Abstract
Epstein–Barr virus (EBV) is implicated in the development of human B cell lymphomas and carcinomas. Although related oncogenic herpesviruses were believed to be endemic only in Old World primate species, we now find these viruses to be endemic in New World primates. We have isolated a transforming, EBV-related virus from spontaneous B cell lymphomas of common marmosets (Callithrix jacchus). Sequencing of two-thirds of the genome reveals considerable divergence from the genomes of EBV and Old World primate EBV-related viruses, including differences in genes important for virus-induced cell growth transformation and pathogenesis. DNA related to the C. jacchus herpesvirus is frequently detected in squirrel monkey peripheral blood lymphocytes, indicating that persistent infection with EBV-related viruses is prevalent in both New World primate families. Understanding how these more divergent EBV-related viruses achieve similar biologic outcomes in their natural host is likely to provide important insights into EBV infection, B cell growth transformation, and oncogenesis.
Epstein–Barr virus (EBV) and Kaposi's sarcoma herpesvirus (KSHV, HHV-8) are gammaherpesviruses associated with malignant diseases in humans. EBV is associated with the development of Burkitt's lymphomas, B cell lymphomas in immunosuppressed hosts, nasopharyngeal carcinoma, Hodgkin's disease, and gastric carcinomas, whereas KSHV is associated with Kaposi's sarcoma, body cavity lymphomas, and Castleman's disease. EBV-related herpesviruses have only been found in Old World primate species (1) and are grouped in the lymphocryptovirus (LCV) genera. In contrast, KSHV-related herpesviruses are found in Old World primates, New World primates, and nonprimate species and are grouped in the rhadinovirus (RV) genera.
Investigators at the Wisconsin Regional Primate Research Center (WRPRC) recently used polymerase chain reactions to amplify 178-bp and 378-bp DNA fragments that were most closely related to EBV DNA from peripheral blood lymphocytes (PBLs) and spontaneous B cell lymphomas of common marmosets (Callithrix jacchus) (2). The origin of these sequences was a mystery because these DNA fragments were the only evidence for an EBV-related virus in New World primates despite 30 years of experimental studies of EBV infection in these animals. We have now isolated a herpesvirus from these marmoset lymphomas, sequenced most of its genome, found that the agent has biologic properties similar to those of EBV, and show that EBV-related herpesviruses are endemic in both families of New World primates.
Materials and Methods
Cell Culture.
The CJ0149 cell line was derived by primary culture of a tumor biopsy from the WRPRC. The biopsy specimen was disaggregated and incubated in RPMI medium 1640 containing 10% FBS and 0.5 μg/ml of cyclosporin A.
Viral DNA Cloning and Sequencing.
Genomic DNA prepared from the CJ0149 cell line was partially digested with Sau3AI and cloned into the BamHI site of the Supercos I (Stratagene) vector. The A10 cosmid was isolated from the cosmid library by hybridization to a radiolabeled CalHV-3 DNA polymerase PCR product. Cosmids D6 and D4 were identified by hybridization with oligonucleotide probes from the ends of other cosmids. Cosmid DNA was randomly sheared by sonication, DNA fragments were cloned into pBluescript, and nucleotide sequences were derived from random clones.
Sequence Analysis.
The random nucleotide sequences were assembled with the use of sequencher (genecode, V4.05). The contiguous sequence consisted of at least 5-fold redundancy and was analyzed by using blast (National Center for Biotechnology Information) and mapdraw (DNAstar, Madison, WI) programs. The megalign program (DNAstar) was used to generate the phylogenetic tree and percentage homology for ORFs greater than 180 aa or homologous to known EBV or KSHV ORFs. The contiguous nucleotide sequence derived from the three cosmids has been deposited in GenBank (AF319782).
Induction of NF-κB and AP-1 Activity.
The CalHV-3 C1 expression construct (pCDNA C1) contained the C1 cDNA with an amino-terminal flag epitope tag under the control of a cytomegalovirus early promoter. The pCDNA C1d263 mutant was constructed by inserting a PCR amplification product with a stop codon at AA264. The rhesus LCV LMP1 expression construct (pCDNA rhLMP1) was constructed by reverse transcription–PCR (RT-PCR) amplification of a full-length cDNA clone from LCL8664 cell RNA, insertion of an amino-terminal flag epitope, and cloning into the pCDNA expression vector (Invitrogen). The EBV LMP1 was expressed from the pSG5 vector (Stratagene) as previously described (3). Expression constructs were cotransfected into 293 cells with a luciferase reporter gene under the control of three NF-κB-responsive elements (3xNFkB-Luc) (4) or seven AP-1-responsive elements (7xAP-1-Luc; Invitrogen) and a β-galactosidase expression plasmid to control for transfection efficiency. Data were normalized for β-galactosidase expression and reported as fold induction versus transfection of 3xNFkB-Luc with vector control DNA. Expression levels for LMP1 and C1 were confirmed after each transfection by Western blots probed with an anti-flag antibody (M2; Sigma).
Glutathione S-Transferase (GST)-Pulldown Assay.
GST fusion proteins with the complete (179–355:GST-C1) or truncated (179–263:GST-C1–263) C-terminal cytoplasmic domain of C1 were expressed in Escherichia coli and purified on glutathione–Sepharose beads as previously described (3). The immobilized GST fusion proteins were incubated with in vitro translated, 35S-labeled tumor necrosis factor receptor-associated factor-1 (TRAF1), TRAF2, or TRAF3 proteins. The immobilized GST fusion proteins were washed, and radiolabeled TRAF proteins binding to the GST fusion proteins were detected by SDS-PAGE and autoradiography (3). TRAF binding to GST fusion proteins with EBV LMP1 (GST-LMP1), rhesus LCV LMP1 (GST-rhLMP1), and CD30 (GST-CD30) were included as positive controls (3).
PCR Analysis.
Degenerative PCR primers and amplification conditions for amplifying diverse herpesvirus DNA polymerase sequences were used as described (5). CalHV-3-specific analysis was performed with the use of the following nested PCR primers: DP1739F: TTGACTTCGCCAGCCTCTAT and DP2045R: TTGCAGGTGCACTTGATAGC, DP1767F: CATTCAGGCCCACAATCTCT and DP1894R: TAACAAAGTGGAAGGTGCCC. The PCR products were analyzed by agarose gel electrophoresis, blotted to nylon filter, and probed with a 32P-labeled, CalHV-3-specific oligonucleotide (DP1821F: GTAACGCCGGGAGAAGAGGGGAA).
Results
A Cell Line Derived from a Common Marmoset B Cell Lymphoma Is Infected with a Transforming Herpesvirus.
A cell line was established from a B cell lymphoma biopsy on a WRPRC marmoset and was positive by PCR for the DNA polymerase sequence reported by Ramer et al. (2). To test whether the cell line was infected with and produced a transforming virus, cell-free filtered supernatants were used to infect marmoset, rhesus monkey, and human peripheral blood lymphocytes. The cell-free supernatants induced continuous proliferation of marmoset lymphocytes with kinetics similar to that of EBV-induced immortalization of human peripheral blood lymphocytes. Virus transmission was confirmed by PCR amplification of the DNA polymerase sequence from the resultant marmoset cell lines. No cell proliferation of marmoset lymphocytes was observed with control culture medium. Furthermore, the cell line culture supernatants did not induce the proliferation of rhesus monkey or human lymphocytes. These data indicate that the lymphoma-derived cell line is productive of a herpesvirus that specifically or preferentially immortalizes marmoset lymphocytes.
Cloning and Sequencing of the Marmoset Lymphoma Herpesvirus.
To further identify the transforming herpesvirus, DNA fragments from total cell genomic DNA were cloned into a cosmid vector, and a viral DNA clone was identified by hybridization to the labeled DNA polymerase PCR product. Two additional viral DNA cosmid clones were identified by sequential hybridization with sequences from the end of each cosmid clone. A total of 104,678 bp of contiguous viral DNA was sequenced with an overall GC content of 50%. A GC-rich (75%), 328-bp repeat sequence was found at one end that is similar to the terminal repeats found at the ends of most herpesvirus genomes. The viral DNA sequence differed from that of known herpesviruses. In accordance with herpesvirus nomenclature, this virus is named CalHV-3 because it is the third herpesvirus identified in the Callitrichidae subfamily of New World primates.
CalHV-3 Is a Gammaherpesvirus in the LCV Genera.
The viral DNA sequence of 105 kb encodes for 60 potential ORFs. The arrangement and amino acid homology of these ORFs indicate that CalHV-3 is a gammaherpesvirus. As shown in Fig. 1 and Table 1, 50 ORFs have homologues common among herpesviruses and are organized in the same order as the two human gammaherpesviruses, EBV and KSHV. Moreover, each of these 50 common ORFs have greater homology to EBV and KSHV genes than to that of any other alpha- or betaherpesviruses. This comparison suggests that approximately two-thirds of the CalHV-3 genome has been cloned and sequenced.
Figure 1.
Schema of the partial CalHV-3 genome and comparison with EBV and KSHV. Solid boxes represent ORFs common among all gammaherpesviruses, and the role for the conserved ORFs is shown where known. CalHV-3 ORFs with homologues restricted to EBV and other LCVs are shown as shaded boxes. ORFs unique to CalHV-3, EBV, or KSHV are shown as open boxes, wavy boxes, or hatched boxes, respectively. The size of the coding and intergenic regions is not proportional to the actual genome sequences. The schema represents approximately 105 kbp, 96 kbp, and 84 kbp of the CalHV-3, EBV, and KSHV genomes, respectively, absent the terminal repeats (TR).
Table 1.
Homology of CaIHV-3, EBV, and KSHV ORFs
| CaIHV-3 name | CaIHV-3 vs.
|
Description | |||
|---|---|---|---|---|---|
| EBV
|
KSHV
|
||||
| Name | % Hom | Name | % Hom | ||
| C1 | LMP1 | 18.8 | Transforming gene | ||
| K1 | Transforming gene | ||||
| BARF1 | CSF1R homologue | ||||
| ORF4 | Complement binding | ||||
| ORF1 | BALF1 | 27.6 | bcl-2 homologue | ||
| ORF2 | BALF2 | 68.3 | ORF6 | 40.3 | DNA binding protein |
| ORF3 | BALF3 | 55.2 | ORF7 | 34.8 | |
| ORF4 | BALF4 | 58.7 | ORF8 | 34.9 | Glycoprotein B |
| ORF5 | BALF5 | 73.5 | ORF9 | 48.6 | DNA polymerase |
| ORF6 | BILF1 | 38.2 | Membrane protein | ||
| ORF7 | Raji LF1 | 24.1 | |||
| ORF10 | |||||
| ORF8 | Raji LF2 | 63.6 | ORF11 | 22.4 | |
| Raji LF3 | |||||
| BILF2 | Glycoprotein (gp55) | ||||
| C2 | Membrane protein | ||||
| K2 | IL-6 homologue | ||||
| ORF02 | Dihydrofolate reductase | ||||
| K3 | IE-1 homologue | ||||
| ORF70 | Thymidylate synth. | ||||
| K4 | vMIP-II | ||||
| K5 | IE-1 homologue | ||||
| K6 | vMIP-I | ||||
| K7 | |||||
| ORF16 | bcl-2 homologue | ||||
| ORF9 | BVRF2 | 38.3 | ORF17 | 28.4 | Capsid protein |
| ORF10 | BVLF1.5 | 36.2 | ORF18 | 29.8 | |
| ORF11 | BVRF1 | 47.4 | ORF19 | 33.5 | Tegument protein |
| ORF12 | BXRF1 | 37.1 | ORF20 | 25.2 | |
| ORF13 | BXLF1 | 47.0 | ORF21 | 24.8 | Thymidine kinase |
| ORF14 | BXLF2 | 44.6 | ORF22 | 20.9 | Glycoprotein H |
| ORF15 | BTRF1 | 57.0 | ORF23 | 24.8 | |
| ORF16 | BcRF1 | 36.5 | ORF24 | 27.8 | |
| ORF17 | BcLF1 | 75.5 | ORF25 | 55.7 | Capsid protein |
| ORF18 | BDLF1 | 70.1 | ORF26 | 50.0 | Capsid protein |
| ORF19 | BDLF2 | 26.3 | ORF27 | 13.1 | |
| BDLF3 | Glycoprotein (gp 150) | ||||
| ORF28 | |||||
| ORF20 | BDRF1 | 74.6 | ORF29b | 57.8 | Packaging protein |
| ORF21 | BDLF4 | 51.9 | ORF31 | 28.3 | |
| ORF22 | BGLF1 | 30.6 | ORF32 | 15.0 | |
| ORF23 | BGLF2 | 57.4 | ORF33 | 24.5 | |
| ORF24 | BGRF1 | 63.7 | ORF29a | 45.3 | Packaging protein |
| ORF25 | BGLF3 | 48.2 | ORF34 | 30.0 | |
| ORF26 | BGLF3.5 | 35.6 | ORF35 | 26.2 | |
| ORF27 | BGLF4 | 59.9 | ORF36 | 24.1 | Kinase |
| ORF28 | BGLF5 | 69.6 | ORF37 | 36.9 | Alk. exonuclease |
| ORF29 | BBLF1 | 38.7 | ORF38 | 16.4 | |
| ORF30 | BBRF3 | 55.6 | ORF39 | 40.5 | Glycoprotein M |
| ORF31 | BBLF2 | 20.3 | ORF40 | 13.6 | Helicase complex |
| ORF32 | BBLF3 | 22.9 | ORF41 | 14.4 | Helicase complex |
| ORF33 | BBRF2 | 55.8 | ORF42 | 27.7 | |
| ORF34 | BBRF1 | 70.6 | ORF43 | 46.6 | Capsid protein |
| ORF35 | BBLF4 | 65.3 | ORF44 | 47.8 | Helicase complex |
| ORF36 | BKRF4 | 27.7 | ORF45 | 15.2 | |
| ORF37 | BKRF3 | 73.7 | ORF46 | 46.7 | Uracil DNA glyc. |
| ORF38 | BKRF2 | 50.4 | ORF47 | 18.1 | Glycoprotein L |
| ORF39 | EBNA-1 | 36.0 | Episomal maint. | ||
| ORF40 | BRRF2 | 22.4 | ORF48 | 14.2 | |
| ORF41 | BRRF1 | 46.7 | ORF49 | 16.2 | |
| ORF42 | BRLF1 | 39.0 | ORF50 | 13.8 | Transactivator |
| ORF43 | BZLF1 | 29.0 | K8 | 13.9 | Transactivator |
| ORF44 | BZLF2 | 33.6 | |||
| EBNA3A | Nuclear protein | ||||
| EBNA3B | Nuclear protein | ||||
| EBNA3C | Nuclear protein | ||||
| C3 | |||||
| ORF45 | gp350 | 22.0 | Glycoprotein | ||
| K8.1 | |||||
| ORF46 | BLRF2 | 40.4 | ORF52 | 22.1 | |
| ORF47 | BLRF1 | 44.1 | ORF53 | 34.6 | |
| ORF48 | BLLF3 | 54.3 | ORF54 | 26.8 | dUTPase |
| ORF49 | BSRF1 | 61.5 | ORF55 | 40.0 | |
| ORF50 | BSLF1 | 46.9 | ORF56 | 27.1 | DNA replication |
| ORF51 | BMLF1 | 38.8 | ORF57 | 18.9 | Transactivator |
| K9 | |||||
| K10 | |||||
| K11 | |||||
| ORF52 | BMRF2 | 46.2 | ORF58 | 21.8 | |
| ORF53 | BMRF1 | 40.4 | ORF59 | 18.2 | DNA replication |
| ORF54 | BaRF1 | 73.5 | ORF60 | 55.4 | Ribonucleotide red. |
| ORF55 | BORF2 | 55.5 | ORF61 | 38.8 | Ribonucleotide red. |
| ORF56 | BORF1 | 46.8 | ORF62 | 29.9 | DNA maturation |
| ORF57 | BOLF1 | 37.5 | ORF63 | 18.4 | Tegument protein |
In each case, the 50 common ORFs are more similar to the EBV than they are to the KSHV homologues (Table 1), indicating that CalHV-3 belongs to the LCV genera, rather than RV genera. CalHV-3 also encodes for seven genes that have homologues found only in EBV and other LCVs and are not present in any RVs. One of these LCV-restricted ORFs, ORF39, shows strong homology to the EBV nuclear antigen 1, a viral protein important for maintenance of the EBV episome (6), and no homology to the KSHV latency-associated antigen, LANA, which is important for the maintenance of the KSHV episome (7). ORF39 has a conserved carboxy-terminal DNA binding domain (42% similarity to EBNA-1) but only a single arginine–glycine-rich domain and no significant glycine–alanine repeat region. Furthermore, CalHV-3 does not encode any ORFs that are restricted to RVs and not found in LCVs. Thus, CalHV-3 is a LCV found to be naturally infecting a New World primate.
CalHV-3 Encodes Genes Unique from Other LCV.
The CalHV-3 genome has several striking differences from EBV that have not been previously observed in other members of the oncogenic LCV. Three CalHV-3 genes have no significant amino acid homology by BLAST search to known viral or cellular genes and are referred to as C1, C2, and C3. All three of these genes are located in divergent regions of the gammaherpesvirus genome, where genes restricted to LCV or RV are found (Fig. 1, open boxes). For example, C2 is located at a position comparable to where EBV and rhesus LCV encode a glycoprotein (BILF2) and where KSHV encodes several RV-restricted ORFs, including IL-6, bovine herpesvirus type 4 immediate early gene, and macrophage inflammatory protein homologues (8, 9). C2 has Ig-like domains, but a potential function for C2 remains unknown.
C1 Has Structural and Functional Similarities to the EBV LMP1 Oncogene.
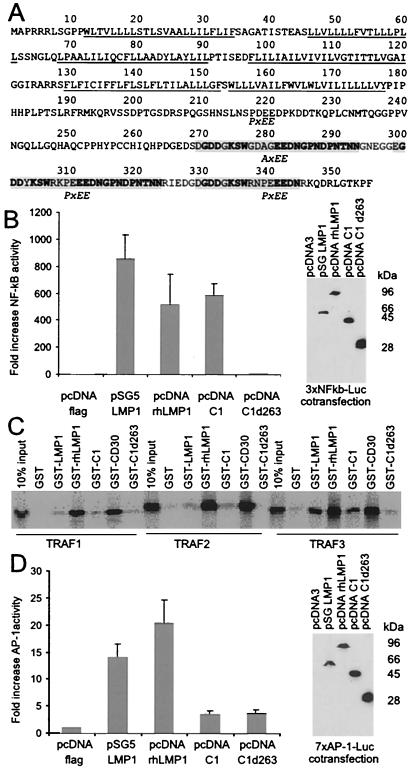
C1 is located near the terminal repeats where all gammaherpesviruses sequenced to date encode an oncogene (10). EBV and simian LCVs encode for a membrane protein expressed during latent infection, LMP1 (3). LMP1 is essential for EBV-induced growth transformation and signals through the tumor necrosis factor receptor pathway to activate NF-κB and AP-1 activity (11–13). C1 is predicted to be a spliced gene with two short exons and one long exon, based on computer analysis. This finding was confirmed by RT-PCR analysis of RNA from CalHV-3-infected cells, with the use of primers spanning the first and second introns (data not shown), and the C1 coding sequence was verified by sequencing the RT-PCR products (Fig. 2A). C1 is predicted to be similar to LMP1 in containing six hydrophobic transmembrane domains, a large carboxy-terminal domain, and amino and carboxy termini that both reside in the cytoplasm. However, C1 has little primary amino acid sequence homology to LMP1. C1 lacks sequences related to the known transformation effector site 1 (PXQXS/T motif) or transformation effector site 2 (PXQXXYYD/A) sequences present in EBV and rhesus LCV LMP1 that interact with TRAFs or tumor necrosis factor receptor-associated death domain protein (TRADD), respectively, to activate NF-κB (3, 11, 14).
Figure 2.
CalHV-3 C1 amino acid sequence, activation of NF-κB, TRAF interaction, and lack of AP-1 induction. (A) The six hydrophobic transmembrane domains are underlined. Repeat elements in the carboxy terminus containing putative TRAF-binding sequence motifs (P/AxEE) are indicated by shading, with conserved residues shown in bold. (B) C1 induces NF-κB activity. A luciferase reporter gene under the control of three NF-κB-responsive elements (3xNFkB-Luc) was cotransfected into 293 cells with flag epitope-tagged expression vectors for EBV LMP1 (pSG5 LMP1), rhesus LCV LMP1 (pcDNA rhLMP1), CalHV-3 C1 (pcDNA C1), and a CalHV-3 C1 mutant truncated at aa 263 (pcDNA C1d263). Data were normalized for β-galactosidase expression and reported as fold induction versus transfection of 3xNFkB-Luc with the pcDNA-Flag as vector control DNA. Results represent the average of four independent transfections. Expression levels for LMP1 and C1 were confirmed after each transfection by Western blots probed with an anti-flag antibody (M2). A representative immunoblot is shown. (C) The C1 carboxy-terminal cytoplasmic domain can bind to TRAF proteins. GST fusion proteins with the complete (aa 179–355: GST-C1) or truncated (aa 179–263: GST-C1d263) C-terminal cytoplasmic domain of C1 were used to precipitate in vitro translated, 35S-labeled TRAF1, TRAF2, or TRAF3 proteins. TRAF binding to GST fusion proteins with EBV LMP1 (GST-LMP1), rhesus LCV LMP1 (GST-rhLMP1), and CD30 (GST-CD30) were included as positive controls. (D) C1 does not induce AP-1 activity. The same expression constructs as in B were cotransfected with a luciferase reporter gene under the control of seven AP-1-responsive elements (7xAP-1-Luc).
However, C1 can still induce NF-κB activity to levels comparable to those of the rhesus LCV and EBV LMP1 when transfected into human 293 cells (Fig. 2B). C1 may signal through the TRAF pathway by way of consensus TRAF binding motifs (P/S/A/T)x(Q/E)E (15) in the C1 carboxy terminus that are different from those in EBV and other Old World LCV. Transfection of a C1 mutant with a stop codon at amino acid 264 (C1d263) failed to induce NF-κB activity above background despite comparable C1 protein expression levels detected by epitope tags in the expression constructs (Fig. 2B). These data suggest that three of four potential (P/A)xEE consensus TRAF binding motifs located in the repeated region of the C1 carboxy terminus are important for NF-κB signaling.
Furthermore, the C1 carboxy terminus can interact with TRAFs that are important for inducing NF-κB activity (11, 16, 17). As shown in Fig. 2C, the GST-C1 fusion protein was capable of binding TRAF1, -2, and -3 in pulldown assays, in a manner similar to that of the GST proteins fused with EBV LMP1, rhesus LCV LMP1, and CD30 and used as positive controls. TRAF2 is well conserved between the human and mouse genomes (84% amino acid similarity); thus it is likely that the primate TRAFs are highly conserved. TRAF2 and TRAF3 (but not TRAF1) binding was reduced with the use of a GST fusion with a mutated C1 truncated at aa 263 (Fig. 3D; by densitometry, GST-C1d263 bound 61%, 0%, and 5% of TRAF1, -2, and -3, respectively, compared with GST-C1), suggesting that TRAF2/3 interactions with sites in the repeat elements are important for C1 induction of NF-κB activity.
Figure 3.
Prevalence of persistent CalHV-3 infection in two different common marmoset colonies. CalHV-3-specific primers for the DNA polymerase gene were used for nested PCR amplification of PBL genomic DNA from common marmosets at the WRPRC and NERPRC. The EBV-negative human B cell line BJAB and water were used as negative controls.
However, C1 was different from LMP1 in its inability to induce AP-1 activity. As shown in Fig. 2D, both EBV and rhesus LCV LMP1s induced AP-1 activity 15- to 20-fold over vector control, but similar levels of C1 expression failed to induce significant levels of AP-1 activity. Thus, C1 has functional similarities to LMP1 in its ability to induce NF-κB and bind TRAFs but differs from LMP1 in its inability to activate AP-1.
C3 Is a Positional Homologue for the EBV EBNA-3A, -3B, and -3C Genes.
C3 is a unique CalHV-3 gene encoded between homologues for the EBV major membrane antigen and the lytic infection Zebra transactivator (ORF 44 and 43; Table 1). At a similar position, both EBV and rhesus LCV encode three distantly related latent infection genes (EBNA-3A, -3B, and -3C), each containing more than 900 aa (18–21). EBNA-3A and -3C are indispensable for EBV-induced B lymphocyte growth transformation (22, 23), but the functions for the EBNA-3s remain poorly defined. A spliced C3 transcript from two exons was detected by RT-PCR from CalHV-3-infected cell RNA, and an 825-aa ORF was confirmed by sequencing of the RT-PCR product (data not shown). C3 has no homology to any viral or cellular genes or to any specific domains in the EBNA-3s. Because CalHV-3 is also able to efficiently immortalize B lymphocytes, the substitution of three EBNA-3 genes with a single C3 gene suggests that C3 is likely to be important for virus-induced growth transformation. It remains to be determined whether C3 provides a growth signal comparable to those of EBNA-3A and -3C or whether C3 contributes to growth transformation through a unique molecular mechanism.
Other Unique Features of the CalHV-3 Genome.
CalHV-3 is also notable for the absence of three genes, BARF1, BDLF3, and LMP2, that are likely to be important for EBV pathogenesis in vivo. BARF1 is a secreted homologue of the CSF1 receptor that is highly conserved in EBV and rhesus LCV (ref. 24 and P.R., Y.-G.C., and F.W., unpublished data). BDLF3 is a viral glycoprotein. BARF1 and BDLF3 are expressed during lytic viral replication but are not essential for virus replication in tissue culture (25, 36). Thus these viral gene products are most likely important for EBV infection in vivo but appear to be unnecessary for CalHV-3 infection. There is also no apparent homologue for the LMP2A first exon encoded adjacent to BARF1 in the EBV genome. LMP2A interacts with src protein tyrosine kinases, modulates src family tyrosine kinase signaling, and prevents signaling-induced activation of lytic virus infection via a well-conserved immunoreceptor tyrosine-based activation motif in the first exon of EBV and rhesus LCV (26–28). LMP2A is not essential for EBV-induced B cell growth transformation but may be important for preventing viral reactivation in vivo (26). However, an immunoreceptor tyrosine-based activation motif or LMP2A homologue is not evident at the corresponding locus in CalHV-3. Whether a functional LMP2 homologue is encoded in the remaining exons or whether there may alternative ways in which CalHV-3 inhibits viral reactivation remain to be determined.
CalHV-3 Infection Is Prevalent in Marmosets at Two Different Primate Centers.
The sequence analysis demonstrates that CalHV-3 is a previously uncharacterized LCV; however, natural LCV infection has never previously been recognized in New World primates (1). Therefore, we tested for the presence of CalHV-3 infection at the New England Regional Primate Research Center (NERPRC) to determine whether CalHV-3 infection is indigenous to marmoset populations or whether infection is limited to WRPRC marmosets, suggesting a potential cross-species jump of an Old World LCV to marmosets during captivity. DNA was prepared from the peripheral blood lymphocytes of random healthy marmosets at the two primate centers, and evidence for CalHV-3 infection was determined by PCR amplification with the use of primers specific for the CalHV-3 DNA polymerase. CalHV-3 DNA was detected in 6 of 10 healthy marmosets at the WRPRC and 18 of 30 healthy marmosets at the NERPRC (16 samples shown in Fig. 3). The nucleotide sequence of PCR products from eight random NERPRC animals was >96% identical to the CalHV-3 DNA polymerase sequence derived from WRPRC animals. These results show that persistent CalHV-3 infection is prevalent at similar levels in two geographically separated marmoset populations and suggests that CalHV-3 infection is indigenous to this New World primate species.
LCV Infection Is Prevalent in Other New World Primate Species.
The discovery of CalHV-3 in marmosets suggests that LCV infection is not restricted to Old World primates and that LCV are more widespread than currently believed. Because marmosets belong to the Callitrichidae family of New World primates, we looked for evidence of LCV infection in squirrel monkeys (Saimiri scireus), which belong to the other major New World primate family, Cebidae. Five randomly selected squirrel monkeys were determined to be positive with the use of degenerate PCR primers to amplify herpesvirus DNA polymerase sequences from PBL DNA, and two DNA PCR clones from each of four animals were sequenced. A previously uncharacterized herpesvirus DNA polymerase sequence was obtained from each animal, and the consensus amino acid sequence derived from the PCR sequences has 68%, 70%, and 49% homology with the CalHV-3, EBV, and KSHV DNA polymerase sequences, respectively (Fig. 4A), indicating that these animals are also infected with a previously uncharacterized LCV. Phylogenetic analysis shows that this sequence is more closely related to CalHV-3 and other LCVs than to RVs (Fig. 4B). By convention, it is named Saimiriine herpesvirus-3 for the third herpesvirus in the Saimirinae subfamily. One PCR clone from animal 4 had a different sequence with 95% identity to the DNA polymerase gene from a known RV, Saimiriine herpesvirus-2. Thus, LCV infection is prevalent in multiple New World species, and persistent coinfection with LCV and RV occurs in nonhuman primates as well as humans.
Figure 4.
LCV infection in squirrel monkeys (Saimiri scireus) and phylogenetic relationship of LCVs and RVs in human and primates. (A) Herpesvirus DNA polymerase sequences detected in squirrel monkey PBLs. Degenerative oligonucleotide primer sets targeted to highly conserved regions of the herpesvirus DNA polymerase were used in nested PCR reactions with squirrel monkey PBL genomic DNA (5). The consensus sequence from a putative LCV in squirrel monkeys, Saimiriine herpesvirus-3, is shown at the top, and amino acid differences are shown for each clone sequenced from four animals. Conserved and missing amino acid residues are indicated by dots and dashes. Amino acid differences between animal 4, clone 2, and the RV Saimiriine herpesvirus-2 DNA polymerase sequence in GenBank (X64346) are underlined. (B) Phylogenetic relationship of LCVs from New World primates to other LCVs (EBV: V01555; baboon LCV: AF091051; rhesus LCV: AF091053) and RV (KSHV: U75698, HVS:X64346; RRV: 2625044; MHV-68: U97553). The New World primate LCVs are shown in italics.
Discussion
These studies represent a definitive identification of LCV infection in New World primates and demonstrate that oncogenic LCVs are more widespread than previously believed. It remains to be determined whether LCVs exist in additional nonprimate species, as is the case for RV. Careful investigation for potential pathogens and reassessing historical concepts of virus host range become increasingly important as the push for xenotransplantation progresses.
These studies also establish phylogenetic relationships between EBV, Old World, and New World LCVs. LCVs all share common biologic properties of B lymphocyte growth transformation in vitro, a high prevalence of infection in the natural host, immune evasion, persistent infection, and the potential for oncogenic transformation in vivo. The New World LCVs, as typified by CalHV-3, appear to have evolved with a genetic repertoire that is slightly different from those of EBV and Old World LCVs in response to the selective pressure for these common biologic properties.
For example, B lymphocyte growth transformation is a common property shared among all LCVs. Old World LCVs are known to have homologues with moderate sequence homology to each of the latent infection genes expressed in EBV-immortalized B cells, and these homologues can functionally substitute for the EBV gene in nearly every in vitro assay examined to date (3, 21, 27–31). In contrast, CalHV-3 encodes a different repertoire of potential latent infection genes as compared with EBV and Old World LCVs. The most dramatic difference is the substitution of the C3 gene for the three EBNA-3 genes. Despite these differences in gene repertoire, the selective pressure for B lymphocyte growth transformation most likely resulted in the evolution of similar, if not identical, molecular strategies for interrupting normal cell growth processes by these related herpesviruses. This is the case for the C1 gene, which shares little sequence homology with the EBV and Old World LCV LMP1s, but has evolved with a common strategy for signal transduction through the tumor necrosis factor receptor pathway involving TRAFs and NF-κB. Similarly, C3 may function through the use of molecular pathways in common with the EBNA-3 genes, despite the lack of any significant sequence homology. It is interesting to note that the three EBNA-3 genes share a similar intron–exon structure, distant homology, and a common interaction with the transcription factor RBP-Jk, suggesting a possible common origin through gene duplication. Thus, the fundamental molecular role for this genetic locus may become more evident in CalHV-3, where the evolution of a single gene is sufficient for the transforming virus.
CalHV-3 infection in marmosets can also provide a valuable animal model for EBV pathogenesis and associated malignancies. Persistent virus infection, immune evasion, and association with spontaneous malignant transformation are key features of EBV infection in vivo. These studies show that like EBV infection in humans, asymptomatic CalHV-3 infection is persistent and prevalent in common marmosets. However, the CalHV-3 gene repertoire is different and lacks two EBV homologues, BARF1 and LMP2A, that are hypothesized to be important for immune evasion and persistent infection. Thus, other mechanisms for immune evasion must be essential for persistent infection and immune evasion by New World LCV, and infection of marmosets with mutant viruses can be used to test which LCV genes are essential for persistent infection in the natural host.
The development of spontaneous CalHV-3-positive lymphomas in otherwise healthy and immunocompetent marmosets is also an important phenomenon and suggests that other cofactors contribute to CalHV-3-associated lymphomagenesis. The marmoset lymphomas typically arise in the abdominal cavity, and the association of endemic CalHV-3 infection and recurrent enteritis in marmosets is reminiscent of the two-step model for Burkitt's lymphoma, where persistent EBV infection and c-myc translocation, perhaps associated with endemic malaria infection, result in malignant B cell transformation. CalHV-3-positive B cell lymphomas also occur in marmosets at the NERPRC, although not with as high a frequency as observed at the WRPRC (M. Simon, Y.-G.C., and F.W., unpublished observations). Because the prevalence of CalHV-3 infection is similar at the two primate centers, this difference in frequency of occurrence of B cell lymphomas suggests that a second event contributing to LCV-associated malignancies in marmosets occurs more frequently at the WRPRC. LCV-positive B cell lymphomas also occur in Old World macaque species, but almost always in the setting of simian immunodeficiency virus infection and immunosuppression (32). These LCV-associated malignancies in Old World primates resemble the development of EBV-positive lymphomas in AIDS, posttransplant, and genetically immunodeficient patients (33–35), where a compromised immune system results in uncontrolled growth of LCV-infected B cells. Thus the identification of both Old World and New World LCVs provides important animal model systems for studying these different pathogenetic pathways for EBV-induced lymphomas in humans.
Acknowledgments
We thank Drs. Keith Mansfield and Angela Carville for their assistance with the NERPRC marmoset colony. This work was funded by grants from the U.S. Public Health Service (CA68051 and CA65319 to F.W.) and American Heart Association (9740129N). Services by the NERPRC and the WRPRC were supported by base grants to these institutions (U.S. Public Health Service Grants P51RR00168 and RR00167).
Abbreviations
- EBV
Epstein–Barr virus
- KSHV
Kaposi's sarcoma herpesvirus
- LCV
lymphocryptovirus
- RV
rhadinovirus
- WRPRC
Wisconsin Regional Primate Research Center
- PBL
peripheral blood lymphocyte
- RT-PCR
reverse transcription–PCR
- GST
glutathione S-transferase
- TRAF
tumor necrosis factor receptor-associated factor
- NERPRC
New England Regional Primate Research Center
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF319782).
References
- 1.Frank A, Andiman W A, Miller G. Adv Cancer Res. 1976;23:171–201. doi: 10.1016/s0065-230x(08)60546-1. [DOI] [PubMed] [Google Scholar]
- 2.Ramer J C, Garber R L, Steele K E, Boyson J F, O'Rourke C, Thomson J. Comp Med. 2000;50:59–68. [PubMed] [Google Scholar]
- 3.Franken M, Devergne O, Rosenzweig M, Annis B, Kieff E, Wang F. J Virol. 1996;70:7819–7826. doi: 10.1128/jvi.70.11.7819-7826.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchell T, Sugden B. J Virol. 1995;69:2968–2976. doi: 10.1128/jvi.69.5.2968-2976.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.VanDevanter D R, Warrener P, Bennett L, Schultz E R, Coulter S, Garber R L, Rose T M. J Clin Microbiol. 1996;34:1666–1671. doi: 10.1128/jcm.34.7.1666-1671.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yates J L, Warren N, Sugden B. Nature (London) 1985;313:812–815. doi: 10.1038/313812a0. [DOI] [PubMed] [Google Scholar]
- 7.Ballestas M E, Chatis P A, Kaye K M. Science. 1999;284:641–644. doi: 10.1126/science.284.5414.641. [DOI] [PubMed] [Google Scholar]
- 8.Russo J J, Bohenzky R A, Chien M C, Chen J, Yan M, Maddalena D, Parry J P, Peruzzi D, Edelman I S, Chang Y, Moore P S. Proc Natl Acad Sci USA. 1996;93:14862–14867. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baer R, Bankier A T, Biggin M D, Deininger P L, Farrell P J, Gibson T J, Hatfull G, Hudson G S, Satchwell S C, Seguin C, et al. Nature (London) 1984;310:207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- 10.Damania B, Choi J K, Jung J U. J Virol. 2000;74:1593–1601. doi: 10.1128/jvi.74.4.1593-1601.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosialos G, Birkenbach M, Yalamanchili R, VanArsdale T, Ware C, Kieff E. Cell. 1995;80:389–399. doi: 10.1016/0092-8674(95)90489-1. [DOI] [PubMed] [Google Scholar]
- 12.Kaye K M, Izumi K M, Kieff E. Proc Natl Acad Sci USA. 1993;90:9150–9154. doi: 10.1073/pnas.90.19.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eliopoulos A G, Blake S M, Floettmann J E, Rowe M, Young L S. J Virol. 1999;73:1023–1035. doi: 10.1128/jvi.73.2.1023-1035.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Izumi K M, Kaye K M, Kieff E D. Proc Natl Acad Sci USA. 1997;94:1447–1452. doi: 10.1073/pnas.94.4.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye H, Park Y C, Kreishman M, Kieff E, Wu H. Mol Cell. 1999;4:321–330. doi: 10.1016/s1097-2765(00)80334-2. [DOI] [PubMed] [Google Scholar]
- 16.Wallach D, Varfolomeev E E, Malinin N L, Goltsev Y V, Kovalenko A V, Boldin M P. Annu Rev Immunol. 1999;17:331–367. doi: 10.1146/annurev.immunol.17.1.331. [DOI] [PubMed] [Google Scholar]
- 17.Devergne O, Hatzivassiliou E, Izumi K M, Kaye K M, Kleijnen M F, Kieff E, Mosialos G. Mol Cell Biol. 1996;16:7098–7108. doi: 10.1128/mcb.16.12.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hennessy K, Wang F, Bushman E W, Kieff E. Proc Natl Acad Sci USA. 1986;83:5693–5697. doi: 10.1073/pnas.83.15.5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petti L, Sample J, Wang F, Kieff E. J Virol. 1988;62:1330–1338. doi: 10.1128/jvi.62.4.1330-1338.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petti L, Kieff E. J Virol. 1988;62:2173–2178. doi: 10.1128/jvi.62.6.2173-2178.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang H, Cho Y, Wang F. J Virol. 2000;74:5921–5932. doi: 10.1128/jvi.74.13.5921-5932.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomkinson B, Kieff E. J Virol. 1992;66:2893–2903. doi: 10.1128/jvi.66.5.2893-2903.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomkinson B, Robertson E, Kieff E. J Virol. 1993;67:2014–2025. doi: 10.1128/jvi.67.4.2014-2025.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strockbine L D, Cohen J I, Farrah T, Lyman S D, Wagener F, DuBose R F, Armitage R J, Spriggs M K. J Virol. 1998;72:4015–4021. doi: 10.1128/jvi.72.5.4015-4021.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen J I, Lekstrom K. J Virol. 1999;73:7627–7632. doi: 10.1128/jvi.73.9.7627-7632.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller C L, Lee J H, Kieff E, Longnecker R. Proc Natl Acad Sci USA. 1994;91:772–776. doi: 10.1073/pnas.91.2.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franken M, Annis B, Ali A N, Wang F. J Virol. 1995;69:8011–8019. doi: 10.1128/jvi.69.12.8011-8019.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rivailler P, Quink C, Wang F. J Virol. 1999;73:8867–8872. doi: 10.1128/jvi.73.10.8867-8872.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blake N W, Moghaddam A, Rao P, Kaur A, Glickman R, Cho Y G, Marchini A, Haigh T, Johnson R P, Rickinson A B, et al. J Virol. 1999;73:7381–7389. doi: 10.1128/jvi.73.9.7381-7389.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ling P D, Ryon J J, Hayward S D. J Virol. 1993;67:2990–3003. doi: 10.1128/jvi.67.6.2990-3003.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng R, Gordadze A V, Fuentes Panana E M, Wang F, Zong J, Hayward G S, Tan J, Ling P D. J Virol. 2000;74:379–389. doi: 10.1128/jvi.74.1.379-389.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feichtinger H, Putkonen P, Parravicini C, Li S T, Kaya E E, Bottiger D, Biberfeld P. Am J Pathol. 1990;137:1311–1315. [PMC free article] [PubMed] [Google Scholar]
- 33.Purtilo D T, DeFlorio D J, Hutt L M, Bhawan J, Yang J P, Otto R, Edwards W. N Engl J Med. 1977;297:1077–1080. doi: 10.1056/NEJM197711172972001. [DOI] [PubMed] [Google Scholar]
- 34.Ziegler J L, Miner R C, Rosenbaum E, Lennette E T, Shillitoe E, Casavant C, Drew W L, Mintz L, Gershow J, Greenspan J, et al. Lancet. 1982;2:631–633. doi: 10.1016/s0140-6736(82)92740-4. [DOI] [PubMed] [Google Scholar]
- 35.Cleary M L, Warnke R, Sklar J. N Engl J Med. 1984;310:477–482. doi: 10.1056/NEJM198402233100801. [DOI] [PubMed] [Google Scholar]
- 36.Borza C M, Hutt-Fletcher L. J Virol. 1998;72:7577–7582. doi: 10.1128/jvi.72.9.7577-7582.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]