Abstract
In the adult peripheral nerve, microvillous processes of myelinating Schwann cells project to the nodes of Ranvier; their composition and physiologic function have not been established. As the ezrin-radixin-moesin (ERM) proteins are expressed in the microvilli of many epithelial cells, we have examined the expression and distribution of these proteins in Schwann cells and neurons in vitro and in vivo. Cultured Schwann cells express high levels of all three proteins and the ezrin-binding protein 50, whereas neurons express much lower, although detectable, levels of radixin and moesin. Ezrin is specific for Schwann cells. All three ERM proteins are expressed predominantly at the membrane of cultured Schwann cells, notably in their microvilli. In vivo, the ERM proteins are concentrated strikingly in the nodal processes of myelinating Schwann cells. Because these processes are devoid of myelin proteins, they represent a unique compartment of the myelinating Schwann cell. During development, the ERM proteins become concentrated at the ends of Schwann cells before myelin basic protein expression, demonstrating that Schwann cells are polarized longitudinally at the onset of myelination. ERM-positive Schwann cell processes overlie and are associated closely with nascent nodes of Ranvier, identified by clusters of ankyrin G. Ankyrin accumulation at the node precedes that of Caspr at the paranodes and therefore does not depend on the presence of mature paranodal junctions. These results demonstrate that nodes of Ranvier in the peripheral nervous system form in contact with specialized processes of myelinating Schwann cells that are highly enriched in ERM proteins.
Nodes of Ranvier are the regularly spaced gaps between myelin segments where action potentials regenerate and propagate via saltatory conduction (1, 2). Central to their role in nerve conduction, the nodal axolemma exhibits a high concentration of sodium channels. Ultrastructural and electrophysiological studies have estimated the concentration of sodium channels at up to 1,500/μm2, compared with less than 100/μm2 in the adjacent internodes (3–5). In addition to sodium channels, other proteins such as the cytoskeletal protein ankyrin G and the neural cell adhesion molecules, NrCAM and neurofascin (the 186-kDa isoform), are also concentrated in the nodal axolemma where they are likely to form a multiprotein complex (reviewed in refs. 6 and 7).
The mechanism(s) by which these axonal proteins concentrate and are retained at the node is not yet known. These proteins are distributed uniformly along axons before myelination (8). With development, clusters of sodium channels first appear adjacent to Schwann cell processes expressing the myelin-associated glycoprotein (MAG), a glial adhesion molecule expressed at the onset of myelination (9, 10). With further development, pairs of clusters associated with neighboring Schwann cells, or heminodes, appear to fuse into single mature nodes. A similar pattern of sodium channel clustering has been observed in myelinating cocultures of dorsal root ganglia (DRG) neurons and Schwann cells (11). These findings, together with studies of channel clustering during remyelination, indicate that the formation and localization of nodes in the peripheral nervous system (PNS) require signals provided by myelinating Schwann cells (reviewed in refs. 7 and 12).
The nature of the glial clustering signal(s) is not known. Under normal conditions, formation of the node in the PNS appears to require contact-dependent signals from the Schwann cell (11), although in certain pathologic conditions soluble factors may be able to substitute (13). In contrast, clustering of sodium channels was found to be promoted by soluble factors released by oligodendrocytes (14), suggesting that node formation in the central nervous system and PNS may be mechanistically distinct.
These apparent discrepancies between the mechanisms of sodium channel clustering in the central nervous system and PNS may reflect important differences in the structural and cellular composition of central and peripheral nodes. Of particular relevance to the current study is the presence of numerous microvilli at PNS nodes (15). These project radially from the outer collar of adjacent, myelinating Schwann cells, closely approaching and surrounding the axon at the node (16, 17). They appear morphologically similar to microvilli of other cell types, with a diameter of 70–80 nm and a central core of 4–6 microfilaments; in larger fibers, these microvilli are organized into a regular hexagonal array (17, 18). In some cases, a direct connection to the nodal axolemma has been observed (16, 17, 19). Their function is unknown, and the time course of their development relative to the formation of the node has not been delineated precisely. Roles in the buffering of extracellular ions, production of the nodal gap substance (reviewed in ref. 18), and development and maintenance of the nodal axolemma (20) have been suggested.
Ezrin, radixin, and moesin, also known as ERM proteins, are members of the band 4.1 superfamily of proteins and are expressed differentially in the microvilli of many cell types (21). Members of this family share a highly conserved amino-terminal domain, known as the Four-point-one/ezrin/radixin/moesin (FERM) domain (22), which binds directly or indirectly to the plasma membrane, and a carboxyl-terminal domain that interacts laterally with actin filaments. ERM proteins have been regarded as “linkers” between membrane proteins and the actin cytoskeleton, concentrated in and/or involved in the morphogenesis of microvilli as well as filopodia, membrane ruffles, and cell adhesion sites (reviewed in refs. 23–25).
We now report that Schwann cells express high levels of all three ERM proteins at the plasma membrane. Antibodies to each of these proteins specifically stain the Schwann cell microvilli at the node of Ranvier both in vivo and in vitro. By using ezrin antibodies, we further demonstrate that clusters of ankyrin G in the axolemma develop in close association with overlying Schwann cell processes. These results support the hypothesis that contact between Schwann cells and the axon regulates the formation and localization of the node of Ranvier in the PNS.
Materials and Methods
Preparation of Sciatic and Optic Nerve Sections and Teased Fibers.
Teased fibers and frozen sections from sciatic and optic nerves were prepared as described (26). Briefly, sciatic nerves were removed from Sprague–Dawley rats (Taconic Farms) of specified ages, fixed in 4% (vol/vol) paraformaldehyde, and teased in ice-cold Dulbecco's PBS by using fine needles. For frozen sections, sciatic and optic nerves were dissected, fixed in 4% (vol/vol) paraformaldehyde, cryoprotected in 30% (vol/vol) sucrose, frozen in OCT compound (VWR Scientific), and cut in 10-μm-thick cryostat sections. Tissue slides were stored at −80°C until used.
Tissue Culture Methods.
Primary rat Schwann cells, DRG neurons, and myelinating Schwann cell/DRG cocultures were established as described (27). Schwann cell cultures were kept in serum-containing media consisting of DMEM (BioWhittaker), 10% (vol/vol) FBS (HyClone), and 2 mM l-glutamine (Life Technologies, Rockville, MD) until used. Neuronal cultures were prepared from embryonic day n (E)16-E17 rat DRG, which were either dissociated with trypsin or grown directly as explants on collagen-coated glass coverslips. Cultures were maintained in standard neuronal media, which consisted of MEM (Life Technologies), 10% (vol/vol) FBS, 2 mM glutamine, 0.4% (vol/vol) glucose (Sigma), and 50 ng/ml 2.5S nerve growth factor (Bioproducts for Science, Indianapolis). During the first 2.5 weeks, cultures were cycled with antimitotic agents to remove nonneuronal cells. Schwann cell/DRG cocultures were established by adding 200,000 Schwann cells to purified DRG neurons in standard media. After 24 h, the standard neuronal media were replaced with N2 media, consisting of 5 mg/ml insulin, 100 μM putrescine, 30 nM selenium, 20 nM progesterone (Sigma), 10 mg/ml transferrin (Jackson ImmunoResearch), and 2 mM glutamine and nerve growth factor in a 1:1 mixture of DMEM and Ham's F-12 (Life Technologies). After 3 days in N2 media, the standard media were supplemented with 50 μg/ml ascorbic acid to promote basal lamina formation and myelination.
Antibodies and Immunofluorescence.
Rabbit polyclonal antibodies against human placental ezrin and moesin (B22 and B40), recombinant rat GST-radixin (B50), and human recombinant ezrin-binding protein (EBP50) (B60) have been described (21, 28, 29). A mouse monoclonal antibody to MAG (gift of J. Roder, Mount Sinai Hospital, Toronto), a guinea pig antiserum to the carboxyl terminus of mouse Neurexin IV/Caspr (gift of M. Bhat, Mount Sinai Medical Center, New York), and a chicken antibody to ankyrin G (9) also were used. Monoclonal antibodies against MBP (SMI 94) and neurofilaments (SMI 31, SMI 32) were purchased from Sternberger Monoclonals (Lutherville, MD). Secondary antibodies included rhodamine-conjugated goat anti-rabbit, coumarin-conjugated goat anti-mouse (Jackson ImmunoResearch), and Alexa 488 goat anti-chicken and anti-guinea pig antibodies from Molecular Probes.
Fixed samples (Schwann cells, myelinating cocultures, and teased sciatic nerves) were permeabilized with acetone at −20°C, washed with Dulbecco's PBS, and blocked in Dulbecco's PBS/5% (vol/vol) BSA/1% (vol/vol) normal goat serum/0.2% (vol/vol) Triton X-100. Primary antibodies diluted in blocking solution were added and left overnight in a humidifying chamber at 4°C. After washing with Dulbecco's PBS/0.2% (vol/vol) Triton X-100, the tissue was incubated with the corresponding secondary antibodies at a dilution of 1:100 for 1 h at room temperature. The tissue was washed several times with Dulbecco's PBS, and a final wash of distilled water before mounting in Citifluor (Ted Pella, Redding, CA). The tissue was examined by epifluorescence on a Zeiss Axiophot microscope and a Zeiss LSM 510 confocal microscope.
Cell Extracts and Immunoblotting.
Cultures of DRG neurons, Schwann cells, and myelinating cocultures were lysed in a solution containing 1% (vol/vol) SDS, 150 mM NaCl, 10 mM EDTA, 1 mM PMSF, 10 μg/ml aprotinin, and 20 μM leupeptin in 50 mM Tris, pH 7.4. Lysates were cleared by centrifugation at 12,000 × g for 10 min. Protein concentrations were determined by using the bicinchoninic acid method (Pierce). Lysates (10–20 μg of total protein) were subjected to SDS/PAGE and blotted onto nitrocellulose. Appropriate regions of the blots were cut and incubated with ERM (1:10,000) or EBP50 (1:5,000) antibodies and developed by using the SuperSignal chemiluminescent substrate (Pierce).
Results and Discussion
ERM Proteins Are Concentrated in Schwann Cell Microvilli at the Node of Ranvier.
Staining of teased rat sciatic nerves with antibodies against ezrin, radixin, moesin, and EBP50 [a PSD-95, Discs-large, ZO-1-containing phosphoprotein that interacts with ERM proteins (28)] showed that all four proteins were localized strikingly to the node of Ranvier. Confocal microscopy demonstrated that ezrin and EBP50 staining forms a collar around, and in apparent contact with, the nodal axolemma, which is identified by its specific staining with ankyrin G antibodies (Fig. 1A). A similar staining pattern was observed for radixin and moesin (data not shown). Ezrin was also present at significant levels in the outer mesaxon (arrowheads in Fig. 1B), particularly in nerves from younger animals postnatal day n (P4–P11). These results are consistent with and extend a recent report in which a pan-ERM antibody was shown to stain the nodal region of peripheral nerves (30). In contrast, staining of adult rat optic nerve with ezrin antibodies showed a completely different distribution. In particular, ezrin was expressed in astrocytic processes (Fig. 1C), identified by double staining with a monoclonal antibody against glial fibrillary acidic protein (data not shown), but did not colocalize with ankyrin at the nodes. Endothelial cells in blood vessels in the optic nerve also intensely stained for ezrin (data not shown). These results indicate that all three ERM proteins are concentrated specifically at peripheral nodes of Ranvier.
Figure 1.
ERM expression in adult rat sciatic and optic nerves. (A) Confocal images of adult rat sciatic nerves double stained for ankyrin (green) and EBP50 (teased fibers; red) or ezrin (frozen sections; red). EBP50 and ezrin are concentrated in a collar of staining around the axolemma at the node of Ranvier, which is stained specifically with ankyrin antibodies; overlap of staining appears yellow in the merged images. The rotated images (right, Middle and Bottom) show that ezrin and EBP50 form a ring just external to that of ankyrin. (Bar = 10 μm.) (B) Teased sciatic nerve from P11 stained with ezrin (red) and Caspr (green) antibodies. In addition to its localization at the node of Ranvier, ezrin can also be detected in the outer mesaxonal spiral (arrowheads); Caspr is concentrated in the paranodes. (Bar = 10 μm.) (C) Confocal image of adult rat optic nerve double stained for ezrin (red) and ankyrin (green). In the CNS, ezrin is found in elongated astrocytic processes not associated with central nodes. (Bar = 10 μm.)
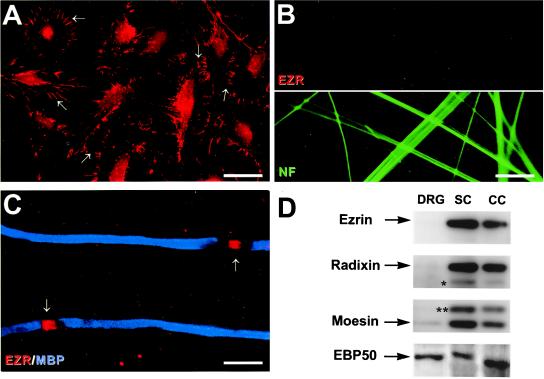
The staining pattern for ERM proteins in the PNS suggested that these proteins were localized in the Schwann cell membrane, particularly the nodal microvilli. To determine whether the ERM proteins indeed were localized specifically to Schwann cells, we analyzed the expression of these proteins by Schwann cells, DRG neurons, and myelinating cocultures. As shown in Fig. 2A, ezrin staining was concentrated in Schwann cell membrane processes and microvilli, which were quite abundant even under basal culture conditions; some faint nuclear staining was also observed. In contrast, no expression of ezrin by cultured DRG neurons was detected by immunostaining (Fig. 2B Upper). In cocultures, ezrin staining was distributed diffusely along the Schwann cell membrane shortly after plating Schwann cells onto neurons (data not shown), whereas 2–3 days after switching to media that promote myelination, ezrin had redistributed to nascent nodes. An example of ezrin at the nodes of Ranvier in the cocultures is indicated by the arrows in Fig. 2C. Taken together, these data strongly suggest that Schwann cell microvilli, including those at the node of Ranvier, are highly enriched in ezrin. In agreement with these findings, recent immunoelectron microscopy studies have shown that ezrin is localized throughout the microvilli of Schwann cells in rat peripheral nerves (S. Scherer, personal communication).
Figure 2.
Expression of ERM proteins in vitro. (A) Ezrin exhibits a striking localization in the microvilli of primary rat Schwann cells (arrows). Schwann cell nuclei are also stained. (Bar = 34 μm.) (B) Double staining of DRG neurons with antibodies against ezrin (Upper) and neurofilaments (Lower). Ezrin was not detected in the axons or neuronal nuclei. (Bar = 15 μm.) (C) Myelinating DRG/Schwann cell cocultures stained for MBP (blue) and ezrin (red). Ezrin is concentrated at the nodes (arrows). (Bar = 12.5 μm.) (D) Western blot analysis of lysates prepared from DRG neurons (DRG), Schwann cells (SC) and myelinating cocultures (CC) probed with antibodies against ERM proteins and EBP50. ERM proteins were present mainly in the lysates of Schwann cells and cocultures; EBP50 was present in all lysates. Low levels of moesin and radixin were detectable in neurons. Crossreactivity of antibodies to radixin with moesin (asterisk) and of antibodies to moesin with ezrin (double asterisk) are indicated.
Schwann cell microvilli in culture also stained strongly for moesin and radixin (data not shown). In contrast to ezrin, moesin and radixin were also expressed diffusely in DRG neurites, although at substantially reduced levels compared with Schwann cells (data not shown). Interestingly, antibodies to EBP50 did not stain the microvilli of Schwann cells in culture but showed a more diffuse cytoplasmic and nuclear staining pattern (data not shown). Because EBP50 was concentrated at the node together with ERM proteins in rat peripheral nerve, translocation of this protein from the cytoplasmic compartment to the nodal microvilli presumably occurred during development, possibly under axonal control.
Western blotting analysis provided further evidence that ERM proteins are expressed predominantly by Schwann cells (Fig. 2D). Antibodies to ezrin recognized a single band with a molecular mass of 80 kDa in lysates of Schwann cells and myelinating cocultures; no expression by DRG neurons was apparent. Similarly, antibodies to radixin (80-kDa band) and moesin (77-kDa band) demonstrated strong expression of these proteins in Schwann cells and cocultures. Low levels of moesin and radixin also were detected in neurons. Antibodies to radixin crossreact slightly with moesin (band indicated with an asterisk), whereas moesin antibodies strongly crossreact with ezrin (band indicated with two asterisks), but only in Schwann cells and cocultures, in agreement with the results obtained with anti-ezrin serum. Finally, EBP50 was present at high levels in Schwann cells, in cocultures as well as in DRG neurons. A slight difference in the apparent molecular weight of EBP50 in neurons and Schwann cells was observed, possibly reflecting a difference in the extent of phosphorylation as reported for placental forms of EBP50 (28).
Ankyrin G Clusters in Association with ERM-Positive Schwann Cell Processes.
We next examined when the ERM proteins accumulate at the node of Ranvier during peripheral nerve development relative to initial node formation. We used ankyrin G as a marker for nascent nodes of Ranvier, as it accumulates with the same time course as sodium channels (9). We analyzed the expression of these proteins in sciatic nerves of rats from P0–P8, comparing their staining to that of MAG, which is expressed at the onset of myelination, and to MBP, a marker of compact myelin.
At P0, ankyrin and ezrin were distributed diffusely along nerve fibers and at the Schwann cell surface, respectively; notably, a few clusters of ezrin were also detected at this stage (data not shown). As ankyrin was not clustered and MBP was not yet expressed, it was not possible to determine whether these ezrin clusters were sites of developing nodes. These results do suggest that as Schwann cells extend along the axon, even before myelination, they are polarized longitudinally with ERM proteins concentrated at either end of the cell. As previous morphologic studies demonstrated that microvilli develop from the outer collar of Schwann cell cytoplasm during later stages of myelin maturation (18, 31), ezrin is likely to be concentrated in the tips of Schwann cell processes that are extending along the axon rather than in the mature microvilli. These findings are also consistent with the known role of ERM proteins in the morphogenesis of membranous structures (23, 25).
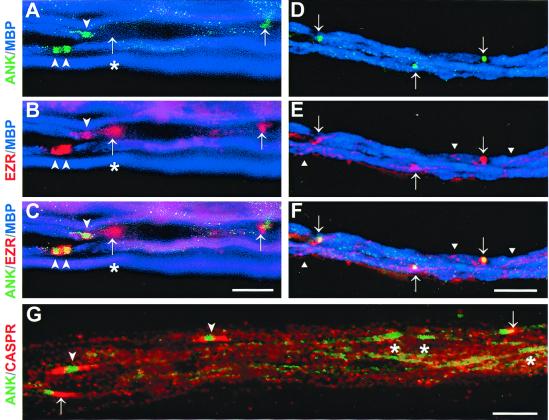
By P1–P2, clusters of ankyrin were detected clearly, surrounded by ezrin-positive Schwann cell processes (Fig. 3 A, B, and C). Ankyrin had a broad distribution, and occasional heminodes were observed (arrowheads). Many of the coclusters of ezrin and ankyrin were adjacent to MBP-positive internodes, but others were not (see clusters indicated with arrows, Fig. 3 A, B, and C). Thus, ankyrin and ezrin clustering seems to precede compact myelin formation, a result consistent with previous reports of ankyrin and sodium-channel clustering during development (9, 10). Interestingly, ezrin-positive processes without associated ankyrin clusters were also observed (asterisks, Fig. 3 A, B, and C). By P8, ankyrin clusters were much tighter and more closely resembled those in the adult nerve; at this stage, almost all of the ankyrin and ezrin clusters overlapped (Fig. 3 D, E, and F). Initial analysis of EBP50 and moesin during development suggested that they were enriched similarly in Schwann cell processes associated with early ankyrin clusters (data not shown).
Figure 3.
Coclustering of ankyrin and ezrin at the node of Ranvier during peripheral nerve development. Sciatic nerves from P2 (A–C and G) and P8 (D–F) rats stained for ankyrin (green), ezrin (red, A–F), MBP (blue, A–F), and Caspr (red, G). (A–C) Early clusters of ankyrin and ezrin at P2 are indicated by arrowheads. The merged image (C) shows ezrin-positive processes overlying nascent nodes of Ranvier. A single heminode and two closely apposed heminodes (double arrowheads) can be seen at this stage. Clusters are found at the edges of MBP-positive internodes but also in association with an MBP-negative segment (arrows). Of note, an ezrin-only cluster (asterisk) is associated with the MBP-negative segment and adjacent to a more mature myelinating internode and heminode. (Bar = 10 μm.) (D–F) By P8, the distribution of ezrin and ankyrin is similar to that observed in adult nerves, with both proteins colocalizing at the nodes (arrows). Staining of the mesaxonal spiral (triangles) by ezrin antibodies is also seen at this stage. (Bar = 25 μm.) (G) At P2, clustering of ankyrin at nascent nodes (asterisks) frequently precedes Caspr accumulation in the paranodes. Asymmetric Caspr staining at one side of the node is also evident at this stage (arrows). More mature nodes with Caspr staining in both paranodal regions (arrowheads) are also seen. (Bar = 10 μm.)
These data are quantified in Table 1. The majority of the clusters found at P1 (69%) were positive for both ankyrin and ezrin. However, a substantial number of ezrin-only clusters were observed (26%), whereas ankyrin-only clusters were much less frequent (5%). By P2, the number of coclusters of ankyrin and ezrin increased substantially (82%), whereas the numbers of ezrin- or ankyrin-only clusters both decreased (15 and 3%, respectively). By P8, the latest time point examined, over 96% of the clusters counted were positive for both ankyrin and ezrin. These results suggest ankyrin and ezrin clustering is highly correlated, with ezrin-positive processes developing just before ankyrin accumulation at the node.
Table 1.
Quantitation of ezrin and ankyrin clusters during development
| Age | Total number* | Ezrin+ only | Ankyrin+ only | Both |
|---|---|---|---|---|
| P1 | 225 | 59 (26%) | 10 (5%) | 156 (69%) |
| P2 | 567 | 86 (15%) | 19 (3%) | 462 (82%) |
| P8 | 916 | 47 (5%) | 13 (1%) | 856 (94%) |
Numbers represent pooled data from two independent experiments per developmental stage. A total of 10–12 high-power fields (×200 and ×400) were counted in each experiment.
Ezrin-Positive Processes Define a Distinct Schwann Cell Compartment.
We have also found that at various developmental times, the ezrin-positive Schwann cell processes associated with ankyrin clusters were MAG-negative (Fig. 4). Thus these early Schwann cell processes, and microvilli in the adult, define a specialized Schwann cell compartment enriched in ERM proteins from which myelin proteins including MAG or MBP are excluded (Fig. 5). These findings are also consistent with previous reports that ankyrin and sodium channels cluster beyond MAG-positive regions of the Schwann cell (9, 10). These results had been interpreted as suggesting that ankyrin clustered beyond the end of the Schwann cell itself, and that clustering might therefore reflect exclusion of these proteins from the internodal regions, possibly as a consequence of wrapping of a myelinating Schwann cell (12). Our results indicate that MAG-negative/ERM-positive Schwann cell processes overlie the early nodal axolemma throughout development and in the adult and support the alternative possibility that node formation is directed via contact with Schwann cell processes.
Figure 4.
Ezrin-positive processes at the node of Ranvier do not express MAG. A P4 sciatic nerve triple-stained for ezrin (blue), ankyrin (green), and MAG (red) is shown. Ezrin staining is restricted to the nodal area, as shown by its colocalization with ankyrin. MAG staining is limited to neighboring regions of the Schwann cell (arrowheads), which do not express ezrin. (Bar = 10 μm.)
Figure 5.

Organization of Schwann cell compartments. A cross section through a myelinated axon at the node is illustrated schematically, indicating the location of markers used in this study. The external mesaxon, compact myelin, paranodal loops with their septate-like junctions, and radially oriented array of Schwann cell microvilli contacting the nodal axolemma are shown.
Temporal Sequence of Clustering: Nodal Markers Precede Paranodal Markers.
It has also been suggested that ankyrin and sodium-channel clustering at the node requires paranodal junction formation (12, 32), possibly by creating a lateral barrier at the edge of the elongating Schwann cell. In potential support of this possibility, formation of the paranodal junctions and the nodes of Ranvier are correlated tightly during postnatal development in the optic nerve (33). We therefore compared the temporal relationship of ankyrin and ezrin clustering during peripheral nerve development to that of Caspr, an axonal constituent of the paranodal junctions (27, 34).
At P2, Caspr staining was detected adjacent to clusters of ankyrin and ezrin in only about half the cases (e.g., 106 of 204 nodes; see Fig. 3G). Of interest, paranodal accumulation of Caspr was frequently much more advanced on one side of the forming nodes, consistent with the tendency of the paranodal junctions to form asymmetrically (35). By P4, 94% (821/873) of the ankyrin clusters had associated Caspr staining in the paranodal region and at later time points, the association was even higher. These results indicate that, in the PNS, ankyrin accumulation at the node precedes that of Caspr at the paranodes and therefore does not depend on the presence of mature paranodal junctions. These results agree with previous observations of myelinating cocultures of Schwann cells and DRG neurons, which indicate that sodium channel and ankyrin clusters are detected 3 days before Caspr accumulation at the paranodes (11, 27). They are also consistent with earlier morphologic studies that demonstrated nodal specialization precedes frank paranodal junction formation during development (35–37). Finally, recent studies of the ceramide galactosyl transferase knockout mouse, which is unable to synthesize galactocerebroside and lacks paranodal junctions, demonstrate that sodium channels cluster appropriately at the node in the absence of normal paranodal junctions (38).
Implications for Node Formation.
Taken together, these results suggest that in the PNS, node formation normally occurs in direct association with overlying Schwann cell processes rather than adjacent to the Schwann cell as hypothesized (12, 39). These findings are in strong agreement with earlier ultrastructural studies (31, 35, 36) that suggested Schwann cell processes overlie early nodal specializations defined by their characteristic cytoplasmic undercoating and/or accumulation of intramembranous particles. Interestingly, the zone of apposition between the overlying Schwann cell process and the axolemmal specialization was correlated tightly (35), consistent with a functional relationship. As neurofascin and NrCAM accumulate just before and may serve to recruit ankyrin and sodium channels to nodes, clusters of these CAMs represent the earliest known nodal intermediates on the axon (9). In the future, it may be of considerable interest to determine whether these ezrin-positive Schwann cell processes contact such early nodal intermediates and whether they express receptors for these CAMs, including the possibility that such receptors interact directly or indirectly with the ERM proteins.
Acknowledgments
We thank Manzoor Bhat for anti-Caspr antibodies, Lee Cohen-Gould for assistance with confocal microscopy, and Jill Gregory for artwork. These studies were supported by National Institutes of Health Grant NS 38208 (to J.L.S.) and National Scientific Foundation Major Instrumentation Award 9977430. C.M.V. is a recipient of an advanced postdoctoral fellowship from the National Multiple Sclerosis Society.
Abbreviations
- ERM
ezrin-radixin-moesin
- MBP
myelin basic protein
- CAM
cell adhesion molecule
- PNS
peripheral nervous system
- MAG
myelin-associated glycoprotein
- DRG
dorsal root ganglia
- En
embryonic day n
- EBP50
ezrin-binding protein 50
- Pn
postnatal day n
References
- 1.Salzer J L. Neuron. 1997;18:843–846. doi: 10.1016/s0896-6273(00)80323-2. [DOI] [PubMed] [Google Scholar]
- 2.Stämpfli R. Physiol Rev. 1954;34:101–112. doi: 10.1152/physrev.1954.34.1.101. [DOI] [PubMed] [Google Scholar]
- 3.Chiu S Y, Schwarz W. J Physiol (London) 1987;391:631–649. doi: 10.1113/jphysiol.1987.sp016760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenbluth J. J Neurocytol. 1976;5:731–745. doi: 10.1007/BF01181584. [DOI] [PubMed] [Google Scholar]
- 5.Shrager P. J Physiol (London) 1988;404:695–712. doi: 10.1113/jphysiol.1988.sp017314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett V, Lambert S. J Neurocytol. 1999;28:303–318. doi: 10.1023/a:1007005528505. [DOI] [PubMed] [Google Scholar]
- 7.Peles E, Salzer J L. Curr Opin Neurobiol. 2000;10:558–565. doi: 10.1016/s0959-4388(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 8.Black J A, Kocsis J D, Waxman S G. Trends Neurosci. 1990;13:48–54. doi: 10.1016/0166-2236(90)90068-l. [DOI] [PubMed] [Google Scholar]
- 9.Lambert S, Davis J Q, Bennett V. J Neurosci. 1997;17:7025–7036. doi: 10.1523/JNEUROSCI.17-18-07025.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vabnick I, Novakovic S D, Levinson S R, Schachner M, Shrager P. J Neurosci. 1996;16:4914–4922. doi: 10.1523/JNEUROSCI.16-16-04914.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ching W, Zanazzi G, Levinson S R, Salzer J L. J Neurocytol. 1999;28:295–301. doi: 10.1023/a:1007053411667. [DOI] [PubMed] [Google Scholar]
- 12.Vabnick I, Shrager P. J Neurobiol. 1998;37:80–96. [PubMed] [Google Scholar]
- 13.Deerinck T J, Levinson S R, Bennett G V, Ellisman M H. J Neurosci. 1997;17:5080–5088. doi: 10.1523/JNEUROSCI.17-13-05080.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan M R, Meyer-Franke A, Lambert S, Bennett V, Duncan I D, Levinson S R, Barres B A. Nature (London) 1997;386:724–728. doi: 10.1038/386724a0. [DOI] [PubMed] [Google Scholar]
- 15.Robertson J D. J Physiol. 1957;137:8–9. [PubMed] [Google Scholar]
- 16.Berthold C H, Rydmark M. J Neurocytol. 1983;12:475–505. doi: 10.1007/BF01159386. [DOI] [PubMed] [Google Scholar]
- 17.Raine C S. J Neurocytol. 1982;11:935–947. doi: 10.1007/BF01148309. [DOI] [PubMed] [Google Scholar]
- 18.Landon D N, Hall S. In: The Peripheral Nerve. Landon D N, editor. London: Chapman & Hall; 1976. pp. 1–105. [Google Scholar]
- 19.Ichimura T, Ellisman M H. J Neurocytol. 1991;20:667–681. doi: 10.1007/BF01187068. [DOI] [PubMed] [Google Scholar]
- 20.Rosenbluth J. Freeze-Fracture Approaches to Ionophore Localization in Normal and Myelin-Deficient Nerves. New York: Raven; 1981. [PubMed] [Google Scholar]
- 21.Berryman M, Franck Z, Bretscher A. J Cell Sci. 1993;105:1025–1043. doi: 10.1242/jcs.105.4.1025. [DOI] [PubMed] [Google Scholar]
- 22.Chishti A H, Kim A C, Marfatia S M, Lutchman M, Hanspal M, Jindal H, Liu S C, Low P S, Rouleau G A, Mohandas N, et al. Trends Biochem Sci. 1998;23:281–282. doi: 10.1016/s0968-0004(98)01237-7. [DOI] [PubMed] [Google Scholar]
- 23.Bretscher A. Curr Opin Cell Biol. 1999;11:109–116. doi: 10.1016/s0955-0674(99)80013-1. [DOI] [PubMed] [Google Scholar]
- 24.Mangeat P, Roy C, Martin M. Trends Cell Biol. 1999;9:187–192. doi: 10.1016/s0962-8924(99)01544-5. [DOI] [PubMed] [Google Scholar]
- 25.Tsukita S, Yonemura S. J Biol Chem. 1999;274:34507–34510. doi: 10.1074/jbc.274.49.34507. [DOI] [PubMed] [Google Scholar]
- 26.Rios J, Melendez-Vasquez C V, Einheber S, Lustig M, Grumet M, Hemperly J, Peles E, Salzer J L. J Neurosci. 2000;20:8354–8364. doi: 10.1523/JNEUROSCI.20-22-08354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Einheber S, Zanazzi G, Ching W, Scherer S, Milner T A, Peles E, Salzer J L. J Cell Biol. 1997;139:1495–1506. doi: 10.1083/jcb.139.6.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reczek D, Berryman M, Bretscher A. J Cell Biol. 1997;139:169–179. doi: 10.1083/jcb.139.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shcherbina A, Bretscher A, Kenney D M, Remold-O'Donnell E. FEBS Lett. 1999;443:31–36. doi: 10.1016/s0014-5793(98)01674-3. [DOI] [PubMed] [Google Scholar]
- 30.Hayashi K, Yonemura S, Matsui T, Tsukita S. J Cell Sci. 1999;112:1149–1158. doi: 10.1242/jcs.112.8.1149. [DOI] [PubMed] [Google Scholar]
- 31.Berthold C H. Microsc Res Tech. 1996;34:399–421. doi: 10.1002/1097-0029(19960801)34:5<399::aid-jemt1070340502>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 32.Trapp B D, Kidd G J. J Cell Biol. 2000;150:F97–F100. doi: 10.1083/jcb.150.3.f97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rasband M N, Peles E, Trimmer J S, Levinson S R, Lux S E, Shrager P. J Neurosci. 1999;19:7516–7528. doi: 10.1523/JNEUROSCI.19-17-07516.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menegoz M, Gaspar P, Bert M L, Galvez T, Burgaya F, Palfrey C, Ezan P, Arnos F, Girault J-A. Neuron. 1997;19:319–331. doi: 10.1016/s0896-6273(00)80942-3. [DOI] [PubMed] [Google Scholar]
- 35.Tao-Cheng J H, Rosenbluth J. Brain Res. 1983;285:251–263. doi: 10.1016/0165-3806(83)90023-8. [DOI] [PubMed] [Google Scholar]
- 36.Tao-Cheng J-H, Rosenbluth J. Dev Brain Res. 1982;3:577–594. doi: 10.1016/0165-3806(82)90055-4. [DOI] [PubMed] [Google Scholar]
- 37.Waxman S G, Foster R E. Proc. R. Soc. London Ser. B. 1980. , 441–446. [DOI] [PubMed] [Google Scholar]
- 38.Dupree J L, Girault J A, Popko B. J Cell Biol. 1999;147:1145–1152. doi: 10.1083/jcb.147.6.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Novakovic S D, Deerinck T J, Levinson S R, Shrager P, Ellisman M H. J Neurocytol. 1996;25:403–412. doi: 10.1007/BF02284811. [DOI] [PubMed] [Google Scholar]