Abstract
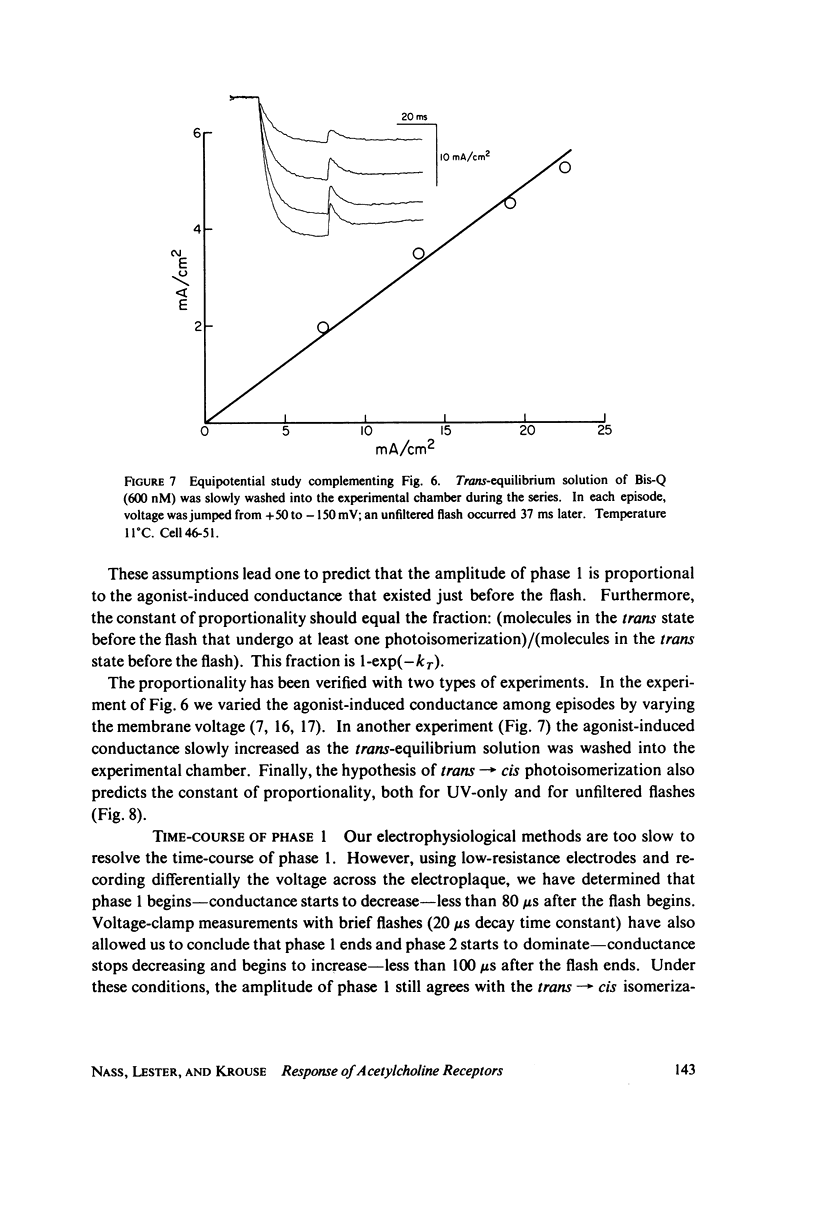
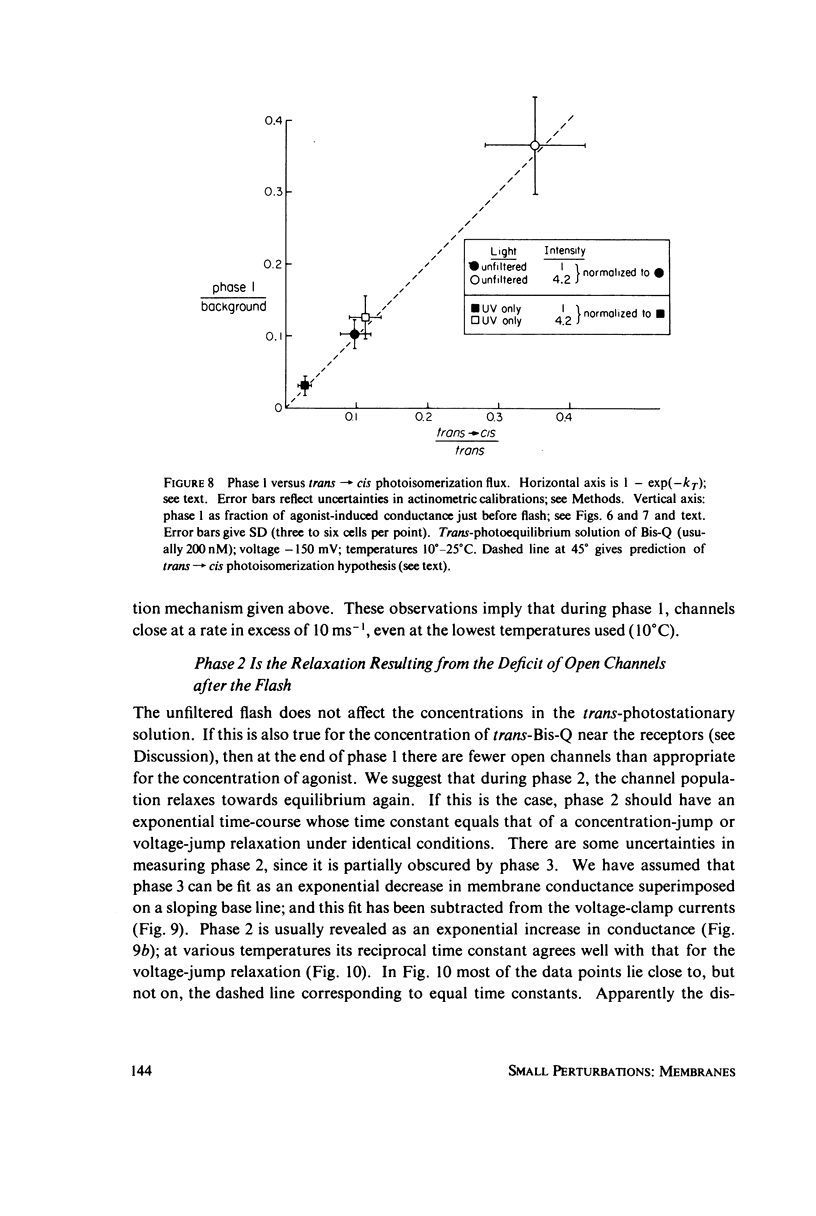
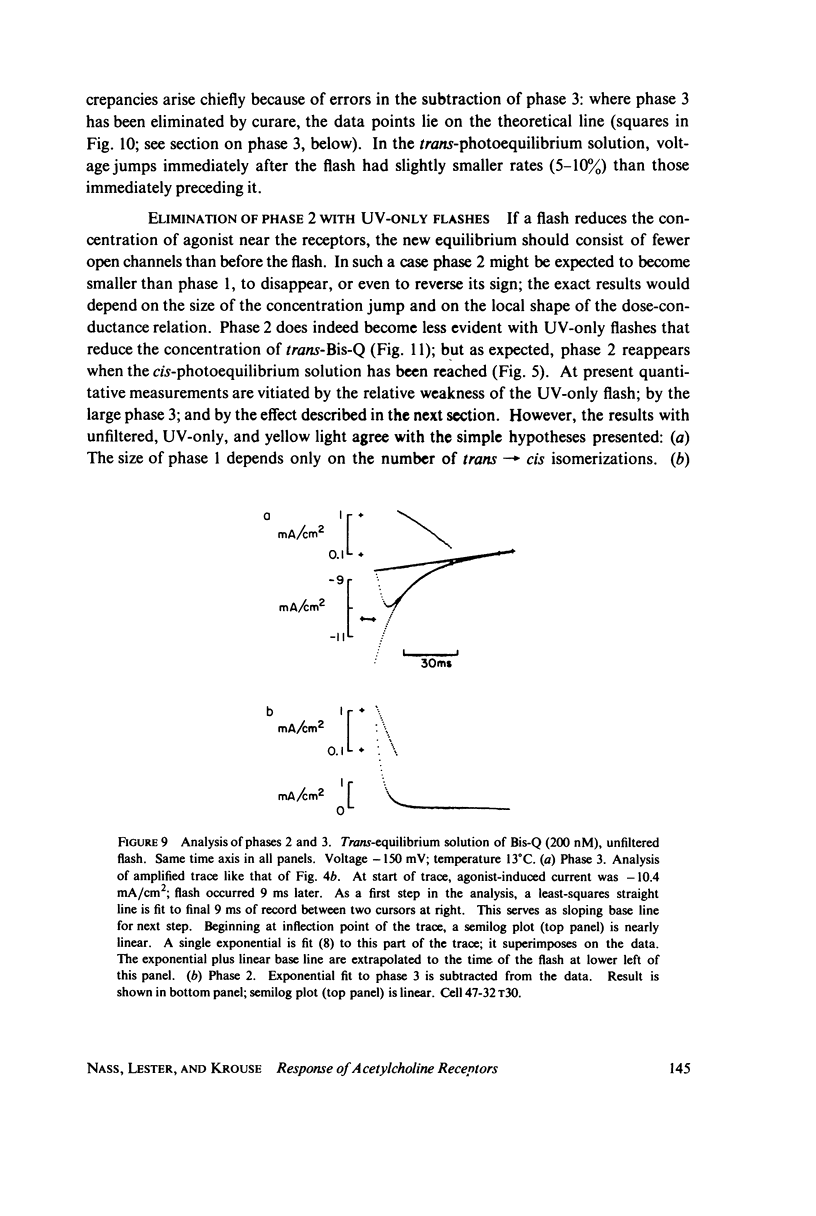
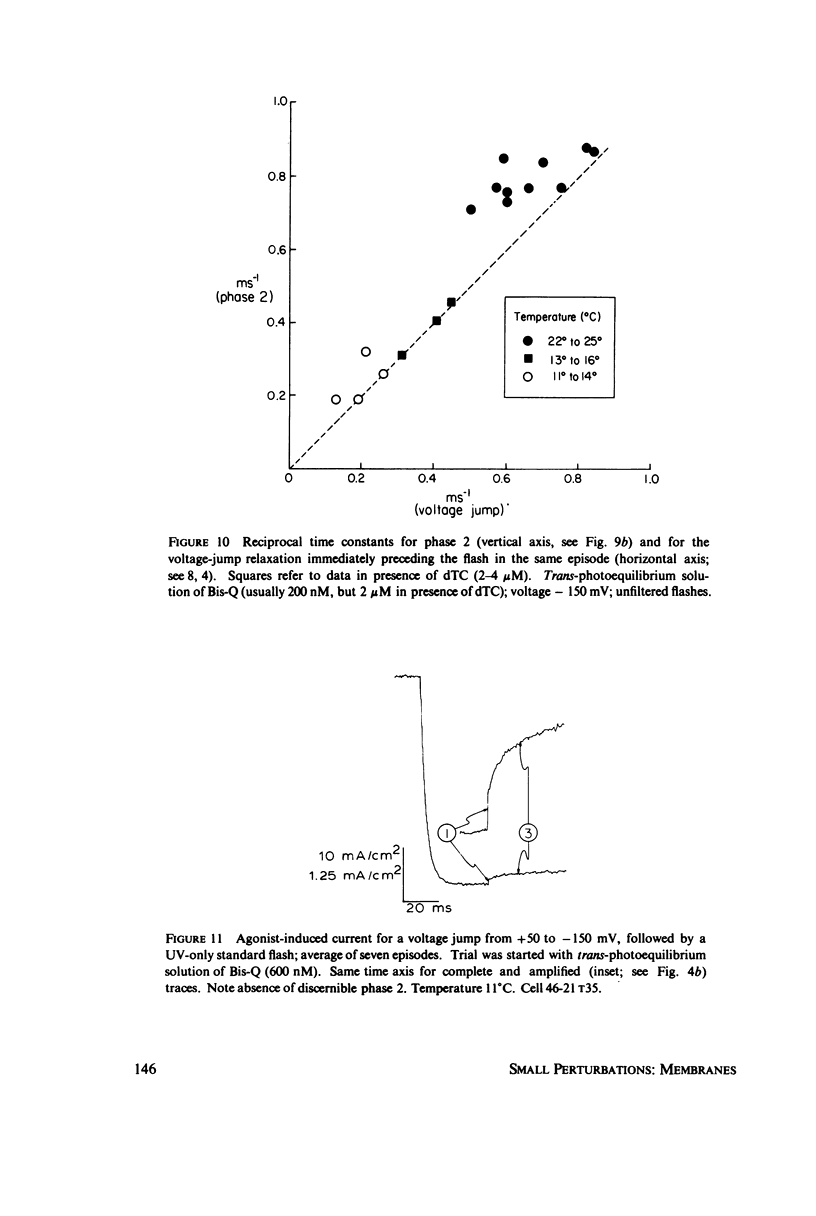
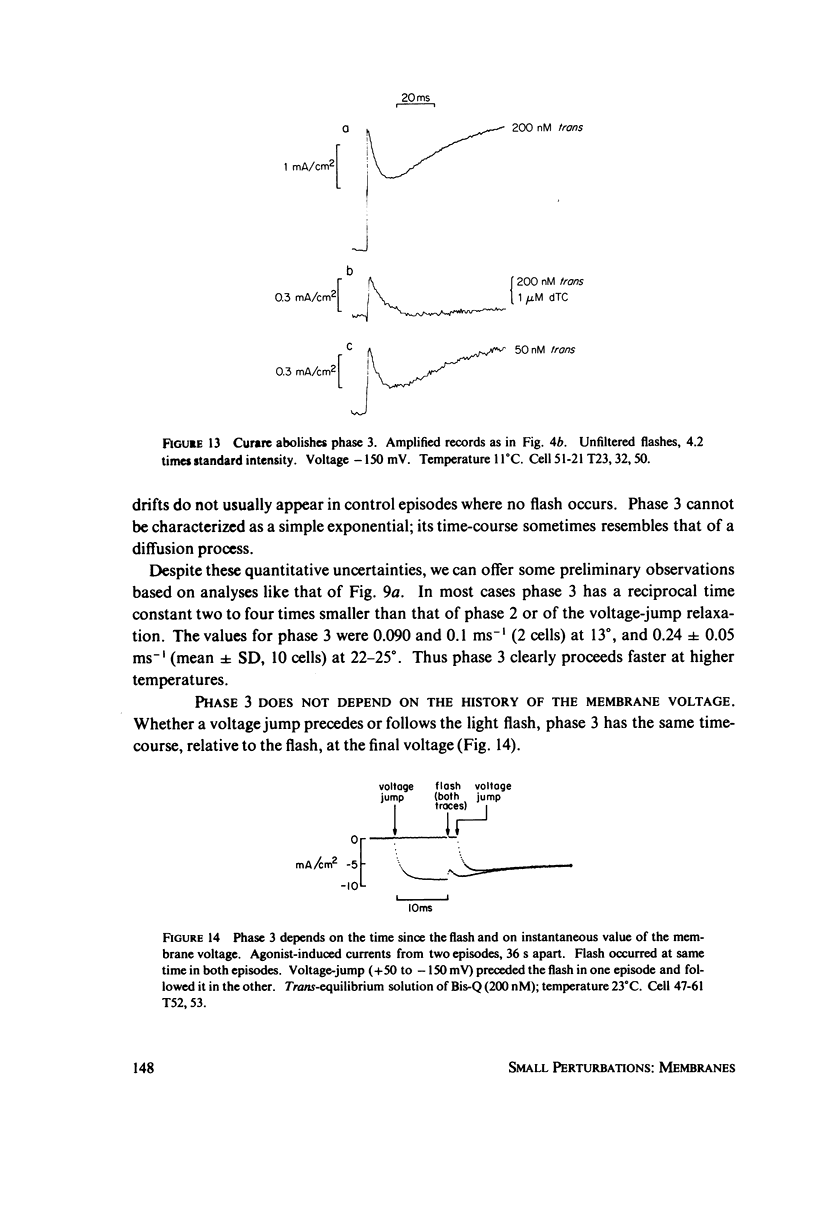
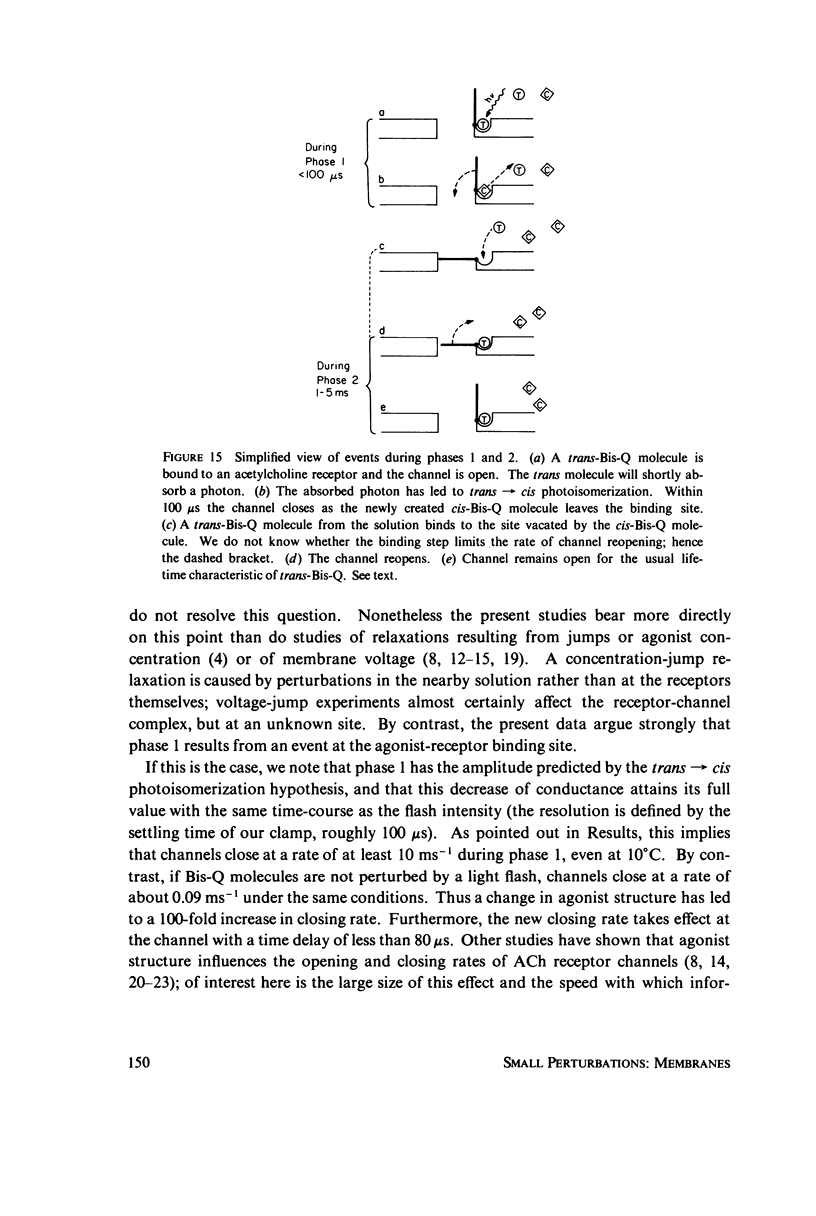
In these experiments, agonist-induced conductance is measured while a sudden perturbation is produced at the agonist-receptor binding site. A voltage-clamped Electrophorus electroplaque is exposed to trans-Bis-Q, a potent agonist. Some channels are open; these receptors have bound agonist molecules. A light flash isomerizes 3(-35)% of the trans-Bis-Q molecules to their cis form, a far poorer agonist. This causes a rapid decrease of membrane conductance (phase 1), followed by a slower increase (phase 2). Phase 1 has the amplitude and wavelength dependence expected if the channel closes within 100 mus after a single bound trans-Bis-Q is isomerized, and if the photochemistry of bound Bis-Q resembles that in solution. Therefore, the receptor channel responds rapidly, and with a hundred-fold greater closing rate, after this change in the structure of a bound ligand. Phase 2 (the conductance increase) seems to represent the relaxation back toward equilibrium after phase 1, because (a) phase 2 has the same time constant (1(-5) ms) as a voltage- or concentration-jump relaxation under identical conditions; and (b) phase 2 is smaller if the flash has led to a net decrease in (trans-Bis-Q). Still slower signals follow: phase 3, a decrease of conductance (time constant 5(-10 ms); and phase 4, an equal and opposite increase (several seconds). Phase 3 is abolished by curare and does not depend on the history of the membrane voltage. We consider several mechanisms for phases 3 and 4.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. Drug blockade of open end-plate channels. J Physiol. 1976 Sep;260(3):531–552. doi: 10.1113/jphysiol.1976.sp011530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R. Kinetics of agonist conductance changes during hyperolarization at frog endplates. Br J Pharmacol. 1975 Feb;53(2):308–310. doi: 10.1111/j.1476-5381.1975.tb07364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R. Relaxation experiments using bath-applied suberyldicholine. J Physiol. 1977 Jun;268(2):271–289. doi: 10.1113/jphysiol.1977.sp011857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R. Voltage jump analysis of procaine action at frog end-plate. J Physiol. 1977 Jun;268(2):291–318. doi: 10.1113/jphysiol.1977.sp011858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels E., Wassermann N. H., Erlanger B. F. Photochromic activators of the acetylcholine receptor. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1820–1823. doi: 10.1073/pnas.68.8.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Dionne V. E., Steinbach J. H., Stevens C. F. Conductance of channels opened by acetylcholine-like drugs in muscle end-plate. Nature. 1975 Jan 17;253(5488):204–206. doi: 10.1038/253204a0. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. Relaxation and fluctuations of membrane currents that flow through drug-operated channels. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):231–262. doi: 10.1098/rspb.1977.0137. [DOI] [PubMed] [Google Scholar]
- Colquhoun D. Mechanisms of drug action at the voluntary muscle endplate. Annu Rev Pharmacol. 1975;15:307–325. doi: 10.1146/annurev.pa.15.040175.001515. [DOI] [PubMed] [Google Scholar]
- Dionne V. E., Stevens C. F. Voltage dependence of agonist effectiveness at the frog neuromuscular junction: resolution of a paradox. J Physiol. 1975 Oct;251(2):245–270. doi: 10.1113/jphysiol.1975.sp011090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer F., Walther C., Peper K. Junctional and extrajunctional acetylcholine receptors in normal and denervated frog muscle fibres. Noise analysis experiments with different agonists. Pflugers Arch. 1976 Oct 15;366(1):1–9. doi: 10.1007/BF02486555. [DOI] [PubMed] [Google Scholar]
- Gage P. W. Generation of end-plate potentials. Physiol Rev. 1976 Jan;56(1):177–247. doi: 10.1152/physrev.1976.56.1.177. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Kuffler S. W., Yoshikami D. Post-synaptic potentiation: interaction between quanta of acetylcholine at the skeletal neuromuscular synapse. J Physiol. 1975 Oct;251(2):427–463. doi: 10.1113/jphysiol.1975.sp011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE MEASUREMENT OF SYNAPTIC DELAY, AND THE TIME COURSE OF ACETYLCHOLINE RELEASE AT THE NEUROMUSCULAR JUNCTION. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:483–495. doi: 10.1098/rspb.1965.0016. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The binding of acetylcholine to receptors and its removal from the synaptic cleft. J Physiol. 1973 Jun;231(3):549–574. doi: 10.1113/jphysiol.1973.sp010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The characteristics of 'end-plate noise' produced by different depolarizing drugs. J Physiol. 1973 May;230(3):707–717. doi: 10.1113/jphysiol.1973.sp010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordas M. An attempt at an analysis of the factors determining the time course of the end-plate current. II. Temperature. J Physiol. 1972 Jul;224(2):333–348. doi: 10.1113/jphysiol.1972.sp009898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester H. A. Blockade of acetylcholine receptors by cobra toxin: electrophysiological studies. Mol Pharmacol. 1972 Nov;8(6):623–631. [PubMed] [Google Scholar]
- Lester H. A., Chang H. W. Response of acetylcholine receptors to rapid photochemically produced increases in agonist concentration. Nature. 1977 Mar 24;266(5600):373–374. doi: 10.1038/266373a0. [DOI] [PubMed] [Google Scholar]
- Lester H. A., Changeux J. P., Sheridan R. E. Conductance increases produced by bath application of cholinergic agonists to Electrophorus electroplaques. J Gen Physiol. 1975 Jun;65(6):797–816. doi: 10.1085/jgp.65.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester H. A., Koblin D. D., Sheridan R. E. Role of voltage-sensitive receptors in nicotinic transmission. Biophys J. 1978 Mar;21(3):181–194. doi: 10.1016/S0006-3495(78)85518-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Presynaptic calcium currents and their relation to synaptic transmission: voltage clamp study in squid giant synapse and theoretical model for the calcium gate. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2918–2922. doi: 10.1073/pnas.73.8.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. A quantitative description of end-plate currents. J Physiol. 1972 May;223(1):173–197. doi: 10.1113/jphysiol.1972.sp009840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Terrar D. A. Factors affecting the time course of decay of end-plate currents: a possible cooperative action of acetylcholine on receptors at the frog neuromuscular junction. J Physiol. 1975 Jan;244(2):467–495. doi: 10.1113/jphysiol.1975.sp010808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 1976 Apr 29;260(5554):799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Voltage-dependence of drug-induced conductance in frog neuromuscular junction. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2140–2144. doi: 10.1073/pnas.72.6.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberry T. L., Chang H. W., Chen Y. T. Purification of acetylcholinesterase by affinity chromatography and determination of active site stoichiometry. J Biol Chem. 1972 Mar 10;247(5):1555–1565. [PubMed] [Google Scholar]
- Ruiz-Manresa F., Grundfest H. Synaptic electrogenesis in eel electroplaques. J Gen Physiol. 1971 Jan;57(1):71–92. doi: 10.1085/jgp.57.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan R. E., Lester H. A. Rates and equilibria at the acetylcholine receptor of Electrophorus electroplaques: a study of neurally evoked postsynaptic currents and of voltage-jump relaxations. J Gen Physiol. 1977 Aug;70(2):187–219. [PMC free article] [PubMed] [Google Scholar]
- Sheridan R. E., Lester H. A. Relaxation measurements on the acetylcholine receptor. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3496–3500. doi: 10.1073/pnas.72.9.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach A. B. A kinetic model for the action of xylocaine on receptors for acetylcholine. J Gen Physiol. 1968 Jul;52(1):162–180. doi: 10.1085/jgp.52.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach A. B. Alteration by xylocaine (lidocaine) and its derivatives of the time course of the end plate potential. J Gen Physiol. 1968 Jul;52(1):144–161. doi: 10.1085/jgp.52.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach J. H. Local anesthetic molecules transiently block currents through individual open acetylcholine receptor channels [proceedings]. Biophys J. 1977 Jun;18(3):357–358. doi: 10.1016/S0006-3495(77)85621-X. [DOI] [PMC free article] [PubMed] [Google Scholar]



