Abstract
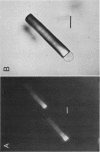
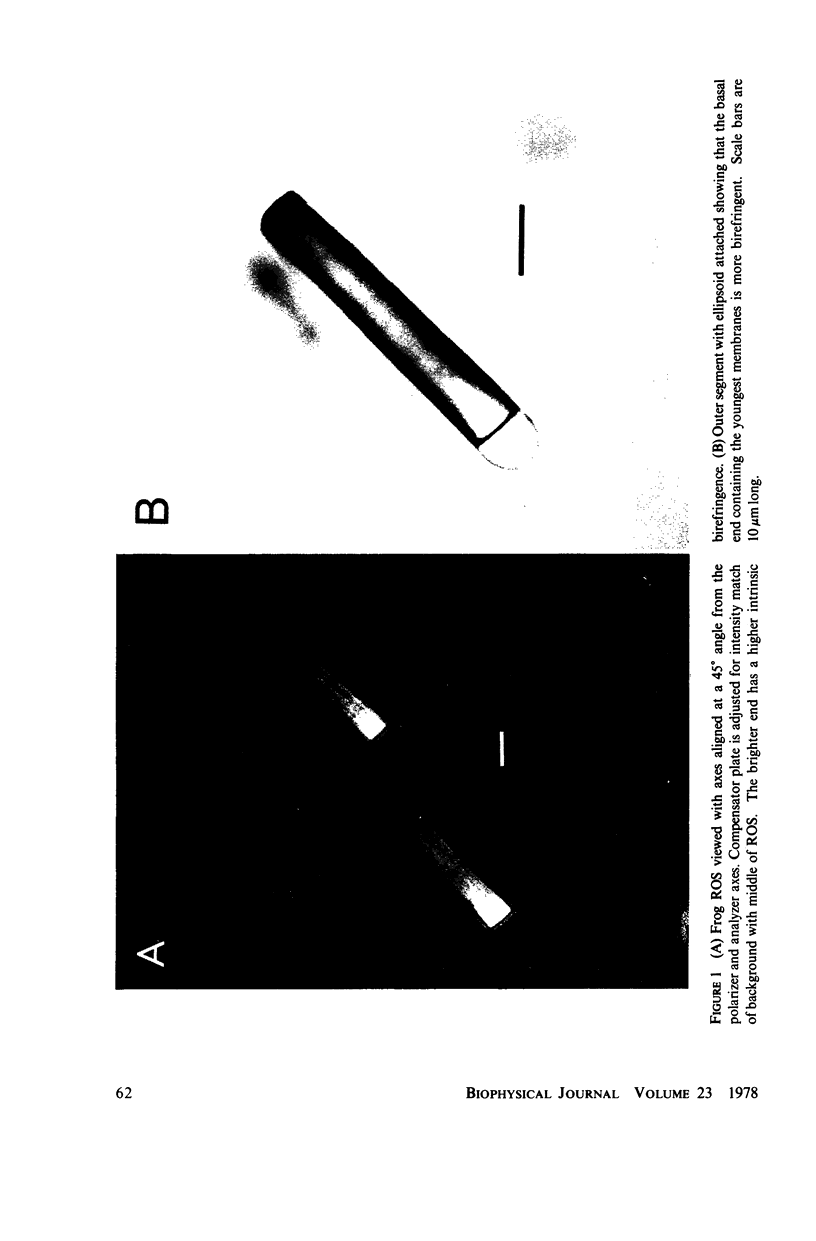
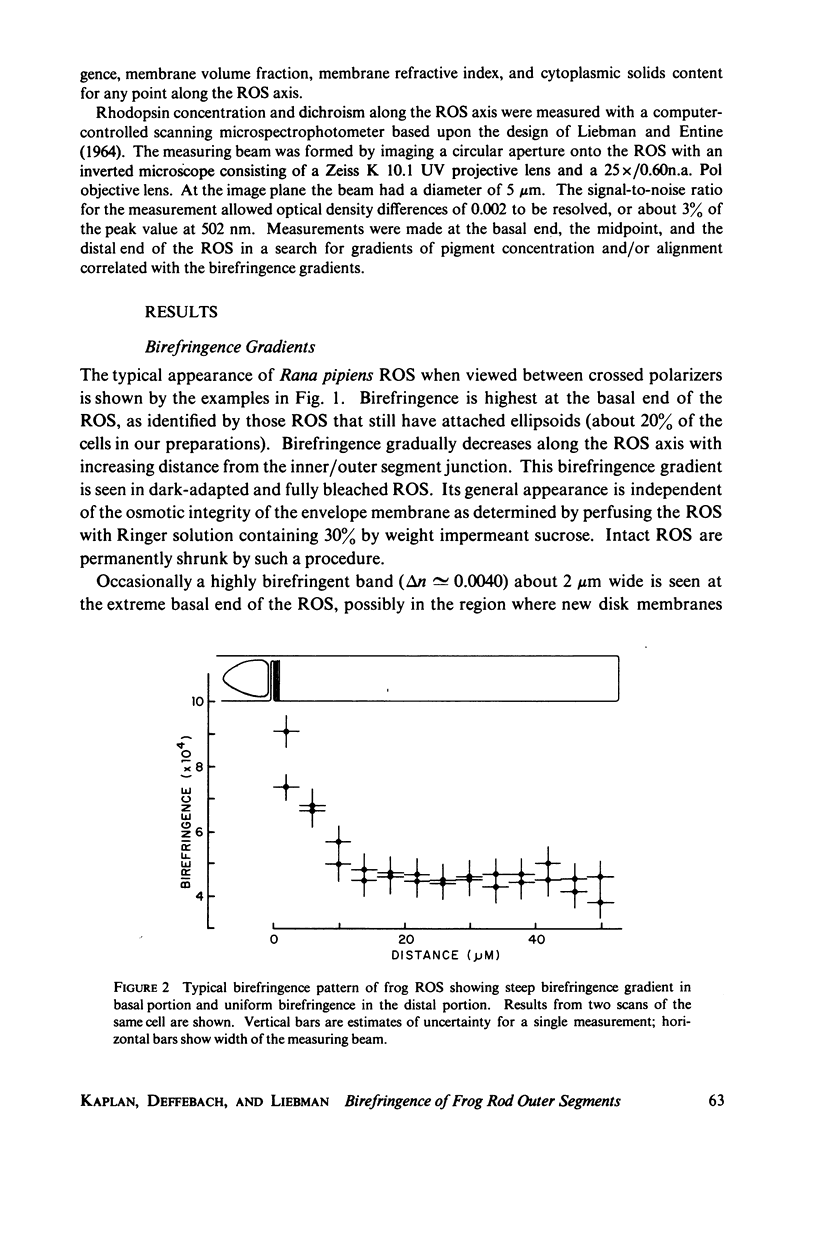
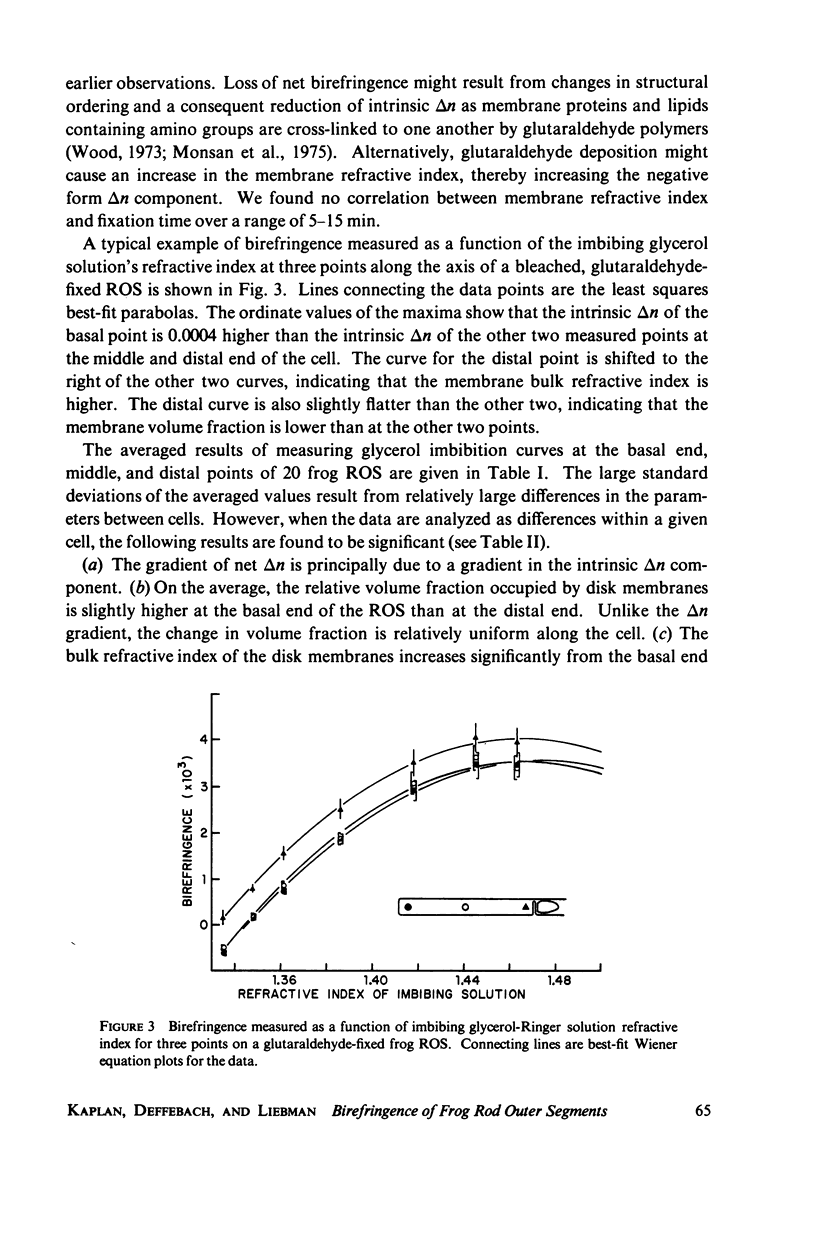
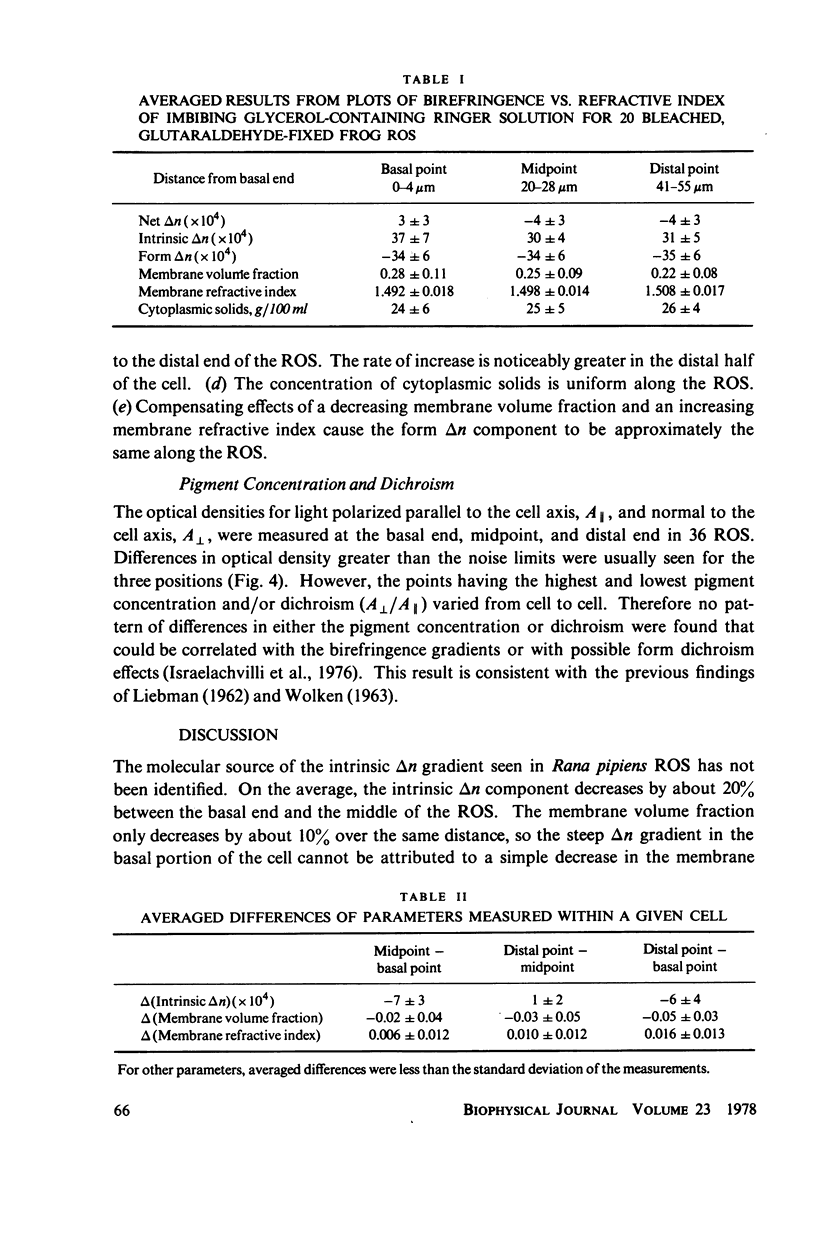
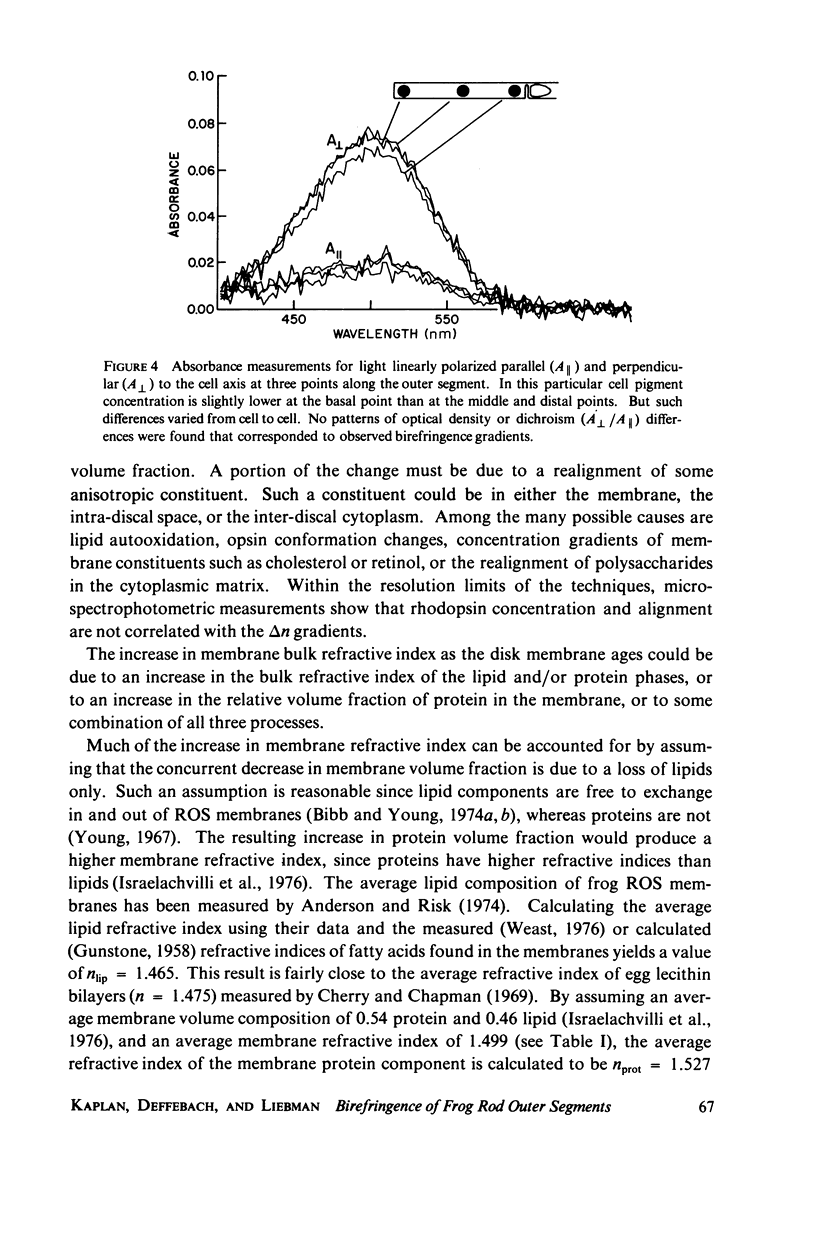
The birefringence (deltan) of Rana pipiens rod outer segments (ROS) reveals microstructure inhomogeneities not seen with other techniques. In the basal 20-30-micron length of the ROS there is a nearly linear axial gradient in deltan of approximately equal to -2 x 10(-5)/micron. No consistent deltan gradients are found in the distal 20-30 micron of the ROS. Using glycerol imbibition to separate the intrinsic and form birefringence components, we found that the basal deltan gradient was principally due to a gradient of the intrinsic birefringence component. The disk membrane volume fraction decreases uniformly from the basal end to the distal end, while the disk membrane refractive index increases. The contributions of these changes to the form birefringence approximately cancel, so that the form component is fairly uniform along the ROS axis. Because its axial distance from the inner segment is a measure of the time since a disk membrane was formed, these gradients may reflect a disk membrane aging process. Occasionally a highly birefringent, 2-micron-wide band is seen at the basal end ot the ROS, possibly where the envelope membrane folds to form new disk membranes.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. E., Risk M. Lipids of ocular tissues. IX. The phospholipids of frog photoreceptor membranes. Vision Res. 1974 Jan;14(1):129–131. doi: 10.1016/0042-6989(74)90127-8. [DOI] [PubMed] [Google Scholar]
- Besharse J. C., Hollyfield J. G., Rayborn M. E. Photoreceptor outer segments: accelerated membrane renewal in rods after exposure to light. Science. 1977 Apr 29;196(4289):536–538. doi: 10.1126/science.300504. [DOI] [PubMed] [Google Scholar]
- Besharse J. C., Hollyfield J. G., Rayborn M. E. Turnover of rod photoreceptor outer segments. II. Membrane addition and loss in relationship to light. J Cell Biol. 1977 Nov;75(2 Pt 1):507–527. doi: 10.1083/jcb.75.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb C., Young R. W. Renewal of fatty acids in the membranes of visual cell outer segments. J Cell Biol. 1974 May;61(2):327–343. doi: 10.1083/jcb.61.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb C., Young R. W. Renewal of glycerol in the visual cells and pigment epithelium of the frog retina. J Cell Biol. 1974 Aug;62(2):378–389. doi: 10.1083/jcb.62.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry R. J., Chapman D. Optical properties of black lecithin films. J Mol Biol. 1969 Feb 28;40(1):19–32. doi: 10.1016/0022-2836(69)90293-9. [DOI] [PubMed] [Google Scholar]
- Farnsworth C. C., Dratz E. A. Oxidative damage of retinal rod outer segment membranes and the role of vitamin E. Biochim Biophys Acta. 1976 Sep 7;443(3):556–570. doi: 10.1016/0005-2736(76)90473-9. [DOI] [PubMed] [Google Scholar]
- Israelachvili J. N., Sammut R. A., Snyder A. W. Birefringence and dichroism of photoreceptors. Vision Res. 1976 Jan;16(1):47–52. doi: 10.1016/0042-6989(76)90075-4. [DOI] [PubMed] [Google Scholar]
- Kagan V. E., Shvedova A. A., Novikov K. N., Kozlov Y. P. Light-induced free radical oxidation of membrane lipids in photoreceptors of frog retina. Biochim Biophys Acta. 1973 Nov 30;330(1):76–79. doi: 10.1016/0005-2736(73)90285-x. [DOI] [PubMed] [Google Scholar]
- Kaplan M. W., Liebman P. A. Slow bleach-induced birefringence changes in rod outer segments. J Physiol. 1977 Mar;265(3):657–672. doi: 10.1113/jphysiol.1977.sp011736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenbrot J. I., Brown D. T., Cone R. A. Membrane characteristics and osmotic behavior of isolated rod outer segments. J Cell Biol. 1973 Feb;56(2):389–398. doi: 10.1083/jcb.56.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIEBMAN P. A. In situ microspectrophotometric studies on the pigments of single retinal rods. Biophys J. 1962 Mar;2:161–178. doi: 10.1016/s0006-3495(62)86847-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman P. A., Entine G. Sensitive low-light-level microspectrophotometer: detection of photosensitive pigments of retinal cones. J Opt Soc Am. 1964 Dec;54(12):1451–1459. doi: 10.1364/josa.54.001451. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Entine G. Visual pigments of frog and tadpole (Rana pipiens). Vision Res. 1968 Jul;8(7):761–775. doi: 10.1016/0042-6989(68)90128-4. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Jagger W. S., Kaplan M. W., Bargoot F. G. Membrane structure changes in rod outer segments associated with rhodopsin bleaching. Nature. 1974 Sep 6;251(5470):31–36. doi: 10.1038/251031a0. [DOI] [PubMed] [Google Scholar]
- MATTHEWS R. G., HUBBARD R., BROWN P. K., WALD G. TAUTOMERIC FORMS OF METARHODOPSIN. J Gen Physiol. 1963 Nov;47:215–240. doi: 10.1085/jgp.47.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsan P., Puzo G., Mazarguil H. Etude du mécanisme d'établissement des liaisons glutaraldéhyde-protéines. Biochimie. 1975 Nov-Dec;57(11-12):1281–1292. [PubMed] [Google Scholar]
- O'Brien P. J. Rhodopsin as a glycoprotein: a possible role for the oligosaccharide in phagocytosis. Exp Eye Res. 1976 Aug;23(2):127–137. doi: 10.1016/0014-4835(76)90196-2. [DOI] [PubMed] [Google Scholar]
- SIDMAN R. L. The structure and concentration of solids in photoreceptor cells studied by refractometry and interference microscopy. J Biophys Biochem Cytol. 1957 Jan 25;3(1):15–30. doi: 10.1083/jcb.3.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saibil H., Chabre M., Worcester D. Neutron diffraction studies of retinal rod outer segment membranes. Nature. 1976 Jul 22;262(5566):266–270. doi: 10.1038/262266a0. [DOI] [PubMed] [Google Scholar]
- Villermet G. M., Weale R. A. The optical activity of bleached retinal receptors. J Physiol. 1969 Apr;201(2):425–435. doi: 10.1113/jphysiol.1969.sp008764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLKEN J. J. Structure and molecular organization of retinal photoreceptors. J Opt Soc Am. 1963 Jan;53:1–19. doi: 10.1364/josa.53.000001. [DOI] [PubMed] [Google Scholar]
- Weale R. A. On the birefringence of rods and cones. Pflugers Arch. 1971;329(3):244–257. doi: 10.1007/BF00586618. [DOI] [PubMed] [Google Scholar]
- Weale R. A. Optical properties of photoreceptors. Br Med Bull. 1970 May;26(2):134–137. doi: 10.1093/oxfordjournals.bmb.a070764. [DOI] [PubMed] [Google Scholar]
- Wood J. G. The effects of glutaraldehyde and osmium on the proteins and lipids of myelin and mitochondria. Biochim Biophys Acta. 1973 Nov 2;329(1):118–127. doi: 10.1016/0304-4165(73)90014-7. [DOI] [PubMed] [Google Scholar]
- Young R. W. The renewal of photoreceptor cell outer segments. J Cell Biol. 1967 Apr;33(1):61–72. doi: 10.1083/jcb.33.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]