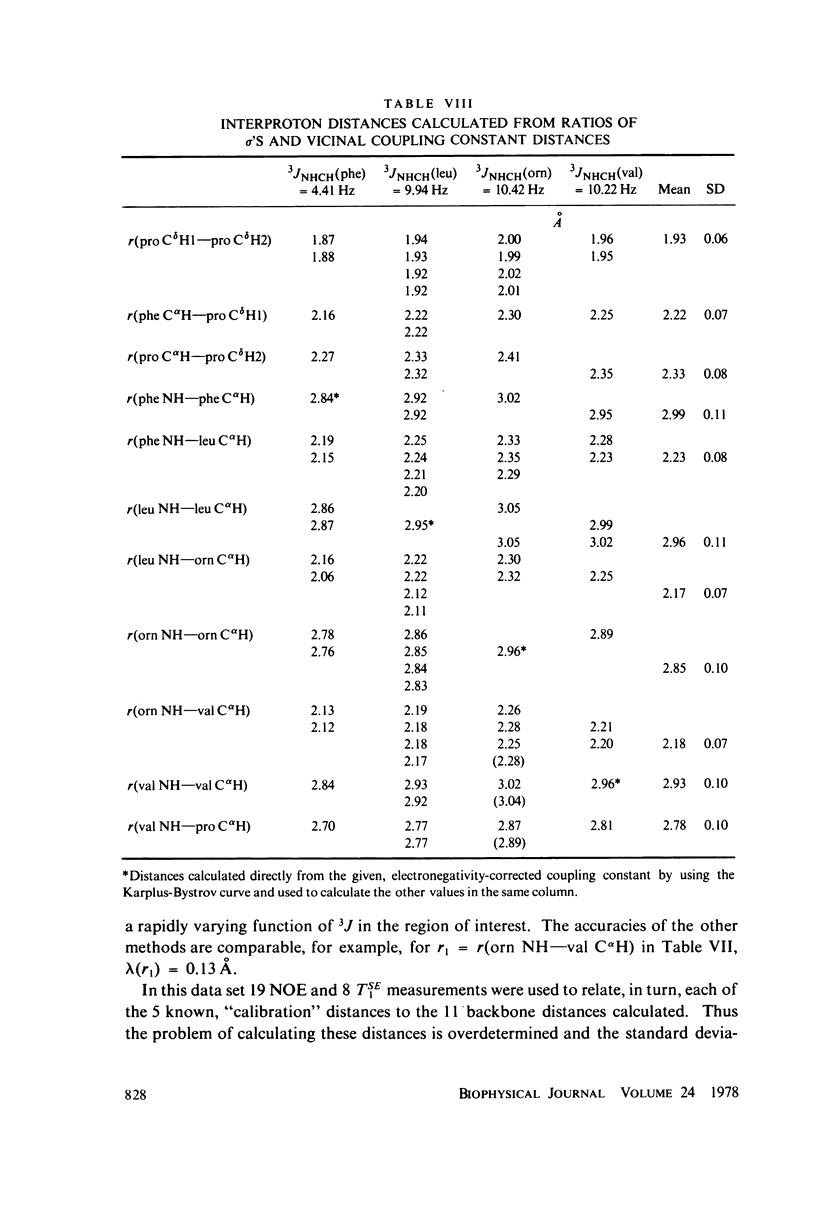
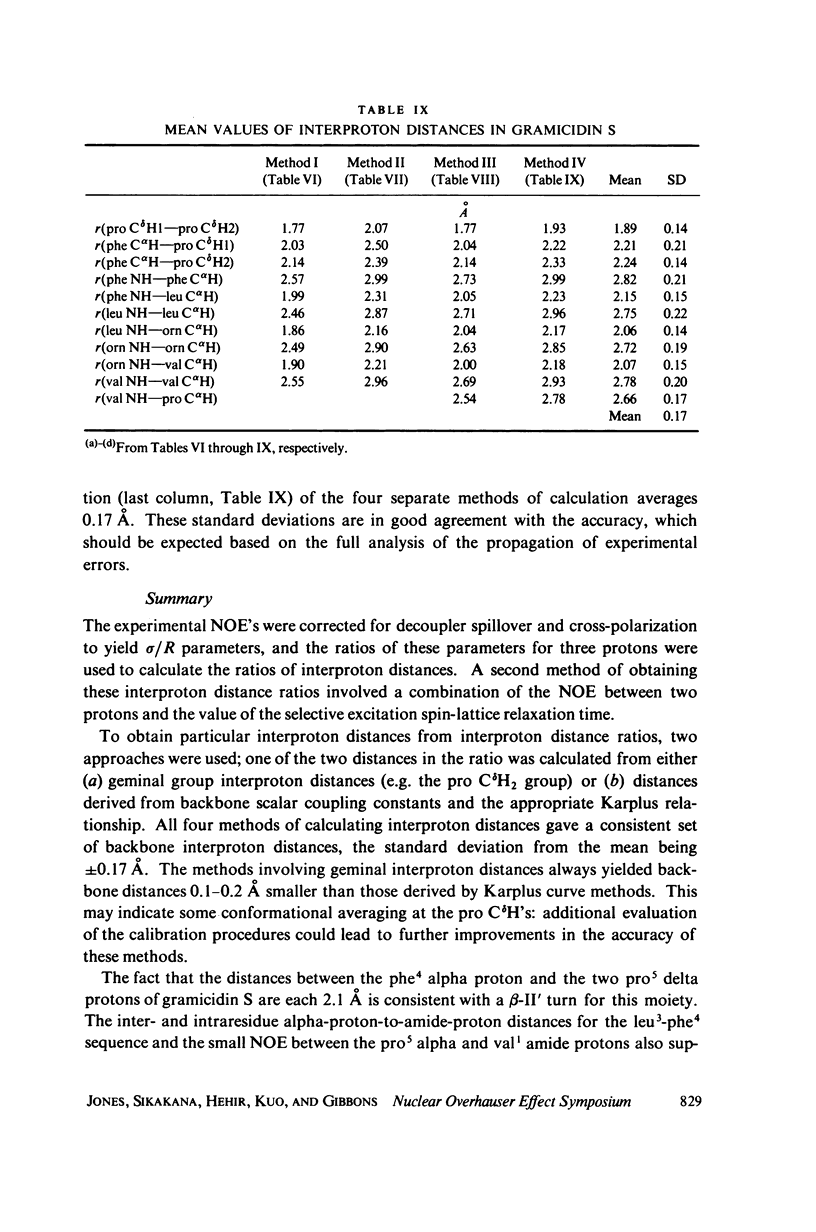
Abstract
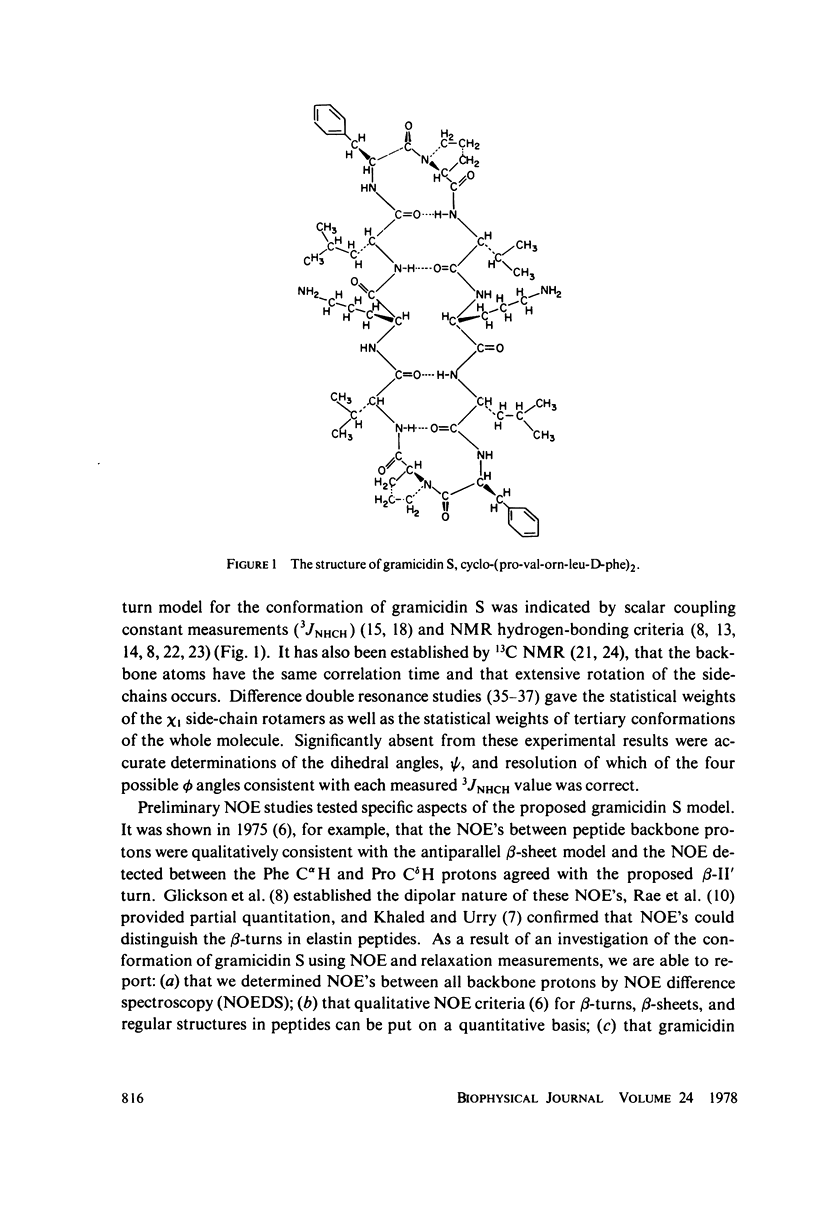
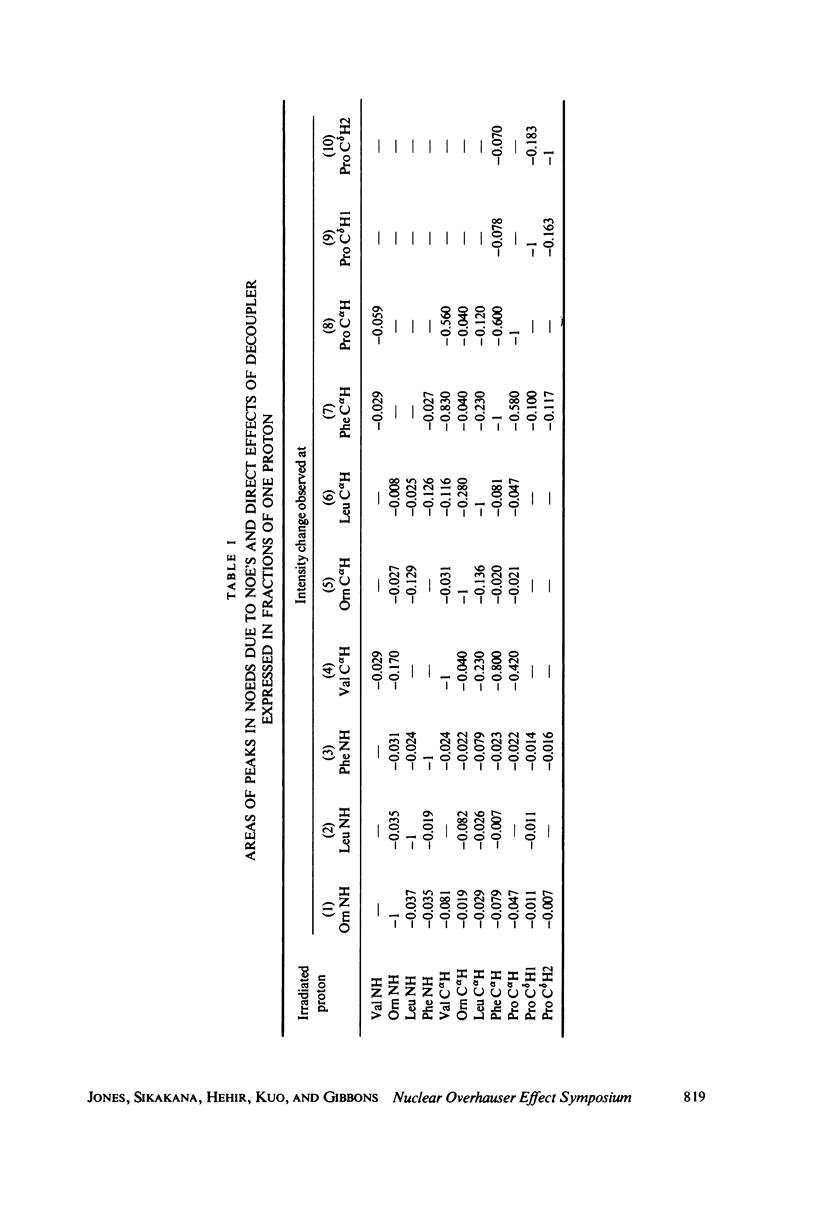
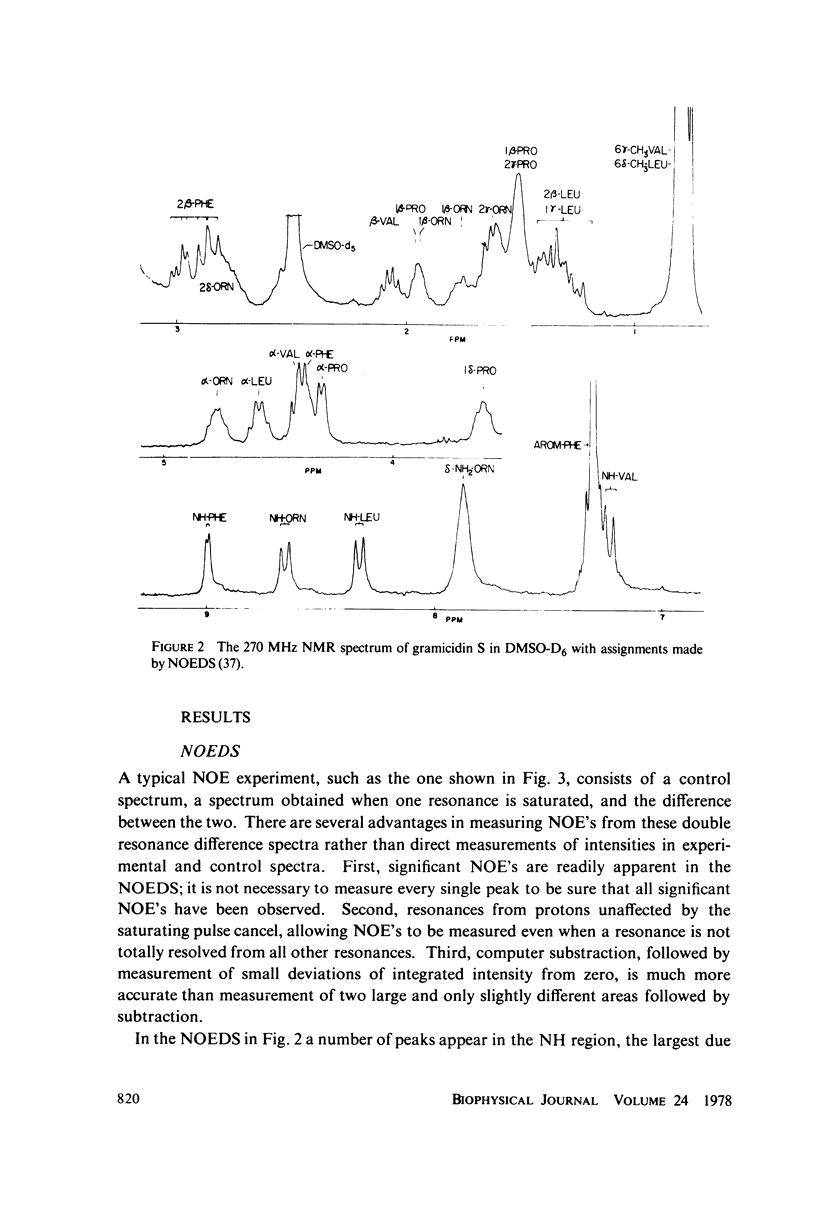
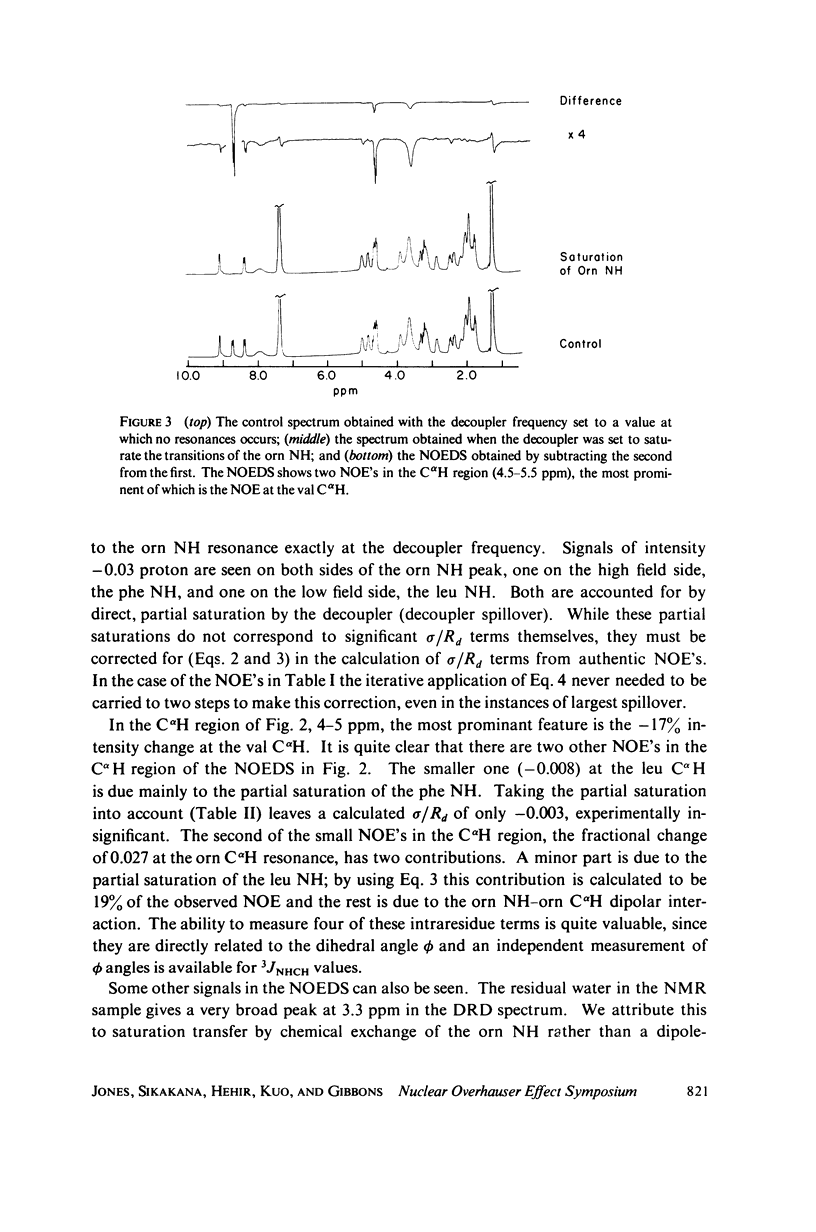
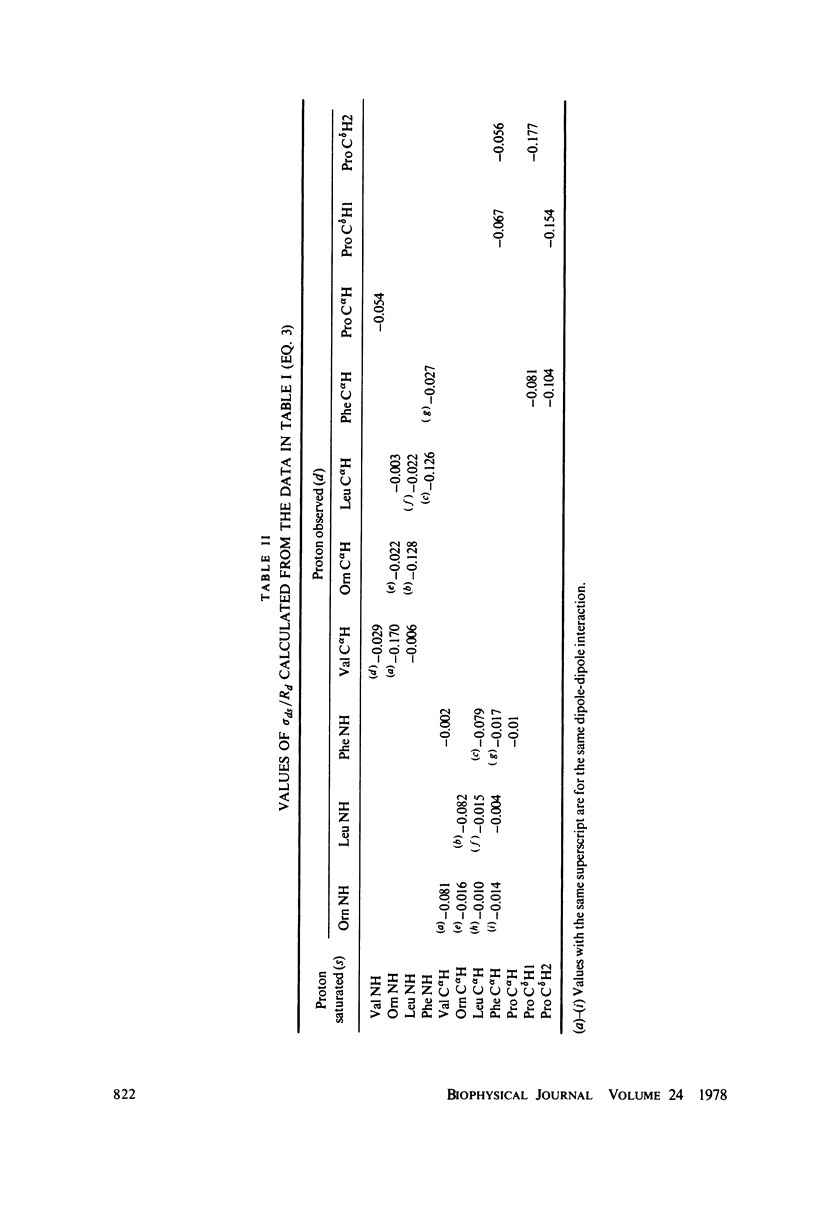
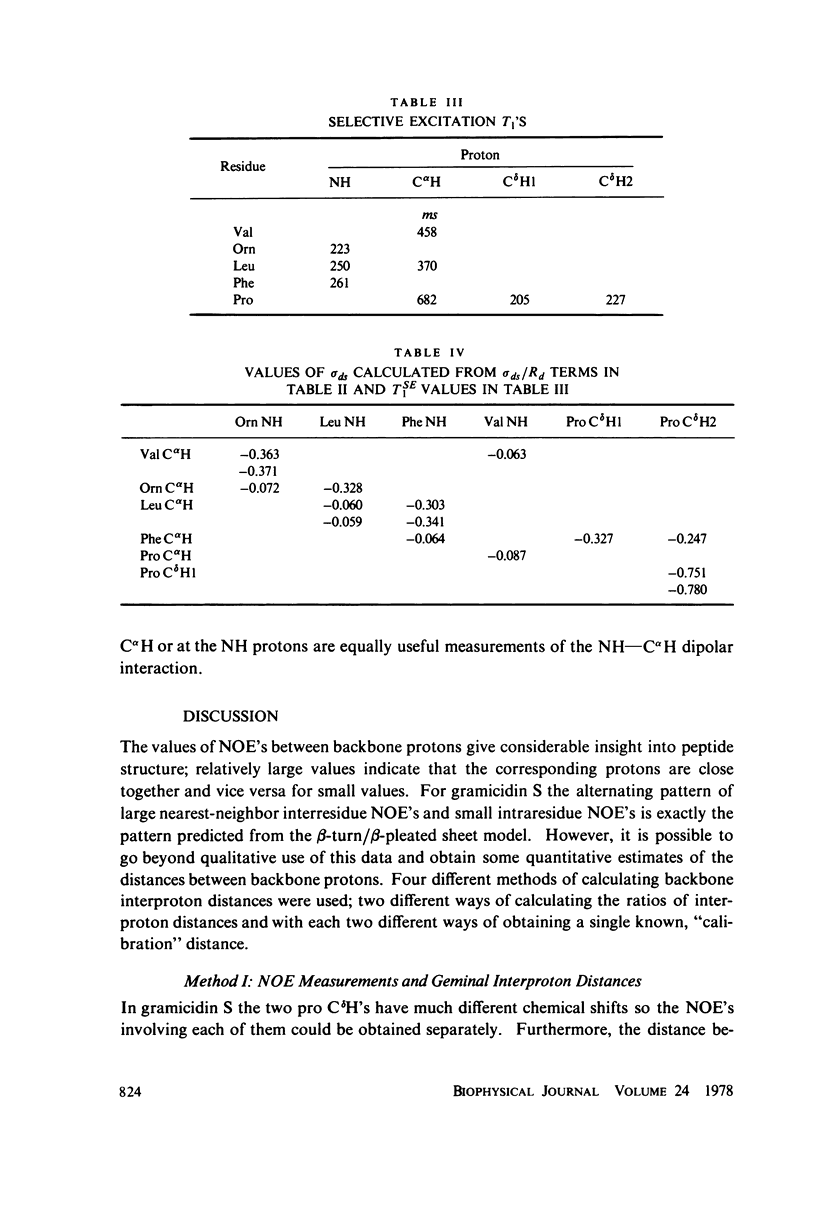
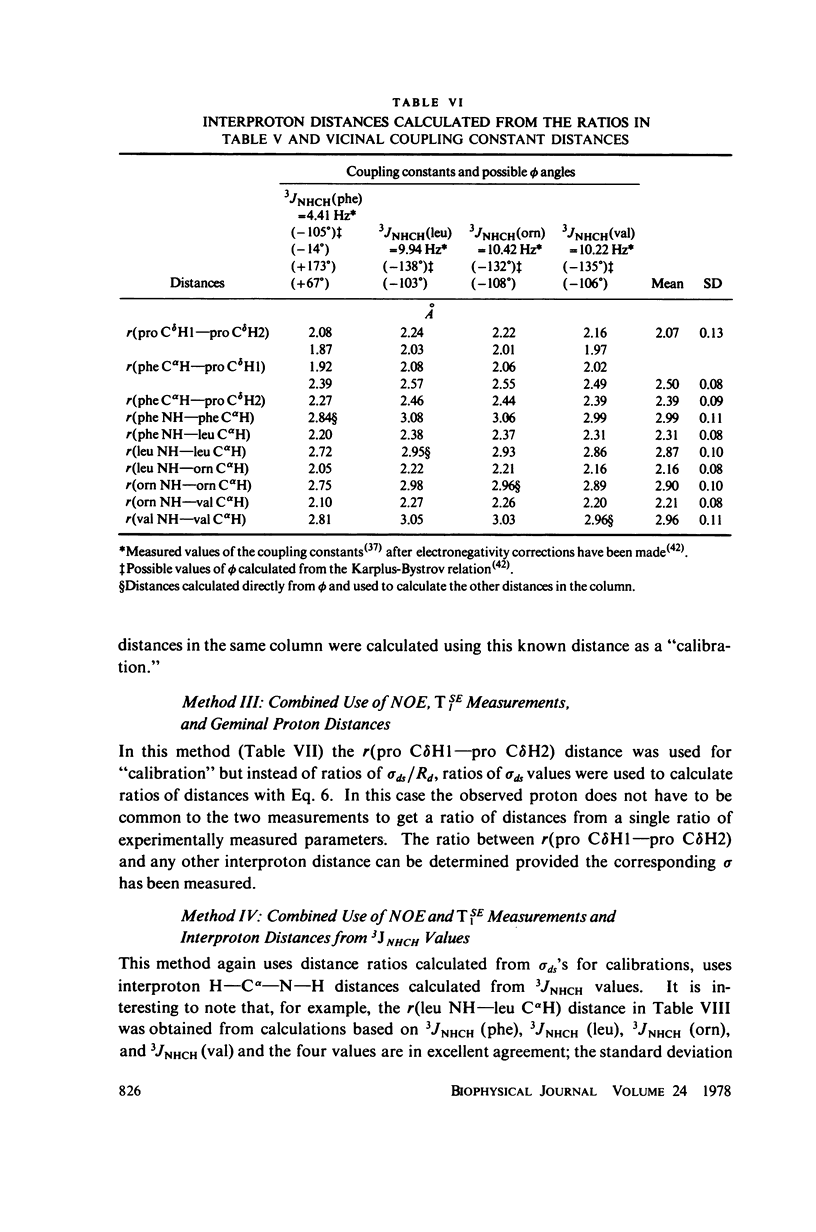
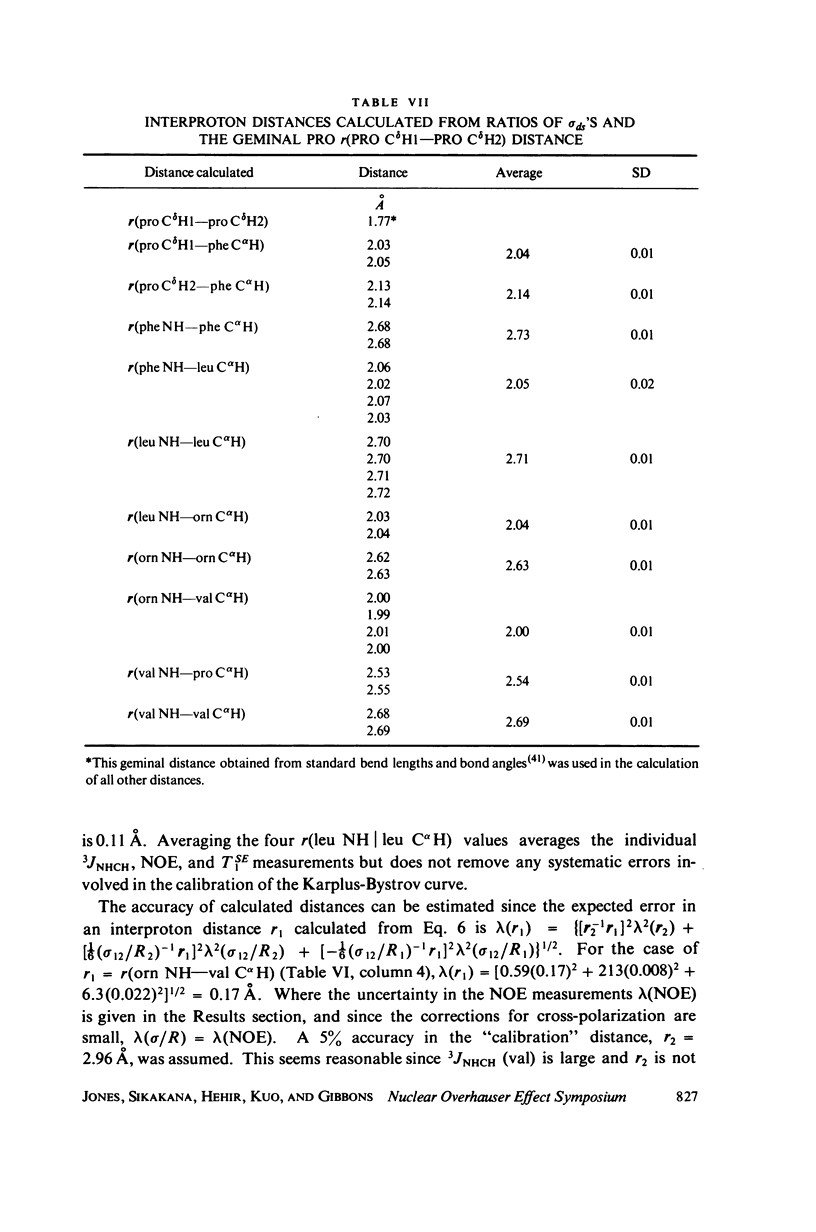
The [1H:1H] nuclear Overhauser effects (NOE's) and spin-lattice relaxation times (T1's) are reported for the backbone protons of the decapeptide gramicidin S. Several methods for calculating interproton distances from these measurements are presented. Ratios of interproton distances were obtained from [1H:1H] NOE's and from the combination of [1H:1H]NOE'S and T1 values. Actual proton-proton distances were calculated from these ratios either by using the known distance between two geminal protons or distances derived from scalar coupling constants. The interproton distances calculated for gramicidin S are consistent with a II' beta-turn/antiparallel beta-sheet conformation.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balaram P., Bothner-By A. A., Breslow E. Nuclear magnetic resonance studies of the interaction of peptides and hormones with bovine neurophysin. Biochemistry. 1973 Nov 6;12(23):4695–4704. doi: 10.1021/bi00747a024. [DOI] [PubMed] [Google Scholar]
- De Santis P., Liquori A. M. Conformation of gramicidin S. Biopolymers. 1971;10(4):699–710. doi: 10.1002/bip.360100408. [DOI] [PubMed] [Google Scholar]
- Dygert M., Gō N., Scheraga H. A. Use of a symmetry condition to compute the conformation of gramicidin S1. Macromolecules. 1975 Nov-Dec;8(6):750–761. doi: 10.1021/ma60048a016. [DOI] [PubMed] [Google Scholar]
- Finer E. G., Hauser H., Chapman D. Nuclear magnetic resonance studies of interactions of phospholipids with cyclic antibiotics. Chem Phys Lipids. 1969 Dec;3(4):386–392. doi: 10.1016/0009-3084(69)90043-7. [DOI] [PubMed] [Google Scholar]
- Gibbons W. A., Alms H., Bockman R. S., Wyssbrod H. R. Homonuclear indor spectroscopy as a means of simplifying and analyzing proton magnetic resonance spectra of peptides and as a basis for determining secondary and tertiary conformations of complex peptides. Biochemistry. 1972 Apr 25;11(9):1721–1725. doi: 10.1021/bi00759a030. [DOI] [PubMed] [Google Scholar]
- Gibbons W. A., Alms H., Sogn J., Wyssbrod H. R. Homonuclear internuclear double resonance spectroscopy as a basis for determination of amino acid conformation. Proc Natl Acad Sci U S A. 1972 May;69(5):1261–1265. doi: 10.1073/pnas.69.5.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickson J. D., Gordon S. L., Pitner P., Agresti D. G., Walter R. Intramolecular 1H nuclear Overhauser effect study of the solution conformation of valinomycin in dimethyl sulfoxide. Biochemistry. 1976 Dec 28;15(26):5721–5729. doi: 10.1021/bi00671a007. [DOI] [PubMed] [Google Scholar]
- Hawkes G. E., Randall E. W., Bradley C. H. Theory and practice for studies for peptides by 15N nuclear magnetic resonance at natural abundance: gramicidin S. Nature. 1975 Oct 30;257(5529):767–772. doi: 10.1038/257767a0. [DOI] [PubMed] [Google Scholar]
- Khaled M. A., Urry D. W. Nuclear Overhauser enhancement demonstration of the type II beta-turn in repeat peptides of tropoelastin. Biochem Biophys Res Commun. 1976 May 17;70(2):485–491. doi: 10.1016/0006-291x(76)91072-x. [DOI] [PubMed] [Google Scholar]
- Komoroski R. A., Peat I. R., Levy G. C. High field carbon-13 NMR spectroscopy. Conformational mobility in gramicidin S and frequency dependence of 13-C spin-lattice relaxation times. Biochem Biophys Res Commun. 1975 Jul 8;65(1):272–279. doi: 10.1016/s0006-291x(75)80089-1. [DOI] [PubMed] [Google Scholar]
- Kumar N. G., Izumiya N., Miyoshi M., Sugano H., Urry D. W. Conformational and spectral analysis of the polypeptide antibiotic N-methylleucine gramicidin S dihydrochloride by nuclear magnetic resonance. Biochemistry. 1975 May 20;14(10):2197–2207. doi: 10.1021/bi00681a024. [DOI] [PubMed] [Google Scholar]
- Leach S. J., Némethy G., Scheraga H. A. Use of proton nuclear Overhauser effects for the determination of the conformations of amino acid residues in oligopeptides. Biochem Biophys Res Commun. 1977 Mar 7;75(1):207–215. doi: 10.1016/0006-291x(77)91310-9. [DOI] [PubMed] [Google Scholar]
- Momany F. A., Vanderkooi G., Tuttle R. W., Scheraga H. A. Minimization of polypeptide energy. IV. Further studies of gramicidin S. Biochemistry. 1969 Feb;8(2):744–746. doi: 10.1021/bi00830a041. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov Y. A., Ivanov V. T., Bystrov V. F., Miroshnikov A. I., Shepel E. N., Abdullaev N. D., Efremov E. S., Senyavina L. B. The conformation of gramicidin S and its N,N'-diacetyl derivative in solutions. Biochem Biophys Res Commun. 1970 Apr 24;39(2):217–225. doi: 10.1016/0006-291x(70)90781-3. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Tonelli A. E. N-methylleucine gramicidin-S and (di-N-methylleucine) gramicidin-S conformations with cis L-Orn-L-N-MeLeu peptide bonds. Biopolymers. 1976 Aug;15(8):1623–1635. doi: 10.1002/bip.1976.360150815. [DOI] [PubMed] [Google Scholar]
- Pitner T. P., Urry D. W. Proton magnetic resonance studies in trifluoroethanol. Solvent mixtures as a means of delineating peptide protons. J Am Chem Soc. 1972 Feb 23;94(4):1399–1400. doi: 10.1021/ja00759a083. [DOI] [PubMed] [Google Scholar]
- Pysh E. S. Conformations at local energy minimums for gramicidin S: optical calculations. Science. 1970 Jan 16;167(3916):290–292. doi: 10.1126/science.167.3916.290. [DOI] [PubMed] [Google Scholar]
- Rae I. D., Stimson E. R., Scheraga H. A. Nuclear Overhauser effects and the conformation of gramicidin S. Biochem Biophys Res Commun. 1977 Jul 11;77(1):225–229. doi: 10.1016/s0006-291x(77)80186-1. [DOI] [PubMed] [Google Scholar]
- Scheraga H. A. Calculation of polypeptide conformation. Harvey Lect. 1969;63:99–138. [PubMed] [Google Scholar]
- Schwyzer R., Ludescher U. Conformational study of gramicidin S using the phthalimide group as nuclear magnetic resonance marker. Biochemistry. 1968 Jul;7(7):2519–2522. doi: 10.1021/bi00847a011. [DOI] [PubMed] [Google Scholar]
- Schwyzer R., Ludescher U. Untersuchungen über die Konformation des cyclischen Hexapeptids cyclo-Glycyl-L-prolyl-glycyl-glycyl-L-prolyl-glycyl mittels protonenmagnetischer Resonanz und Parallelez zum Cyclodecapeptid Gramicidin S. Helv Chim Acta. 1969;52(7):2033–2040. doi: 10.1002/hlca.19690520726. [DOI] [PubMed] [Google Scholar]
- Sogn J. A., Craig L. C., Gibbons W. A. The complete assignment of the 13C nuclear magnetic resonance spectrum of the decapeptide gramicidin S-A by selective biosynthetic enrichment studies. J Am Chem Soc. 1974 May 15;96(10):3306–3309. doi: 10.1021/ja00817a046. [DOI] [PubMed] [Google Scholar]
- Sugano H., Abe H., Miyoshi M., Kato T., Izumiya N. Gramicidin S analogs containing N-methylleucine in place of leucine. Experientia. 1973 Dec;29(12):1488–1489. doi: 10.1007/BF01943872. [DOI] [PubMed] [Google Scholar]
- Zhuze A. L., Kogan G. A., Krit N. A., Andronova T. M., Filatova M. P., Senyavina L. B., Meshcheryakova E. A., Ryabova I. D., Ravdel' G. A., Shchukina L. A. Depsipeptide modification as a method of determining the contribution of intramolecular hydrogen bonds to the conformational stability of peptides (gramicidin S). Mol Biol. 1974 Jul;8(1):69–74. [PubMed] [Google Scholar]


