Abstract
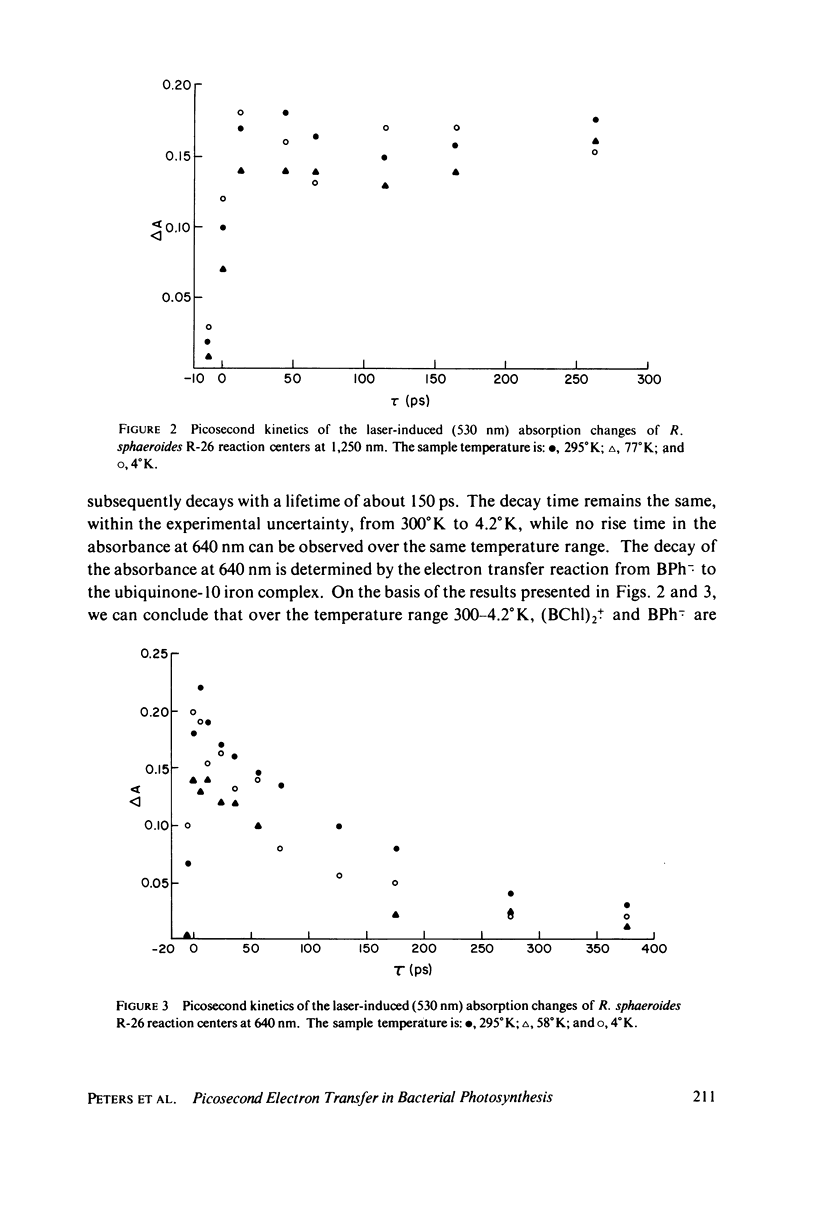
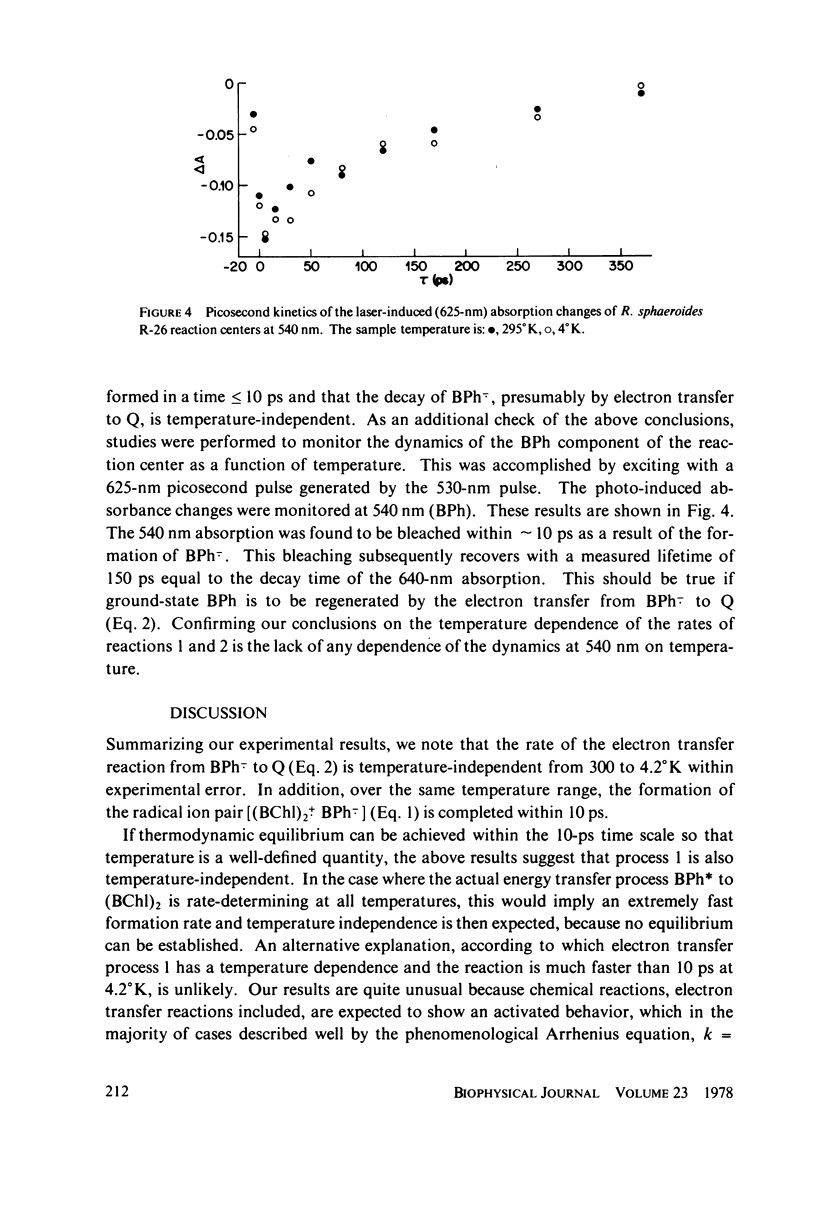
The primary electron transfer processes in Rhodopseudomonas sphaeroides R-26 were studied as a function of temperature by means of picosecond spectroscopy. The first chemical step of the bacterial photosynthesis involves an electron transfer from the excited state of a bacteriochlorophyll a dimer, (BChl)2, to a bacteriopheophytin (BPh) to form the radical ion pair (BChl)2+. BPh-.. The upper limit for the formation time of this ion-pair was found to be 10 ps, at temperatures in the range 300-4.2 degree K. Similarly, the second chemical step, involving electron transfer from BPh-. to an ubiquinone-iron complex (QFe), was found to have a lifetime of approximately 150 ps, also independent of temperature in the same range. We interpret the absence of temperature dependence as indicating that process 2 proceeds via a tunneling mechanism. Utilizing our results in conjunction with electron tunneling theories, we calculate the distance between BPh-. and Q(Fe) to be 9--13 A. Our results also imply a closer proximity between (BChl)2 and BPh.
Full text
PDF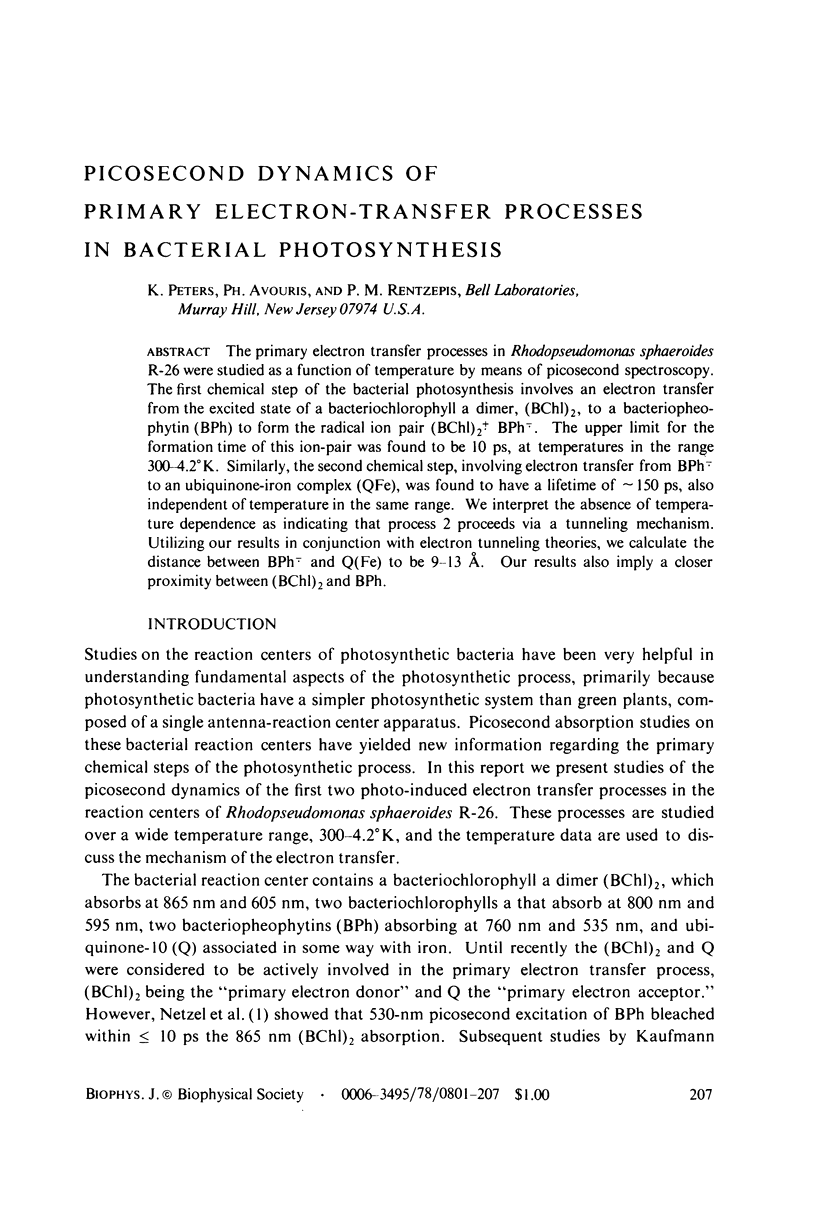







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenfeld L. A., Chernavskii D. S. Tunnelling of electrons in biological processes. J Theor Biol. 1973 Apr;39(1):1–7. doi: 10.1016/0022-5193(73)90201-4. [DOI] [PubMed] [Google Scholar]
- DeVault D., Chance B. Studies of photosynthesis using a pulsed laser. I. Temperature dependence of cytochrome oxidation rate in chromatium. Evidence for tunneling. Biophys J. 1966 Nov;6(6):825–847. doi: 10.1016/s0006-3495(66)86698-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton P. L., Kaufmann K. J., Chance B., Rentzepis P. M. Picosecond kinetics of the 1250 nm band of the Rps. sphaeroides reaction center: the nature of the primary photochemical intermediary state. FEBS Lett. 1975 Dec 15;60(2):275–280. doi: 10.1016/0014-5793(75)80730-7. [DOI] [PubMed] [Google Scholar]
- Dutton P. L., Prince R. C., Tiede D. M., Petty K. M., Kaufmann K. J., Netzel T. L., Rentzepis P. M. Electron transfer in the photosynthetic reaction center. Brookhaven Symp Biol. 1976 Jun 7;(28):213–237. [PubMed] [Google Scholar]
- Fajer J., Brune D. C., Davis M. S., Forman A., Spaulding L. D. Primary charge separation in bacterial photosynthesis: oxidized chlorophylls and reduced pheophytin. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4956–4960. doi: 10.1073/pnas.72.12.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfield J. J. Electron transfer between biological molecules by thermally activated tunneling. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3640–3644. doi: 10.1073/pnas.71.9.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann K. J., Dutton P. L., Netzel T. L., Leigh J. S., Rentzepis P. M. Picosecond kinetics of events leading to reaction center bacteriochlorophyll oxidation. Science. 1975 Jun 27;188(4195):1301–1304. doi: 10.1126/science.188.4195.1301. [DOI] [PubMed] [Google Scholar]
- Kaufmann K. J., Petty K. M., Dutton P. L., Rentzepis P. M. Picosecond kinetics in reaction centers of Rps. sphaeroides and the effects of ubiquinone extraction and reconstitution. Biochem Biophys Res Commun. 1976 Jun 7;70(3):839–845. doi: 10.1016/0006-291x(76)90668-9. [DOI] [PubMed] [Google Scholar]
- Netzel T. L., Rentzepis P. M., Leigh J. Picosecond kinetics of reaction centers containing bacteriochlorophyll. Science. 1973 Oct 19;182(4109):238–241. doi: 10.1126/science.182.4109.238. [DOI] [PubMed] [Google Scholar]
- Parson W. W., Clayton R. K., Cogdell R. J. Excited states of photosynthetic reaction centers at low recox potentials. Biochim Biophys Acta. 1975 May 15;387(2):265–278. doi: 10.1016/0005-2728(75)90109-7. [DOI] [PubMed] [Google Scholar]
- Rockley M. G., Windsor M. W., Cogdell R. J., Parson W. W. Picosecond detection of an intermediate in the photochemical reaction of bacterial photosynthesis. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2251–2255. doi: 10.1073/pnas.72.6.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson E. R., Williams-Smith D. L. Electron paramagnetic resonance spectra of mitochondrial and microsomal cytochrome P-450 from the rat adrenal. Biochim Biophys Acta. 1976 Oct 13;449(1):59–71. doi: 10.1016/0005-2728(76)90007-4. [DOI] [PubMed] [Google Scholar]
- Szent-Györgyi A. TOWARDS A NEW BIOCHEMISTRY? Science. 1941 Jun 27;93(2426):609–611. doi: 10.1126/science.93.2426.609. [DOI] [PubMed] [Google Scholar]
- Tiede D. M., Prince R. C., Dutton P. L. EPR and optical spectroscopic properties of the electron carrier intermediate between the reaction center bacteriochlorophylls and the primary acceptor in Chromatium vinosum. Biochim Biophys Acta. 1976 Dec 6;449(3):447–467. doi: 10.1016/0005-2728(76)90155-9. [DOI] [PubMed] [Google Scholar]
- Vermeglio A., Clayton R. K. Orientation of chromophores in reaction centers of Rhodopseudomonas sphaeroides. Evidence for two absorption bands of the dimeric primary electron donor. Biochim Biophys Acta. 1976 Dec 6;449(3):500–515. doi: 10.1016/0005-2728(76)90159-6. [DOI] [PubMed] [Google Scholar]
- van Grondelle R., Romijn J. C., Holmes N. G. Photoreduction of the long wavelength bacteriopheophytin in reaction centers and chromatophores of the photosynthetic bacterium Chromatium vinosum. FEBS Lett. 1976 Dec 15;72(1):187–192. doi: 10.1016/0014-5793(76)80841-1. [DOI] [PubMed] [Google Scholar]



