbamino adduct of peptide hormones is possible.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnone A. X-ray studies of the interaction of CO2 with human deoxyhaemoglobin. Nature. 1974 Jan 18;247(5437):143–145. doi: 10.1038/247143a0. [DOI] [PubMed] [Google Scholar]
- Colman R. W. Formation of human plasma kinin. N Engl J Med. 1974 Sep 5;291(10):509–515. doi: 10.1056/NEJM197409052911008. [DOI] [PubMed] [Google Scholar]
- Freeman R. H., Davis J. O., Lohmeier T. E., Spielman W. S. [Des-Asp1] angiotensin II: mediator of the renin-angiotensin system? Fed Proc. 1977 Apr;36(5):1766–1770. [PubMed] [Google Scholar]
- Hastings A. B. A biochemist's anabasis. Annu Rev Biochem. 1970;39:1–24. doi: 10.1146/annurev.bi.39.070170.000245. [DOI] [PubMed] [Google Scholar]
- Matthew J. B., Morrow J. S., Wittebort R. J., Gurd F. R. Quantitative determination of carbamino adducts of alpha and beta chains in human adult hemoglobin in presence and absence of carbon monoxide and 2,3-diphosphoglycerate. J Biol Chem. 1977 Apr 10;252(7):2234–2244. [PubMed] [Google Scholar]
- Morrow J. S., Gurd F. R. Nuclear magnetic resonance studies of hemoglobin: functional state correlations and isotopic enrichment strategies. CRC Crit Rev Biochem. 1975 Dec;3(3):221–287. doi: 10.3109/10409237509105453. [DOI] [PubMed] [Google Scholar]
- Morrow J. S., Keim P., Gurd F. R. CO2 adducts of certain amino acids, peptides, and sperm whale myoglobin studied by carbon 13 and proton nuclear magnetic resonance. J Biol Chem. 1974 Dec 10;249(23):7484–7494. [PubMed] [Google Scholar]
- Morrow J. S., Matthew J. B., Wittebort R. J., Gurd F. R. Carbon 13 resonances of 13CO2 carbamino adducts of alpha and beta chains in human adult hemoglobin. J Biol Chem. 1976 Jan 25;251(2):477–484. [PubMed] [Google Scholar]
- Oldfield E., Norton R. S., Allerhand A. Studies of individual carbon sites of proteins in solution by natural abundance carbon 13 nuclear magnetic resonance spectroscopy. Relaxation behavior. J Biol Chem. 1975 Aug 25;250(16):6368–6380. [PubMed] [Google Scholar]
- Oparil S., Haber E. The renin-angiotensin system (first of two parts). N Engl J Med. 1974 Aug 22;291(8):389–401. doi: 10.1056/NEJM197408222910805. [DOI] [PubMed] [Google Scholar]
- Peach M. J. Renin-angiotensin system: biochemistry and mechanisms of action. Physiol Rev. 1977 Apr;57(2):313–370. doi: 10.1152/physrev.1977.57.2.313. [DOI] [PubMed] [Google Scholar]
- Wachsmuth E. D., Fritze I., Pfleiderer G. An aminopeptidase occurring in pig kidney. II. A study on the mechanism of the hydrolysis. Biochemistry. 1966 Jan;5(1):175–182. doi: 10.1021/bi00865a023. [DOI] [PubMed] [Google Scholar]



