Abstract
The model for carbon monoxide or dioxygen recombination with heme proteins developed by the group at the University of Illinois is reexamined. We propose that the carbon monoxide or dioxygen molecule enters the protein at essentially a diffusion-limited rate determined by the solvent viscosity and that the protein offers no important barriers to this entry. The viscosity dependence of the entry rate k(ED), its magnitude (1 x 10(10) M(-1)s(-1), and the rate of quenching of triplet states of protoprophyrin IX in apomyoglobin by dioxygen are used as supporting evidence. Comparison is made to the model of a fluctuating protein developed by G. Weber.
Full text
PDF

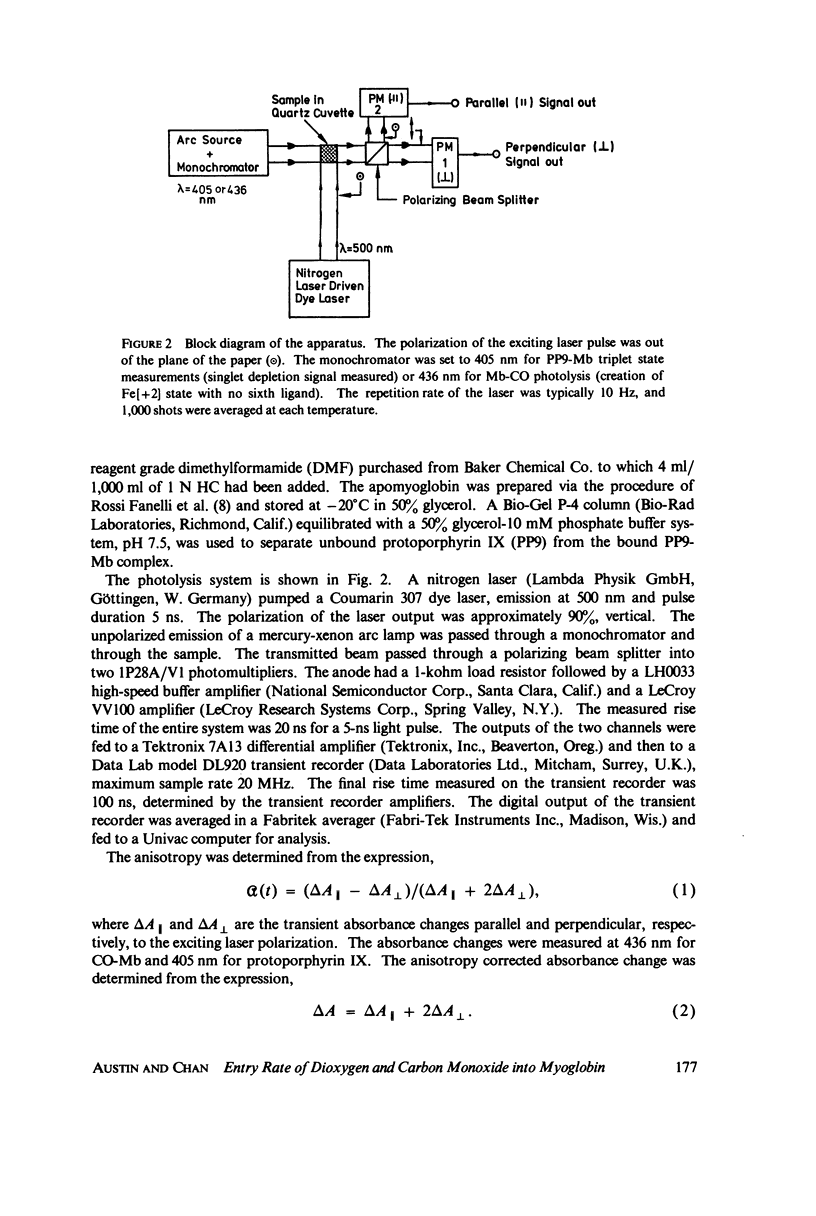





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberding N., Chan S. S., Eisenstein L., Frauenfelder H., Good D., Gunsalus I. C., Nordlund T. M., Perutz M. F., Reynolds A. H., Sorensen L. B. Binding of carbon monoxide to isolated hemoglobin chains. Biochemistry. 1978 Jan 10;17(1):43–51. doi: 10.1021/bi00594a007. [DOI] [PubMed] [Google Scholar]
- Austin R. H., Beeson K. W., Eisenstein L., Frauenfelder H., Gunsalus I. C. Dynamics of ligand binding to myoglobin. Biochemistry. 1975 Dec 2;14(24):5355–5373. doi: 10.1021/bi00695a021. [DOI] [PubMed] [Google Scholar]
- FANELLI A. R., ANTONINI E., CAPUTO A. Studies on the structure of hemoglobin. I. Physicochemical properties of human globin. Biochim Biophys Acta. 1958 Dec;30(3):608–615. doi: 10.1016/0006-3002(58)90108-2. [DOI] [PubMed] [Google Scholar]
- Lakowicz J. R., Weber G. Quenching of protein fluorescence by oxygen. Detection of structural fluctuations in proteins on the nanosecond time scale. Biochemistry. 1973 Oct 9;12(21):4171–4179. doi: 10.1021/bi00745a021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCammon J. A., Gelin B. R., Karplus M. Dynamics of folded proteins. Nature. 1977 Jun 16;267(5612):585–590. doi: 10.1038/267585a0. [DOI] [PubMed] [Google Scholar]
- Saviotti M. L., Galley W. C. Room temperature phosphorescence and the dynamic aspects of protein structure. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4154–4158. doi: 10.1073/pnas.71.10.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]


