Abstract
Purpose. How vitamin A contributes to the maintenance of the wet-surfaced phenotype at the ocular surface is not well understood. We sought to identify vitamin A responsive genes in ocular surface epithelia using gene microarray analysis of cultures of a human conjunctival epithelial cell line (HCjE) grown with all-trans-retinoic acid (RA). The analysis showed that secretory phospholipase A2 Group IIA (sPLA2-IIA) was the gene most upregulated by RA, followed by the membrane-associated mucin MUC16 at a later time point. Since eicosanoids, the product of arachidonic acid generated by the phospholipase A2 family, have been shown to increase mucin production, we sought to determine if sPLA2 mediates the RA induction of MUC16.
Methods. HCjE cells were cultured with or without RA for 3, 6, 24 and 48 hours. Complementary RNA prepared from RNA of the HCjE cells was hybridized to human gene chips (HG-U133A; Affymetrix) and analyzed using Rosetta Resolver software. Microarray data on mucin expression were validated by real-time PCR. To investigate whether sPLA2 is associated with RA-induced MUC16 upregulation, HCjE cells were incubated with RA and the broad spectrum PLA2 inhibitor, aristolochic acid (ArA) or the specific sPLA2-IIA inhibitor LY315920, followed by analysis of MUC16 mRNA and protein by real-time PCR and Western blot analysis.
Results. After RA addition, 28 transcripts were upregulated and 6 downregulated by over 2.0-fold (p < 0.01) at both 3 and 6 hours (early phase). Eighty gene transcripts were upregulated and 45 downregulated at both 24 and 48 hours (late phase). Group IIA sPLA2, significantly upregulated by 24 hours, and MUC16 were the most upregulated RNAs by RA at 48 hours. sPLA2 upregulation by RA was confirmed by Western blot analysis. When HCjE cells were incubated with RA plus ArA or specific inhibitor of sPLA2-IIA, LY315920, the RA-induced MUC16 mRNA was significantly reduced (p < 0.01).
Conclusion. The retinoic acid-associated upregulation of membrane-associated mucin MUC16 at late phase appears to be through sPLA2-IIA. Upregulation of this hydrophilic membrane-associated mucin may be one of the important mechanisms by which vitamin A facilitates maintenance of the wet-surfaced phenotype on the ocular surface.
Keywords: Retinoic acid, conjunctiva, MUC16, sPLA2, microarray
(INTRODUCTION)
Vitamin A and its derivatives, retinoids, are required for normal growth and development of vertebrates. They are absorbed in the small intestine, stored in the liver, and oxidized to all-trans-retinoic acid (RA) in many target cells.1,2 According to a survey by Balmer and Blomhoff, more than 500 genes have been reported as regulatory targets of RA.3 The regulatory effect of RA can be direct or indirect.3,4 Direct regulation of RA is driven by a heterodimer of a retinoic acid receptor and a retinoid X receptor (RAR.RXR dimer) bound to a retinoic acid response element (RARE) on the promoter region of the gene.5,6 Indirect regulation reflects the actions of intermediate transcription factors, such as the POU transcription factor, Brn-2, through which corticotrophin-releasing hormone (CRH) is upregulated.7
It is well known that ocular surface epithelia have an absolute requirement for vitamin A to maintain their wet-surfaced phenotype, and topical vitamin A has been reported to be effective as a treatment for severe squamous metaplasia.8-10 Vitamin A deficiency leads to abnormal differentiation of the ocular surface epithelia resulting in keratinization of both conjunctival and corneal epithelia.11 In a rabbit model, vitamin A deficiency caused conjunctival goblet cell loss and increased epithelial cell stratification in addition to reduced paracellular permeability of the ocular surface.12 Tei et al. demonstrated that vitamin A deficiency in rats results in a decreased expression of mRNA for the membrane-associated mucin rMuc4 and the goblet cell mucin rMuc5AC in conjunctival epithelium, and hypothesized that lack of these hydrophilic mucins contributed to dryness and keratinization of the ocular surface.13
Mucins are high molecular weight and highly O-glycosylated glycoproteins present at the interface between wet-surfaced epithelia and their extracellular environments. At the ocular surface, mucins are believed to attract and hold water due to their hydrophilic character, thus preventing desiccation of the epithelial surface.14 Mucins have been classified as either membrane-associated, including the mucins MUC1, 3A, 3B, 4, 11, 13, 15, 16, 17, and 20,15-21 or secreted. The latter include the gel-forming mucins secreted by goblet cells of various epithelia, MUC2, 5AC, 5B, 6, and 19,15,22 and the small soluble mucins, MUC7 and 9. 15,23 Ocular surface epithelia produce and place at the epithelia-tear film interface at least 3 membrane-associated mucins, MUC1, 4, and 16. Goblet cells of the conjunctiva produce and secrete MUC5AC, and a small soluble mucin, MUC7, is expressed in the lacrimal gland.24-27
Little is known regarding regulation of mucin gene expression by ocular surface epithelia. Dexamethasome has been reported to upregulate MUC1, and serum is a potent upregulator of MUCs 4 and 16.28 Several studies provide evidence suggesting that eicosanoid metabolites, products of arachidonic acid generated by the PLA2 family, can stimulate mucin production in airway epithelia29,30 and in ocular surface tissue.31-33 The specific PLA2 involved in this regulation is unknown.
Only a few studies have examined specific effects of RA in terms of gene expression in ocular surface tissues. Bossenbroek et al. demonstrated that RA induced the β-subunit of the retinoic acid receptor (RAR-β) mRNA in rabbit corneal and conjunctival fibroblasts,34 and Hori et al. reported that RA upregulated the membrane-associated mucins MUC4 and 16 at both the mRNA and protein level in a telomerase-immortalized, human conjunctival epithelial cell line (HCjE).35 Undoubtedly the action of RA on ocular surface epithelia is complex, involving many genes that maintain the wet-surfaced phenotype. The molecular mechanisms or group of genes through which vitamin A acts to maintain a wet-surfaced phenotype on the ocular surface are, however, unknown.
DNA microarray analyses offer a powerful method to identify potential sets of genes induced by RA, by simultaneously screening expression changes in thousands of transcripts in a single experiment.36,37 This technology has the potential to elucidate biologic processes that depend on the interaction of multiple genes and cellular pathways. Recently, several reports analyzed gene expression in corneal tissues using the microarray technique.38-41 These studies compared gene expression patterns in corneal fibroblasts after treatment with interleukin 1,38 in rodent corneas healing after wounding,39,41 and in human donor corneas.40 To our knowledge, there has been no microarray analysis of the effect of RA on human ocular surface epithelial cells.
We recently reported that HCjE cells express two subtypes of retinoic acid receptor mRNA, RAR-α and RAR-γ, and that RA induced MUC4 and 16 in the cell line.35 The purpose of this study was to determine, using microarray technology, which genes in the HCjE conjunctival epithelial cells were regulated by RA over time. Upon learning that retinoic acid upregulates secretory phospholipase A2 Group IIA (sPLA2-IIA) and, at a later time point, the membrane-associated mucin MUC16, and in light of previous data indicating that eicosanoids stimulate mucin production,29,30 we sought to determine if the MUC16 induction was mediated by sPLA2-IIA.
METHODS
Cell Culture
The telomerase-immortalized human conjunctival epithelial cell line, HCjE, was used in this study. The derivation and character of the cell line was previously reported.28,42 Culture of HCjE cells with all-trans-retinoic acid (RA) was based on our previous report.35 Briefly, HCjE cells were cultured in Gibco Keratinocyte-Serum Free Medium (K-sfm) (Gibco-Invitrogen Corp.; Rockville, MD) at 37°C in a 5% carbon dioxide atmosphere, followed by culture in a 1:1 mixture of K-sfm and low calcium DMEM/F12 (Gibco-Invitrogen Corp.) to confluence. At confluence, the cells were cultured in DMEM/F12 for 24 hours (baseline control), and then changed to DMEM/F12 with 100 nM RA dissolved in DMSO (Sigma-Aldrich; St. Louis, MO) for 3, 6, 24, or 48 hours. Experiments were performed in duplicate for each time point. Phase contrast microscopy of cell cultures was done using a Nikon TS100 microscope (Nikon; Melville, NY).
Isolation of RNA
After culture with RA, total RNA was isolated from the cells using TRIzol reagent (Invitrogen; Rockville, MD), following the manufacturer's protocol. Further purification of total RNA was done using the RNeasy Mini Kit (Qiagen; Valencia, CA). The 260/280 nm absorbance ratio of RNA samples used in this experiment ranged consistently from 1.8 to 2.1. The integrity and concentration of total RNA were measured using an Agilent 2100 Bioanalyser (Agilent Technologies; Palo Alto, CA).
Microarray
The microarray experiments were performed at the Bauer Center for Genomics Research of Harvard University (Cambridge, MA). Five μg of total RNA were converted to double-stranded cDNA with T7-(dT)24 oligomer primers (Superscript Choice System; Invitrogen). The cDNA was purified with Eppendorf's Phase Lock Gels (Brinkmann; Westbury, NY), followed by extraction with phenol/chloroform, and then ethanol precipitation. Biotin-labeled complementary RNA (cRNA) was produced by in vitro transcription using the Bioarray High Yield RNA Transcription Labeling Kit (Enzo Diagnostics, Inc.; Farmingdale, NY). The biotinylated cRNA was purified with RNeasy column and fragmented in 40 mM Tris Acetate, pH 8.1, 100 mM KOAC and 30 mM MgOAc (approximately 35 to 200 bases). After the confirmation of quality of the cRNA by hybridizing an aliquot to the Affymetrix Test3 Array, 10 μg of the biotinylated cRNA was hybridized for 16 hours at 45°C to an Affymetrix human microarray chip (Affymetrix GeneChip, HG-U133A; Affymetrix, Inc., Santa Clara, CA). The chip was washed and stained with streptavidin-phycoerythrin in Affymetrix Fluidics Station 400. Two microarray chips were probed for each time point with cRNA from two different experiments (control, 3, 6, 24 and 48 hours). Details on the probe design and sequence information for each gene on the HG-U133A gene chip are available on the manufacturer's web site, (http://www.affymetrix.com/index.affx).
Microarray Data Analysis
The HG-U133A arrays were scanned with the Affymetrix Gene Array Scanner using the Affymetrix Microarray Suite 5.0 software. The corresponding scanned data was deposited into an Enterprise Gene Expression Data Analysis System; the Rosetta Resolver system (Rosetta Biosoftware, Kirkland, W.A). The Rosetta Resolver system uses Affymetrix GeneChip error model to create an intensity profile for each GeneChip with data deposition after pre-processing (background correction and intra-chip normalization). Array data from two individual experiments were combined for each time point (data from replicates are combined by computing group averages, taking into account certain measurement error calculations: http://www.rosettabio.com/tech/Data_processing_and_analysis_methods.pdf), and Rosetta Resolver system Ratio Builder was used to calculate fold changes and ratio p-values for the differential expression of RA treated samples versus control. Detailed information of the Rosetta Resolver system Affymetrix GeneChip error model, Ratio Building error model can be found at http://www.rosettabio.com/tech. Those genes with p-value < 0.01 and fold difference > 2 fold were considered as significantly differentially expressed genes.
Real-time PCR
Real-time PCR experiments were performed to confirm data of mucin gene expression obtained by microarray analysis. Levels of MUC1, 4, and 16 mRNA were determined over the time course by real-time PCR using TaqMan Chemistry with the ABI Prism 7900HT Sequence Detection System (Applied Biosystems; Foster City, CA). The same RNA samples that were used to prepare probes for microarray hybridizations were used for the PCR analysis. Total RNA (2.0 μg) from the HCjE cells was reverse transcribed using the SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen), as previously described.43,44 The primers and double-labeled fluorogenic probes (TaqMan Probes) used for MUC1, 4, 16 and GAPDH amplification in this study have been previously reported.26,43,45 Additionally, new primers and a TaqMan probe for MUC4 (MUC4 C-term) were designed with the assistance of computer software (Primer Express; Applied Biosystems) to amplify the target sequence of the C-terminus of MUC4 used in the GeneChip analysis (Affymetrix Probe Set ID 217110_s_at). The sequences of MUC4 C-term primers and probe were sense: 5′-TAGGCTACCTCAAGACTCACCTCAT-3′, antisense: 5′-TCCCTTTTCCAGTCTCCCAAA-3′, and TaqMan probe: 5′-TACCGCACATTTAAGGCGCCATTGC-3′. BLASTN searches against nucleotide database were performed to confirm the sequence specificity of the MUC4 sequence (BLAST is provided in the public domain by the National Center for Biotechnology Information, Bethesda, MD, and is available at http://www.ncbi.nlm.nih.gov/BLAST/). Conventional RT-PCR experiments were performed to confirm that only a single band is obtained when amplifying conjunctival cDNA with the MUC4 C-term primers. To verify the identity of the MUC4 PCR product, the band in the agarose gel was excised, and the extracted DNA sequenced at the DNA Sequence Center for Vision Research of Massachusetts Eye and Ear Infirmary (Boston, MA).
For relative quantitation in real-time PCR experiments, we used the delta CT method (Applied Biosystems) reported previously.28,35 Samples were assayed in duplicate (N=4), using thermal cycling conditions comprised of 2 minutes at 50°C, 10 minutes at 95°C followed by 40 cycles at 95°C for 15 seconds and 60°C for 1 minute. Controls lacking cDNA template were run in each assay to confirm lack of DNA contamination in reagents used for amplification.
Phospholipase A2 Inhibitor Treatment
To investigate whether RA regulation of MUC16 is associated with sPLA2, the effect of the broad spectrum PLA2 inhibitor, aristolochic acid (ArA) (Sigma-Aldrich),46 on MUC16 mRNA levels was determined in HCjE cells cultured as above with 100 nM RA plus 100 μM of ArA, the inhibitor or vehicle (DMSO) alone for 24 and 48 hours. These experiments were followed up by testing the effect of an inhibitor specific for group IIA secretory phospholipase A2, LY31592047 (gift of D. W. Snyder and J. H. Prather, Lilly Research Laboratories, Eli Lilly and Co., Greenfield, IN). HCjE cells were treated with 100 nM RA, 100 nM RA plus 10 μm LY315920, the inhibitor or vehicle (DMSO) alone for 24 and 48 hours. MUC16 mRNA and protein levels were determined by real-time PCR and Western blot analysis, respectively. The experiments were performed twice for both inhibitors, each experiment being done in duplicate.
SDS-Polyacrylamide Gel Electrophoresis (PAGE) and Western Blot Analysis
Protein from cells cultured with or without RA and/or PLA2 inhibitors was extracted with RIPA buffer (50 nM Tris, 0.1% SDS, 0.5% deoxycholate, 1% NP-40, 150 nM NaCl) plus complete protease inhibitor cocktail (Roche Biochemical; Indianapolis, IN). Culture media were also harvested and centrifuged at 3500 rpm for 4 minutes. Supernatants were collected and concentrated using Nanosep 10K Omega Centrifugal Devices (Pall Life Sciences; Ann Arbor, MI). Protein concentration was determined with the BCA Protein Assay Reagent Kit (Pierce; Rockford, IL). Five micrograms of total protein from cell lysate or 10 μg from conditioned culture medium were diluted in Laemmli sample buffer48 and loaded in each lane. SDS-PAGE was performed under reducing conditions on 4% stacking and 7.5% (for MUC16 and GAPDH) or 15% (for sPLA2) separating gels. Proteins were transferred onto nitrocellulose membranes by conventional methods.49 Primary antibodies against MUC16 (OC125, mouse monoclonal, DAKO; Carpinteria, CA), sPLA2 (sPLA2, mouse monoclonal, Upstate; Lake Placid, NY), and GAPDH (rabbit polyclonal, Abcam; Cambridge, MA) were used. The conditions for immunoblotting with these antibodies have been reported previously.35,50 Protein bands were detected with SuperSignal West Pico Chemiluminescent Substrate (PIERCE; Rockford, IL) after exposure to film (Hyperfilm, Amersham Biosciences; Buckinghamshire, UK). Band intensities were quantified with NIH Image software (v1.62; National Institutes of Health; Bethesda, MD; available in the public domain at http://rsb.info.nih.gov/nih-image/) and 1D Image Analysis Software, Version 2.02 (Eastman Kodak, Co.; Rochester, NY).
Statistical Analysis
Statistical comparisons of results obtained by real-time PCR and Western blot analysis were performed with the Fisher protected, least-significant difference (PLSD) test (Statview 5.0 for Macintosh; SAS Institute, Inc.; Cary, NC). P < 0.05 was considered significant.
RESULTS
Characteristics of HCjE Cells
The growth and mucin characteristics of the human conjunctival epithelial cell line have been described previously.28 When grown to confluence in K-sfm, then in high calcium media DMEM/F12 plus 10% calf serum for seven days, the levels of MUC16 mRNA are comparable to those seen in native tissues and primary cultures.
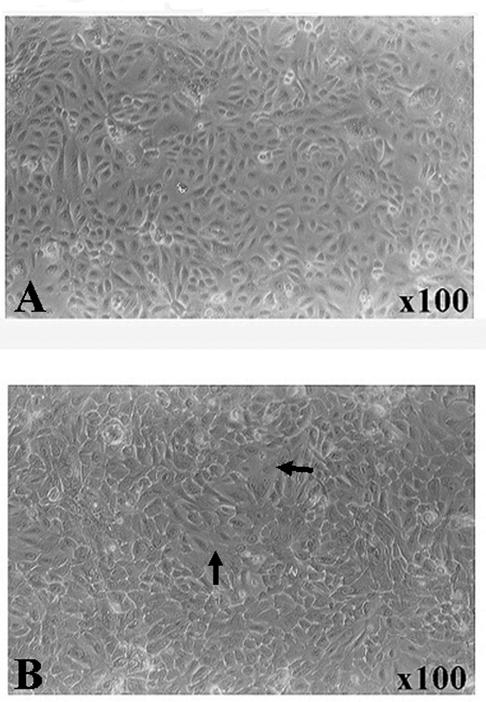
The microscopic appearance of HCjE cells28 grown to confluence, then cultured for 24 hours in DMEM/F12 is shown in Figure 1A. Figure 1B shows microscopic appearance of the HCjE cells cultured as in 1A, then for an additional 48 hours with RA. The cells cultured with RA formed islands with flattened large apical cells (compare Fig. 1A and 1B), indicating further differentiation of the cells. The appearance of cells at 48 hours of culture with RA were similar to those shown in a previous report which demonstrated that MUC16 was present on the apical cells of stratified islands of HCjE cells (see Figure 7D in Ref. 36).
Figure 1.
Phase contrast micrograph of cultures of HCjE cells. (A) Confluent cells before the addition of RA (control) and (B) after 48 hours of RA treatment (100 nM). Note that in B, large flattened cells are present (arrows). These cells have been demonstrated to be the apical cells of islands of stratification.28
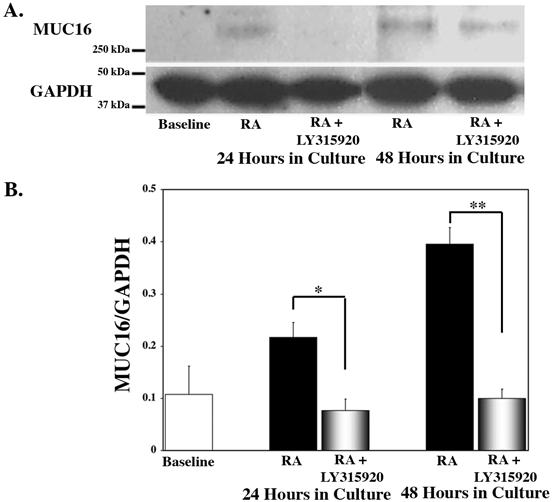
Figure 7.
Analysis of MUC16 protein from HCjE cells cultured with the specific inhibitor of sPLA2-IIA, LY315920. (A) Representative immunoblots of MUC16 mucin and GAPDH protein in HCjE cells at 0 hour (baseline) and at 24 and 48 hours after treatment with 100 nM RA +/- LY315920. (B) Comparisons of amount of MUC16 normalized to GAPDH were obtained by densitometry for each time point. Culture in the presence of RA alone resulted in a significant increase in MUC16 protein at both 24 (p<0.05) and 48 (p<0.0001) hours. The addition of LY315920 along with RA significantly inhibited the RA-induced MUC16 protein expression at both time points. Error bars, SEM. (*p<0.01; **p<0.0001).
Microarray Data
The microarray chip used in this study (HG-U133A, Affymetrix) contains 22,383 genes. Forty-three percent of the genes represented on the chip, 9,516, were detected in HCjE control samples. In response to culture with RA, 114 transcripts were upregulated and 84 downregulated after 3 hours; 102 were upregulated and 212 downregulated after 6 hours; 275 were upregulated and 180 downregulated after 24 hours, and 277 were upregulated and 384 downregulated after 48 hours (p<0.01). For a complete list of results on each individual gene on the microarray, see the Gene Expression Omnibus database at http://www.ncbi.nlm.nih.gov/geo (accession number: GSE2835).
For further analysis, we categorized time points into 2 phases—early (3 and 6 hours) and late (24 and 48 hours). In this analysis, “regulated gene” is defined as a transcript that displayed over a 2-fold change with a p-value < 0.01, at both time points in each phase following RA treatment. In the early phase, we identified 28 genes that were upregulated by 100 nM RA and 6 genes that were downregulated (Table 1). Among the upregulated genes, short-chain dehydrogenase/reductase 1 (GenBank Accession No. NM004753) was the most upregulated gene (10.9-fold increase at 3 hours and 22.6-fold increase at 6 hours). Others included structural molecules keratin 23 (NM015515), keratin 4 (X07695), and involucrin (NM005547). Among the downregulated genes were the cell cycle/cell death genes ubiquitin-specific protease 18 (NM017414), M-phase phosphoprotein 9 (NM022782), and protein phosphatase 1 (N26005).
Table 1.
Changes in gene expression in HCjE cells treated with RA for 3 and 6 hours (p < 0.01)
| Accession Number | Fold Change |
||
|---|---|---|---|
| Gene Name | 3h | 6h | |
| Upregulated | |||
| Blood coagulation | |||
| thrombomodulin | NM000361 | 3.7 | 2.0 |
| Cell proliferation/Differentiation | |||
| epithelial membrane protein 1 | BF445047 | 4.5 | 6.6 |
| sciellin | NM003843 | 3.3 | 4.6 |
| S100 calcium binding protein A7 | NM002963 | 3.3 | 3.3 |
| S100 calcium binding protein P | NM005980 | 2.5 | 3.4 |
| Metabolism | |||
| short-chain dehydrogenase/reductase 1 | NM004753 | 10.9 | 22.6 |
| cytochrome P450, subfamily XXIV | NM000782 | 7.1 | 7.5 |
| serine protease inhibitor, Kazal type, 5 | NM006846 | 2.6 | 2.7 |
| kallikrein 13 | NM015596 | 2.5 | 2.8 |
| kallikrein 10 | AK026045 | 2.5 | 2.1 |
| Oncogenesis | |||
| serine proteinase inhibitor, clade B, member 3 | U19556 | 2.5 | 2.1 |
| Signal transduction/Cell signaling | |||
| leukemia inhibitory factor | NM002309 | 6.1 | 2.5 |
| diphtheria toxin receptor | M60278 | 5.8 | 2.5 |
| putative chemokine receptor | NM006018 | 5.8 | 3.5 |
| CD14 antigen | NM000519 | 5.5 | 6.3 |
| retinoic acid induced 3 (RAI3) | NM003979 | 4.5 | 8.4 |
| FYN binding protein | AF198052 | 3.4 | 3.1 |
| Downregulated | |||
| Cell cycle/Cell death | |||
| ubiquitin specific protease 18 | NM017414 | -12.5 | -12.5 |
| M-phase phosphoprotein 9 | NM022782 | -9.9 | -3.7 |
| protein phosphatase 1 | N26005 | -2.6 | -3.2 |
| Response to stimulus/Immunity | |||
| interferon-induced protein 44 | BE049439 | -3.4 | -3.4 |
| Signal transduction/Cell signaling | |||
| frizzled homolog 2 | L37822 | -12.7 | -6.2 |
| Transcription factor | |||
| promyelocytic leukemia | AF230401 | -3.0 | -3.0 |
| neuroepithelial cell transforming gene 1 | NM005863 | 2.8 | 2.4 |
| A kinase (PRKA) anchor protein 12 | AB003476 | 2.3 | 3.7 |
| Structural molecule | |||
| keratin 23 | NM015515 | 3.7 | 5.9 |
| involucrin | NM005547 | 3.7 | 6.0 |
| keratin 4 | X07695 | 3.1 | 5.9 |
| Transcription factor | |||
| inhibitor of DNA binding 1 | D13889 | 4.9 | 6.3 |
| nuclear receptor interacting protein 1 | NM003489 | 3.4 | 2.1 |
| SRY (sex determining region Y)-box 9 | AI382146 | 2.1 | 2.0 |
| Transport | |||
| carbonic anhydrase II | M36532 | 3.2 | 2.8 |
| Others | |||
| hypothetical protein FLJ22671 | NM024861 | 3.4 | 4.8 |
| Homo sapiens EST from clone 898903 | BG177920 | 2.1 | 2.0 |
In the late phase, 80 genes were upregulated by 100 nM RA (Table 2), while 45 genes were downregulated (Table 3). Among the upregulated genes, a number associated with metabolism were identified, including group IIA phospholipase A2 (NM000300), matrix metalloproteinases MMP7 (NM002423), MMP3 (NM002422), and MMP2 (NM004530), and cytochrome P450 subfamily 4B1 (J02871). Structural molecules such as MUC16 (NM024690), chitinase 3-like 1 (M80927), keratin 23, and keratin 4 were also upregulated (Table 2). Among the downregulated genes, a number of cell cycle/cell death genes were identified, including cell-division-cycle-associated 3 (NM031299), G-2 and S-phase expressed 1 (BF973178), minichromosomal maintenance deficient (MCM)-2 (NM004526), MCM-5 (NM006739), MCM-7 (D55716), and MCM-10 (NM018518) (Table 3).
Table 2.
Upregulated gene expression (80 genes) in HCjE cells treated with RA for 24 and 48 hours (p < 0.01)
| Accession Number | Fold Change |
||
|---|---|---|---|
| Gene Name | 24h | 48h | |
| Up-regulated | |||
| Cell motility | |||
| crystallin, alpha B | AF007162 | 5.0 | 5.0 |
| nebulette | NM006393 | 5.1 | 3.8 |
| Cell proliferation/Differentiation | |||
| kallikrein 7 (chymotryptic, stratum corneum) | NM005046 | 6.7 | 7.1 |
| S100 calcium binding protein P | NM005980 | 9.2 | 6.4 |
| Signal transduction/Cell signaling | |||
| GABA A receptor, pi | NM014211 | 10.0 | 6.2 |
| C-type lectin, superfamily member 12 | AF313468 | 3.7 | 3.3 |
| TNFRSF1A-associated via death domain | L41690 | 2.1 | 2.8 |
| decay accelerating factor for complement | BC001288 | 2.6 | 2.8 |
| phospholipase D1 | U38545 | 2.7 | 2.7 |
| p8 protein (candidate of metastasis 1) | AF135266 | 2.5 | 5.6 |
| glycoprotein (transmembrane) nmb | NM002510 | 4.6 | 5.3 |
| S100 calcium binding protein A7 | NM002963 | 3.7 | 5.0 |
| insulin-like growth factor binding protein 6 | NM002178 | 4.5 | 4.1 |
| sciellin | NM003843 | 3.5 | 2.2 |
| Kruppel-like factor 4 | BF514079 | 2.0 | 2.0 |
| Metabolism | |||
| *phospholipase A2, group IIA | NM000300 | 28.6 | 15.3 |
| matrix metalloproteinase 7 | NM002423 | 7.6 | 8.6 |
| matrix metalloproteinase 3 | NM002422 | 4.2 | 8.4 |
| WAP four-disulfide core domain 2 | NM006103 | 4.0 | 6.1 |
| cytochrome P450, subfamily IVB1 | J02871 | 12.1 | 5.3 |
| myo-inositol 1-phosphate synthase A1 | AL137749 | 2.9 | 4.6 |
| spermidine/spermine N1-acetyltransferase | BE971383 | 2.7 | 3.8 |
| serine protease inhibitor, Kazal type, 5 | NM006846 | 3.1 | 3.6 |
| aldehyde dehydrogenase 2 family | NM000690 | 2.5 | 3.2 |
| hydroxysteroid (17-beta) dehydrogenase 1 | NM000413 | 2.4 | 3.2 |
| death-associated protein kinase 1 | NM004938 | 4.0 | 3.0 |
| sialyltransferase | NM006456 | 2.2 | 3.0 |
| cystatin E/M | NM001323 | 3.4 | 2.8 |
| spermidine/spermine N1-acetyltransferase | M55580 | 2.0 | 2.7 |
| butyrobetaine, 2-oxoglutarate dioxygenase 1 | NM003986 | 2.7 | 2.6 |
| hydroxysteroid (17-beta) dehydrogenase 2 | NM002153 | 2.3 | 2.6 |
| biliverdin reductase B | NM000713 | 2.4 | 2.6 |
| kynureninase (L-kynurenine hydrolase) | BC000879 | 3.1 | 2.4 |
| dual oxidase 1 | NM017434 | 2.0 | 2.3 |
| cytochrome b5 reductase b5R.2 | NM016229 | 2.5 | 2.3 |
| protein phosphatase 2, regulatory | AA974416 | 4.0 | 2.1 |
| B-factor, properdin | NM001710 | 2.1 | 2.6 |
| GABA(A) receptors associated protein 3 | AF180519 | 2.9 | 2.4 |
| A kinase (PRKA) anchor protein 12 | AB003476 | 2.6 | 2.3 |
| Structural molecule | |||
| * Mucin 16 (MUC16) | NM024690 | 4.4 | 11.5 |
| chitinase 3-like 1 | M80927 | 5.0 | 7.7 |
| keratin 23 | NM015515 | 9.2 | 6.5 |
| keratin 4 | X07695 | 7.5 | 6.2 |
| spondin 2, extracellular matrix protein | NM012445 | 3.6 | 4.3 |
| histone 1, H1c | BC002649 | 3.4 | 3.8 |
| transmembrane 7 superfamily member 1 | NM003272 | 3.6 | 3.6 |
| matrilin 2 | NM002380 | 2.7 | 2.4 |
| histone 2, H2be | NM003528 | 2.2 | 2.0 |
| transgelin | AA150165 | 2.1 | 2.0 |
| Transcription factor | |||
| insulin receptor substrate 2 | AF073310 | 4.8 | 2.9 |
| Transport | |||
| ATP-binding cassette, sub-family C | NM020037 | 9.8 | 9.4 |
| lipocalin 2 | NM005564 | 12.7 | 7.7 |
| solute carrier family 16, member 4 | NM004696 | 6.8 | 4.7 |
| retinol binding protein 1, cellular | NM002899 | 3.0 | 3.9 |
| solute carrier family 16, member 2 | NM006517 | 3.6 | 2.9 |
| carbonic anhydrase II | M36532 | 4.2 | 2.1 |
| Unknown | |||
| adipose specific 2 | NM006829 | 8.1 | 4.6 |
| HGFL gene | AL540260 | 3.4 | 3.8 |
| chromosome 14 open reading frame 138 | AI628605 | 2.7 | 2.0 |
| Others | |||
| subunit B | |||
| matrix metalloproteinase | NM004530 | 2.0 | 2.0 |
| Oncogenesis | |||
| serine proteinase inhibitor, clade B, member 3 | U19556 | 4.1 | 5.2 |
| serine proteinase inhibitor, clade B, member 4 | U19557 | 3.9 | 4.9 |
| homeodomain-only protein | AB059408 | 3.3 | 3.9 |
| TBC1 domain family, member 3 | AL136860 | 2.8 | 2.7 |
| lysosomal-associated membrane protein 2 | NM002294 | 2.2 | 2.2 |
| serine proteinase inhibitor, clade B, member 1 | NM030666 | 2.3 | 2.1 |
| Response to stimulus/Immunity | |||
| immune costimulatory protein B7-H4 | NM024626 | 12.8 | 6.2 |
| guanylate binding protein 2 | NM004120 | 3.8 | 3.3 |
| superoxide dismutase 2, mitochondrial | BF575213 | 2.0 | 3.1 |
| dual specificity phosphatase 1 | NM004417 | 2.8 | 2.6 |
| superoxide dismutase 2, mitochondrial | W46388 | 4.4 | 2.6 |
| neutrophil cytosolic factor 2 | BC001606 | 2.5 | 2.5 |
| TP53 activated protein 1 | BC002709 | 4.4 | 2.4 |
| B-cell linker | NM013314 | 3.8 | 2.4 |
| hypothetical protein FLJ21511 | NM025087 | 11.9 | 5.6 |
| DKFZP586H2123 protein | AI671186 | 5.8 | 5.4 |
| hypothetical protein FLJ14675 | BG036668 | 2.4 | 3.1 |
| hypothetical protein LOC54103 | AK026747 | 3.2 | 2.9 |
| hypothetical protein MGC14376 | AF070569 | 2.2 | 2.4 |
| hypothetical protein FLJ23309 | NM024896 | 2.4 | 2.2 |
These genes were further investigated.
Table 3.
Downregulated gene expression (45 genes) in HCjE cells treated with RA for 24 and 48 hours (p < 0.01)
| Accession Number | Fold Change |
||
|---|---|---|---|
| Gene Name | 24h | 48h | |
| Down-regulated | |||
| Cell cycle/Cell death | |||
| cell division cycle associated 3 | NM031299 | -2.2 | -33.8 |
| G-2 and S-phase expressed 1 | BF973178 | -4.8 | -15.6 |
| high-mobility group box 2 | BC000903 | -2.1 | -11.4 |
| aurora kinase B | AB011446 | -3.3 | -7.5 |
| BUB1 | AL137654 | -6.3 | -7.2 |
| MCM10 | NM018518 | -2.1 | -6.7 |
| kinesin family member 2C | AY026505 | -3.3 | -6.1 |
| antigen identified by antibody Ki-67 | AU132185 | -2.0 | -5.8 |
| CDC6 cell division cycle 6 homolog | NM001254 | -2.0 | -5.5 |
| DNA replication factor | AF321125 | -3.0 | -3.0 |
| MCM2 | NM004526 | -2.1 | -3.0 |
| kinesin family member 22 | AC002301 | -2.9 | -2.9 |
| MCM7 | D55716 | -2.0 | -2.9 |
| MCM5 | NM006739 | -3.6 | -2.7 |
| Cell proliferation/Differentiation | |||
| polo-like kinase (Drosophila) | NM005030 | -3.2 | -8.7 |
| TPX2 | AF098158 | -2.0 | -6.5 |
| nucleolar protein ANKT | NM018454 | -2.3 | -6.1 |
| replication factor C (activator 1) 2, 40kDa | M87338 | -2.4 | -3.0 |
| fusion (FUS) | NM004960 | -4.0 | -2.9 |
| Sex determination | |||
| mago-nashi homolog, proliferation-associated | AF067173 | -2.6 | -2.7 |
| Signal transduction/Cell signaling | |||
| neurogranin | NM006176 | -7.0 | -12.8 |
| Spermatogenesis | |||
| ASF1B | NM018154 | -2.3 | -7.6 |
| Structural molecule | |||
| lamin B1 | NM005573 | -5.1 | -23.0 |
| H2A histone family, member X | NM002105 | -2.0 | -4.0 |
| mitochondrial ribosomal protein L24 | NM024540 | -2.4 | -2.2 |
| Lysosomal-associated multispanning membrane protein-5 | NM006762 | -2.0 | -2.1 |
| Transcription factor | |||
| topoisomerase (DNA) II alpha 170kDa | AU159942 | -3.9 | -18.2 |
| interleukin enhancer binding factor 3 | AF147209 | -2.0 | -2.1 |
| Transport | |||
| Treacher Collins-Franceschetti syndrome 1 | NM000356 | -2.4 | -2.6 |
| unknown | |||
| paternally expressed 10 | BE858180 | -18.5 | -4.5 |
| inhibin, beta C | NM005538 | -2.1 | -2.2 |
| DEAD box polypeptide 39 | NM005804 | -2.2 | -2.2 |
| Metabolism | |||
| thymidylate synthetase | NM001071 | -2.0 | -8.6 |
| ligase I, DNA, ATP-dependent | NM000234 | -2.1 | -5.4 |
| thymidine kinase 1, soluble | NM003258 | -2.3 | -5.0 |
| ubiquitin carrier protein | NM014501 | -2.7 | -3.6 |
| ubiquitin specific protease 1 | AW499935 | -5.3 | -3.0 |
| flap structure-specific endonuclease 1 | NM004111 | -2.4 | -2.9 |
| phosphoserine aminotransferase 1 | NM021154 | -2.2 | -2.6 |
| degenerative spermatocyte homolog, lipid desaturase | NM003676 | -2.4 | -2.3 |
| Others | |||
| hypothetical protein DKFZp762E1312 | NM018410 | -2.3 | -21.8 |
| HSPC037 protein | BC003186 | -2.1 | -9.1 |
| hypothetical protein DKFZp762A227 | BC003163 | -2.7 | -5.1 |
| PRO2000 protein | NM014109 | -2.0 | -3.6 |
| hypothetical protein MGC:10200 | BF038461 | -2.2 | -2.2 |
Nine upregulated genes overlapped both the early and late phases: sciellin (NM003843); S100 calcium binding protein A7 (NM002963); S100 calcium binding protein P (NM005980); serine protease inhibitor, Kazal type, 5 (NM006846); serine protease inhibitor, clade B, member 3 (U19556); A kinase (PRKA) anchor protein 12 (AB003476); keratin 23; keratin 4; and carbonic anhydrase II (J03037). No downregulated genes overlapped both early and late phases.
For each time point of culture of the HCjE cells with RA, we used two individual microarray chips. The correlation coefficients of intensities of all genes between the 2 chips at each time point (control, 3, 6, 24, and 48 hours) were at least 0.96 (0.972, 0.961, 0.980, 0.966, and 0.965, respectively). These coefficients indicate that the data from duplicate experiments for each time point were highly reproducible. Therefore, the data from 2 individual experiments for each time point were combined using Rosetta Resolver software, and the ratios for RA treatment at each time point versus control were generated.51
Secretory Phospholipase A2 Protein and MUC16 Expression
From the microarray data, we found that the expression of the group IIA sPLA2 gene was the most upregulated gene by RA at both 24 (28.6-fold change) and 48 (15.4-fold change) hours (Table 2). The expression of the membrane-associated mucin MUC16 gene was the second most upregulated by RA after 48 hours (11.5-fold change) (Table 2). The latter data confirmed our previous report that 100 nMRA upregulated MUC16 expression both at the mRNA and protein levels.35 Since previous reports suggest a role of arachidonic acid metabolites in mucus regulation, we focused on these two genes for further analysis by designing experiments to investigate whether the RA-induced MUC16 upregulation was mediated by sPLA2.
Western blot followed by densitometric analysis of sPLA2-IIA in HCjE cells and their culture media after 24- and 48-hour exposures to RA was compared to control cells and media to confirm the experimental results of the microarray data at the protein level. Cell lysate and conditioned culture medium were examined to look for evidence of synthesis and secretion of sPLA2-IIA by these cells. As shown in Figure 2, sPLA2 protein was not detected in the HCjE cells or their culture media in the control cultures. However, after 24 hours of culture with RA, sPLA2-IIA protein was found in the cells and the amount dramatically increased at 48 hours (Fig. 2A), indicating translation of the sPLA2 mRNA detected at 24 and 48 hours by microarray analysis. sPLA2-IIA was not detected in the culture media until 48 hours after addition of RA (Fig. 2B).
Figure 2.
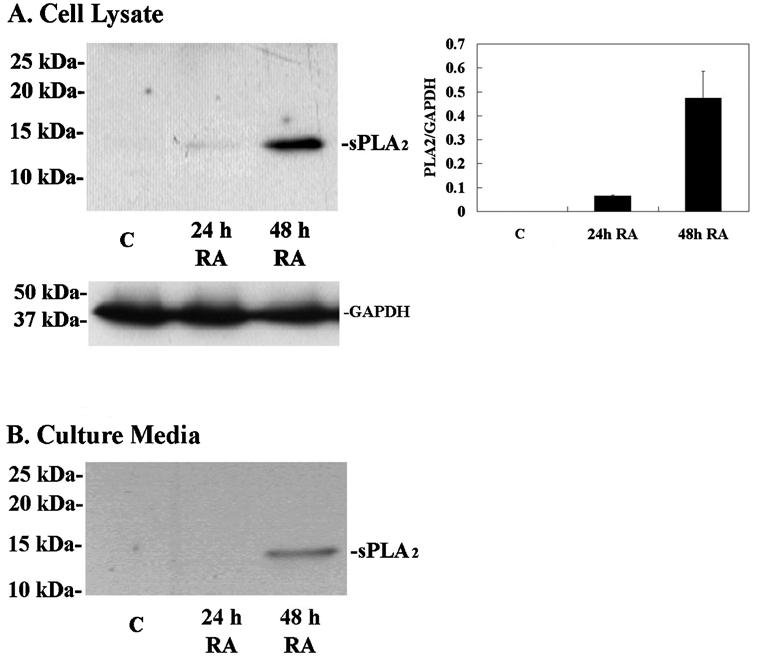
Immunoblot and densitometric analysis of group IIA sPLA2 protein in HCjE cells (A) and culture media (B) grown with 100 nM retinoic acid (RA) for 24 and 48 hours as compared to baseline, C, (0 hour control). (A) Five micrograms of total protein was separated by SDS-PAGE. The expression of sPLA2 protein (14 kDa) was not detected in the control, whereas it was induced by 24 hours after addition of RA and upregulated in a time-dependent manner. Densitometric comparisons of sPLA2 normalized to GAPDH were obtained at each time point. (B) No sPLA2 was detected in the culture medium from control cultures or those treated with RA for 24 hours. Secretion of sPLA2 was induced by 48 hours after addition of RA. Error bars, SEM.
To verify the microarray quantitation of MUC16 mRNA expression, real-time PCR was performed using the same samples that were used to prepare probes for microarray hybridization. Figure 3 shows the expression profile of MUC16 mRNA as measured by these 2 independent methods. In both methods, the level of the control was set at 1, and all other RA cultures were expressed relative to it. Although the relative values of expression amounts were different, the expression patterns of MUC16 mRNA in the two methods were similar over time (Fig. 3).
Figure 3.
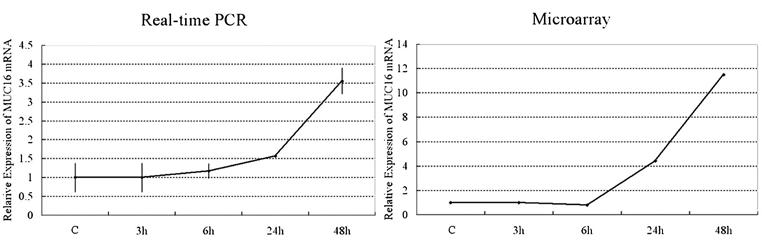
Relative mRNA level of MUC16 in HCjE cells treated with 100 nM retinoic acid (RA) for 0-48 hours. To verify the microarray quantitation of the expression of MUC16 mRNA, relative mRNA levels of MUC16 were measured by real-time PCR (left, n = 2) in the same samples that were used to prepare probe for microarray hybridizations (right). In both methods, the level of control (C) was set at 1, and RA-cultured cells at 3, 6, 24, and 48 hours were expressed relative to it. The expression amounts between these two techniques differed, but quantitation with the two methods showed a similar trend.
Effect of Phospholipase A2 Inhibitors on MUC16 expression
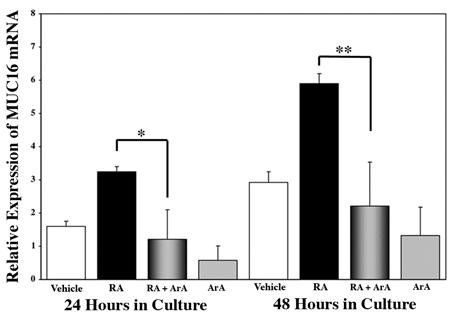
To determine whether sPLA2 mediates the RA induction of MUC16 the MUC16 expression was examined after treatment of HCjE cells with RA in the presence of the broad spectrum PLA2 inhibitor, aristolochic acid (ArA). Figure 4 shows the relative expression of MUC16 mRNA after culture with 100 nM RA plus ArA as determined by real-time PCR. The addition of ArA along with the RA treatment significantly inhibited the RA-induced increase in MUC16 mRNA expression at both 24 and 48 hours (p<0.01, and p<0.0001, respectively). A complete inhibition of the RA-induced MUC16 mRNA expression was observed at both 24 and 48 hours, as compared to vehicle. The reduction of MUC16 after addition of ArA was also confirmed at the protein level. Figure 5 shows that RA-induced MUC16 protein synthesis was significantly inhibited by the addition of 100 μM ArA acid at 24 and 48 hours; 75% less mucin was present at 24 hours and 50% at 48 hours. Thus, the reduction in protein expression follows that of the mRNA.
Figure 4.
Real-time PCR analysis of MUC16 mRNA expression in HCjE cells grown for 24 or 48 hours in the presence of vehicle (DMSO) alone (Vehicle), 100 nM RA plus 100 nM aristolochic acid (ArA), or ArA alone. MUC16 expression is determined relative to the 0-hour baseline value. Culture with RA significantly upregulates MUC16 compared to vehicle control at both 24 (p<0.02) and 48 (p<0.0001) hours. The addition of ArA along with RA significantly inhibited the RA-induced MUC16 mRNA expression at both 24 and 48 hours. ArA alone had no significant effect. Error bars, SEM. (*p<0.01, p <0.0001)
Figure 5.
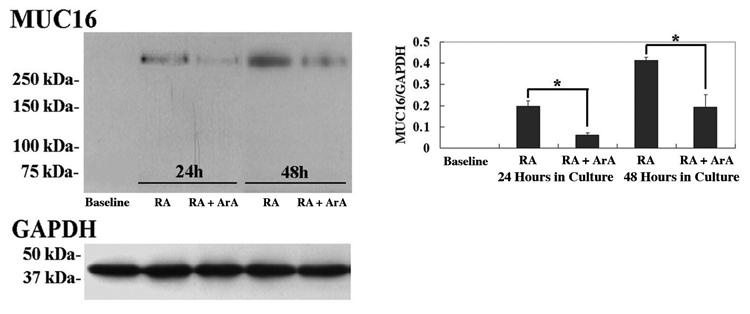
Western blot and densitometric analysis of MUC16 protein from HCjE cells grown without (baseline, 0 hour control) and with 100 nM RA +/- PLA2 inhibitor (100 μM) aristolochic acid (ArA) for 24 and 48 hours. Densitometric comparisons of MUC16 normalized to GAPDH were obtained at each time point. The addition of ArA along with RA significantly inhibited the RA-induced MUC16 expression at both 24 and 48 hours after treatment. *p < 0.05. Error bars, SEM.
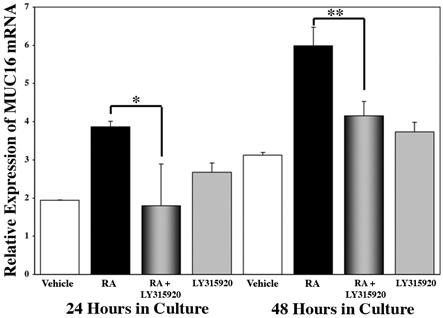
Having shown inhibition of RA-induced MUC16 upregulation using a broad spectrum PLA2 inhibitor, ArA, we sought to determine if a specific inhibitor of group IIA sPLA2, LY315920,47 would affect the RA-induced MUC16 expression. We examined MUC16 mRNA expression levels by real time PCR in HCjE cultures treated for 24 and 48 hours with vehicle (DMSO), 100 nM RA, 100 nM RA plus 10 μm LY315920 or 10 μm LY315920 alone. As shown in Figure 6, addition of 10 μm LY315920 significantly inhibited RA-induced MUC16 expression by 100% at 24 hours and 99% at 48 hours. As shown in Figure 7, the addition of the specific inhibitor for sPLA2-IIA, LY315920, results in complete inhibition of the RA-induced increase in MUC16 protein detected in cell lysates at both 24 (p<0.01) and 48 (p<0.0001) hours.
Figure 6.
Real time PCR analysis of MUC16 mRNA expression in conjunctival cells cultured with the specific inhibitor of sPLA2-IIA, LY315920. HCjE cells were grown for 24 or 48 hours with DMSO (Vehicle), 100 nM RA, RA plus 10 μM LY315920 or LY315920 alone. MUC16 expression is determined relative to the 0-hour value. The addition of LY315920 along with RA results in a significant inhibition of the RA-induced MUC16 expression at both time points. Culture with the inhibitor alone had no significant effect on MUC16 expression. Error bars, SEM (*p<0.01; **p<0.05).
MUC1 and MUC4 mRNA Expression
Analysis of the microarray data showed no upregulation by RA of the mRNAs for the other membrane-associated mucins, MUC1 and 4, expressed by the HCjE cells. This is in agreement with our previous data for MUC1, but not MUC4, as determined by real-time PCR.35 To investigate this difference, real-time PCR analysis was done using previously published primers and probes for MUC1 and 428,43 and the same samples that were used to prepare probes for microarray hybridization. Figure 8 shows the independent verification of the microarray quantitation as determined by real-time PCR. MUC1 mRNA quantitation by the 2 methods yielded similar results (Fig. 8A). However, even though we used aliquots of the same RNA, data for MUC4 mRNA from microarray did not reflect the results obtained with real-time PCR (Fig. 8B). In an attempt to resolve this discrepancy in MUC4 mRNA expression patterns using the 2 methods, we designed new MUC4 primers and probe for real-time PCR (MUC4 C-term), that would amplify the sequence from the same region of the C-terminus of MUC4 as used in the GeneChip. As shown in Figure 8C, MUC4 C-term mRNA was upregulated by RA over the time course. This agrees with the real-time PCR data obtained with the other published MUC4 primers and probe, and differed from the microarray data (Fig. 8B and 8C), suggesting a false negative result for MUC4 using microarray analysis. Figure 8D shows that a unique band, corresponding to the predicted size for the MUC4 C-term (75 bp), was obtained after 40 cycles of cDNA amplification. Sequencing of the PCR product verified that MUC4 was the amplified product.
Figure 8.
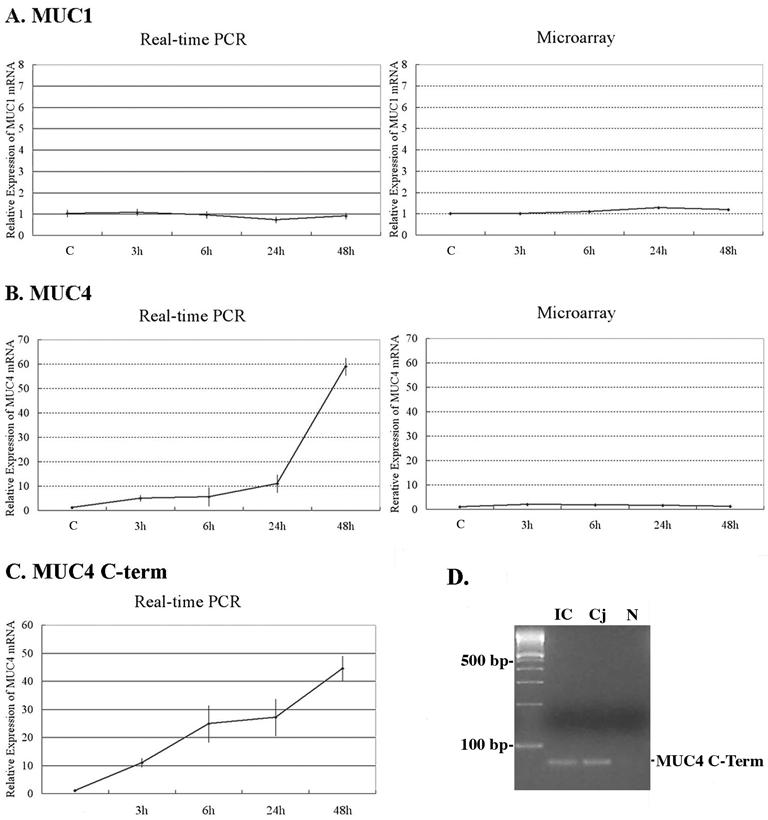
Relative mRNA level of MUC1 and MUC4 in HCjE cells in control (C) cultures and cultures treated with 100 nM retinoic acid (RA) for 3-48 hours. To verify the microarray data, relative mRNA levels of MUC1 (A) and MUC4 (B and C) mucins were measured with real-time PCR (left, n = 2) in the same samples that were used to prepare probe for microarray hybridizations (right). (A) MUC1 mRNA was not upregulated by 100 nM RA based on both microarray and real-time PCR data. (B) Although real-time PCR data demonstrated that RA upregulated MUC4 mRNA, there was no change in the microarray data. (C) For further investigation, we designed new MUC4 primers and probe for real-time PCR (MUC4 C-term) to sequence selected from the same region of the C-terminus of MUC4 as used in the GeneChip. MUC4 C-term mRNA was upregulated by RA over the time course. This expression pattern was again different from that seen by microarray analysis. (D) Conventional RT-PCR analysis demonstrates that the MUC4 primers designed from the C-terminal region (MUC4 C-term) used in real-time PCR produced the expected amplicon size (75 bp) in native conjunctival epithelial and HCjE cells. Samples were electrophoresed on a 4% agarose gel and visualized with ethidium bromide. Sequencing verified that MUC4 was the amplified product. IC: Native human conjunctival epithelium collected by impression cytology. Cj: HCjE culture in presence of serum for 72 hours. N: Negative control (sterile water).
DISCUSSION
This study demonstrates the effect of retinoic acid (RA) on the gene expression profile of human conjunctival epithelia using an immortalized conjunctival epithelial cell line and microarray analysis. In searching through the upregulated genes at both early and late phases for proteins or glycoproteins that may facilitate maintenance of a wet-surfaced phenotype and prevent the keratinization characteristic of keratomileusis, we noted that Group IIA secretory phospholipase A2 (sPLA2-IIA) and MUC16 were the two-most highly upregulated mRNAs after RA treatment in the late phase. We, therefore, focused on the relationship between sPLA2 and RA-associated MUC16 induction in further studies. The major conclusion of these experiments are that retinoic acid upregulates both sPLA2-IIA and MUC16, and that the MUC16 induction is mediated by sPLA2-IIA.
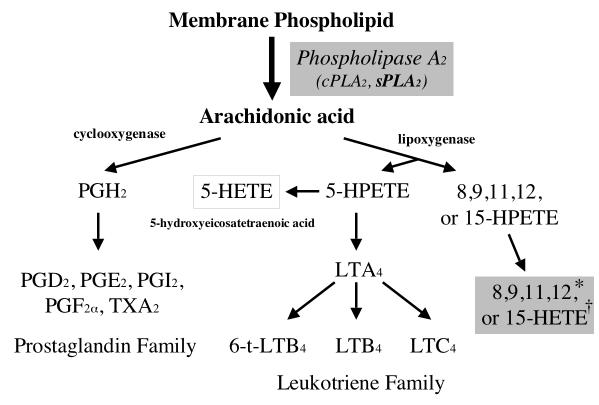
Both molecules are involved in defense of the ocular surface. MUC16 is one of the class of membrane-associated mucins that appear to be major constituents of the glycocalyx of all wet-surfaced epithelial cells,52 where they are hypothesized to facilitate maintenance of fluid on the apical surface and prevent pathogen invasion.53 Group IIA PLA2, a member of the extracellular (secreted) phospholipase A2 (sPLA2) family is a low molecular weight (14 kDa) enzyme.54 All members of the sPLA2 family catalyze the hydrolysis of glycerophospholipids at the sn-2 position to release fatty acids (usually arachidonic acid) and lysophospholipids, important in biosynthesis of lipid mediators (Fig. 9).55,56 sPLA2s also bind a variety of membrane and soluble proteins and can serve as high affinity ligands. The latter include the proteoglycans and the M receptor.55 Both the enzymatic activity and ligand binding appear to mediate a large range of cellular activities. (For review see Six and Dennis, 2000.57) sPLA2-IIA has been reported to be secreted by the lacrimal gland and has been recognized as an antibacterial molecule in tear fluid58-60 where it acts by cleaving arachidonic acid from the bacterial phospholipid membrane.
Figure 9.
Metabolism of arachidonic acid by cyclooxygenase and 5-lipoxigenase pathways. PG, protaglandin;LT, leukotriene; HPTE, hydroperoxyeicosatetraenoic acid. Modified from Diaz BL, Arm JP. Phospholipase A(2). Prostaglandins Leukot Essent Fatty Acids. 2003;69:87-97.
To date, there has been no report of a link between PLA2 and mucin gene expression, nor has there been data reported on RA induction of sPLA2-IIA. PLA2 is a key enzyme for eicosanoid metabolism, due to its regulation of the release of arachidonic acid. Arachidonic acid serves as a precursor to eicosanoids—a group of inflammatory mediators. Previous studies suggest that several lipoxygenase pathway eicosanoid metabolites, hydroxyeicosatetraenoic acids (HETEs), can stimulate mucus production in airway epithelium.29,30 In addition, Jackson et al. reported that topical application of 15(S)-HETE to the rabbit ocular surface increased the thickness of the mucin layer on the surface of the corneal epithelium31 and Jumblatt et al. demonstrated that 15(S)-HETE increased the amount of MUC1 protein, but not MUCs 2, 4, or 5AC in ex vivo human conjunctival tissue.32,33 Because the latter study was done prior to the identification of MUC16 in ocular surface epithelia,26 MUC16 regulation by 15(S)-HETE was not assayed. We found no change in MUC1 expression in response to RA, but did find significant increases in the membrane-associated mucin MUC16 and the eicosanoid metabolic enzyme sPLA2. The previous studies linking eicosanoid metabolites and mucus production led us to hypothesize that sPLA2 may be associated with RA-induced MUC16 regulation. Our data suggest that upregulation of sPLA2 levels in conjunctival cells may result in increased production of arachidonic acid and its lipoxygenase pathway eicosanoid metabolites (HETEs), resulting in increased biosynthesis of the membrane-associated mucin MUC16. The small increase in MUC16 upregulation in cultures treated with the sPLA2 inhibitor but not with RA compared to no increase with the broad spectrum inhibitor of PLA2, suggest that the mechanism of upregulation of MUC16 is not entirely controlled by sPLA2 and RA induction, and that additional PLA2 regulators may be active.
The significant inhibition of the RA-induced MUC16 expression by the broad spectrum PLA2 inhibitor ArA at 24 and 48 hours, for both mRNA and protein in HCjE cells suggests that eicosanoids may be involved in the regulation of MUC16. Use of specific inhibitors of the group IIA sPLA2 in RA-induced MUC16 expression also resulted in a highly significant inhibition at both 24 (100%) and 48 hours (99%) after addition of RA. These data indicated that RA induction of MUC16 is mediated by either eicosanoids or by ligand binding by sPLA2-IIA that signals through the cell membrane. Other factors may also be involved in the regulation of MUC16, since at baseline (0-hour controls), low levels of MUC16 mRNA are expressed, and that level increases over time without RA, albeit at lower levels. Recently Landreville et al.61 reported that group IIA sPLA2 is not expressed in human corneal epithelial cells isolated from fresh donor corneas. These cells normally express high levels of MUC1626, thus, in the corneal epithelium—compared to conjunctival epithelium—MUC16 gene expression is either differentially regulated or exogenous sPLA2-IIA, secreted by the conjunctival or lacrimal cells into the tear film, may effect upregulation of MUC16 expression in corneal epithelium.
Although microarray analysis is a powerful tool, allowing investigators to examine changes in the expression of thousands of genes simultaneously in a single experiment, there must be caution in interpretation of microarray results without confirmation by other techniques. Microarray results are influenced by array production, RNA extraction, probe labeling, hybridization efficiency, and image analysis.62 Therefore, to be assured of the quality of data obtained from microarray analysis, gene expression patterns are confirmed by other methods—real-time PCR or protein expression. In this study, in addition to MUC16 upregulation by RA, the microarray data also confirmed the previously reported real-time PCR data that RA did not regulate the membrane-associated mucin MUC1 in HCjE cells.35 On the other hand, the microarray data did not agree with the real-time PCR data for MUC4,35 even when the same RNA was used to prepare probes for microarray and cDNA for real-time PCR. The primers and probe for gene amplification of MUC4 used in real-time PCR was previously reported and designed from the area flanking the tandem repeat domains, based on GenBank accession No. AF058803.43,63 On the other hand, the target sequences of MUC4 used on the GeneChip were designed from the C-terminal region, which included the transmembrane domain, cytoplasmic tail, and 3′ untranslated sequence (http://www.affymetrix.com/index.affx).64 Then, to investigate whether the difference in expression patterns is a result of the difference in sensitivity between the two methods, or represents the expression of splice variants of MUC4, we designed new MUC4 primers and probe for real-time PCR (MUC4 C-term), which would amplify the region from the C-terminus of MUC4 used in the GeneChip, and compared the expression patterns between the two methods. Interestingly, using the MUC4 C-term primers and probe (Fig. 8 B, C) corroborated the real-time PCR data obtained previously and was different from what was found by microarray, with probes to the same region of the MUC4 gene. Real-time PCR is a sensitive method to detect cDNAs and is reported to require 1000-fold less RNA than conventional assays.62 Since the present real-time PCR data with the new MUC4 C-term primers confirmed our previous data35 on RA induced MUC4 expression, it was determined that the real-time PCR data was more reliable, and that the microarray data gave a false negative. This further emphasizes the need to corroborate microarray data.
Analyzing RA-treated HCjE cells at early and late phases provided insight into the magnitude and time course of changes in gene expression at different stages of RA treatment of human conjunctival epithelial cells. Microarray analysis yielded more transcripts that were up- or downregulated by RA in the late phase than in the early phase. We also found that 9 genes were upregulated in both the early and late phases. Keratin 4, which is known to be present in non-keratinized epithelia and is a non-keratinization marker of epithelial cells, was one of these genes. Expression of the keratin 4 gene increased between 3 and 24 hours (3.1-, 5.9-, and 7.5-fold increases at 3, 6, and 24 hours, respectively) and returned to the 6-hour level at 48 hours (6.2-fold change) (Tables 1 and 2). Since corneal and conjunctival epithelial cells are keratinized in patients with vitamin A deficiency, the continued upregulation of the non-keratinized marker keratin 4 by RA in HCjE cells is reasonable. Curiously, however, involucrin, which is expressed in normal epithelium,65,66 but upregulated in keratinized epithelium,65 was also upregulated in the early phase (3.7- and 6.0-fold increases) (Table 2). Involucrin is reported to be expressed in superficial layers in normal human conjunctival65 and corneal epithelial cells,66 and to be upregulated in superficial and intermediate layers of conjunctiva in patients with Stevens-Johnson Syndrome.65 As described above, the RA-treated HCjE cultures had islands of stratified cells with large apical cells. It is possible that a certain level of upregulation of both proteins is required for the normal differentiation of the HCjE cells from subconfluent to confluent, stratified cultures. Development of an in vitro model for keratinization of these cells may provide new information on the function of both proteins that could be correlated to that of pathological states, such as Stevens-Johnson Syndrome.
In summary, this study identified a number of genes that are up- and downregulated by treatment with retinoic acid (RA) in a cultured human conjunctival epithelial cell line (HCjE). The membrane-associated mucin MUC16 is highly responsive to RA in the late phase of treatment. This is indicative of an indirect effect of RA on MUC16 gene expression. Group IIA sPLA2 is also highly responsive to treatment with RA, being upregulated by 6 hours post treatment, peaking at 24 hours. But remaining highly expressed at 48 hours. Use of the specific Group IIA sPLA2 inhibitor LY315920 demonstrated that sPLA2 (the most upregulated gene by RA) mediates RA upregulation of MUC16 expression.
Acknowledgements
We acknowledge the assistance of the following individuals at Harvard University's Bauer Center for Genomics Research, Boston, MA: Paul Grosu, Reddy Gali, Swati Ranade, Jennifer Couget.
Footnotes
Supported by: NIH/NEI Grant #R01 EY03306
REFERENCES
- 1.Gudas LJ. Molecular mechanisms of retinoid action. Am J Respir Cell Mol Biol. 1990;2:319–320. doi: 10.1165/ajrcmb/2.4.319. [DOI] [PubMed] [Google Scholar]
- 2.Smith J, Steinemann TL. Vitamin A deficiency and the eye. Int Ophthalmol Clin. 2000;40:83–91. doi: 10.1097/00004397-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. J Lipid Res. 2002;43:1773–1808. doi: 10.1194/jlr.r100015-jlr200. [DOI] [PubMed] [Google Scholar]
- 4.Love JM, Gudas LJ. Vitamin A, differentiation and cancer. Curr Opin Cell Biol. 1994;6:825–831. doi: 10.1016/0955-0674(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 5.Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 6.Durand B, Saunders M, Leroy P, Leid M, Chambon P. All-trans and 9-cis retinoic acid induction of CRABPII transcription is mediated by RAR-RXR heterodimers bound to DR1 and DR2 repeated motifs. Cell. 1992;71:73–85. doi: 10.1016/0092-8674(92)90267-g. [DOI] [PubMed] [Google Scholar]
- 7.Ramkumar T, Adler S. A requirement for the POU transcription factor, Brn-2, in corticotropin-releasing hormone expression in a neuronal cell line. Mol Endocrinol. 1999;13:1237–1248. doi: 10.1210/mend.13.8.0327. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan WR, McCulley JP, Dohlman CH. Return of goblet cells after vitamin A therapy in xerosis of the conjunctiva. Am J Ophthalmol. 1973;75:720–725. doi: 10.1016/0002-9394(73)90828-3. [DOI] [PubMed] [Google Scholar]
- 9.Soong HK, Martin NF, Wagoner MD, et al. Topical retinoid therapy for squamous metaplasia of various ocular surface disorders. A multicenter, placebo-controlled double-masked study. Ophthalmology. 1988;95:1442–1446. doi: 10.1016/s0161-6420(88)33009-5. [DOI] [PubMed] [Google Scholar]
- 10.Herbort CP, Zografos L, Zwingli M, Schoeneich M. Topical retinoic acid in dysplastic and metaplastic keratinization of corneoconjunctival epithelium. Graefes Arch Clin Exp Ophthalmol. 1988;226:22–26. doi: 10.1007/BF02172711. [DOI] [PubMed] [Google Scholar]
- 11.Sommer A. Xerophthalmia and vitamin A status. Prog Retin Eye Res. 1998;17:9–31. doi: 10.1016/s1350-9462(97)00001-3. [DOI] [PubMed] [Google Scholar]
- 12.Huang AJW, Tseng SCG, Kenyon KR. Change of paracellular permeability of ocular surface epithelium by vitamin A deficiency. Invest Ophthlmol Vis Sci. 1991;32:633–639. [PubMed] [Google Scholar]
- 13.Tei M, Spurr-Michaud SJ, Tisdale AS, Gipson IK. Vitamin A deficiency alters the expression of mucin genes by the rat ocular surface epithelium. Invest Ophthalmol Vis Sci. 2000;41:82–88. [PubMed] [Google Scholar]
- 14.Gipson IK, Inatomi T. Mucin genes expressed by the ocular surface epithelium. Prog Ret Eye Res. 1997;16:81–98. [Google Scholar]
- 15.Gendler SJ, Spicer AP. Epithelial mucin genes. Annu Rev Physiol. 1995;57:607–634. doi: 10.1146/annurev.ph.57.030195.003135. [DOI] [PubMed] [Google Scholar]
- 16.Williams SJ, McGuckin MA, Gotley DC, Eyre HJ, Sutherland GR, Antalis TM. Two novel mucin genes down-regulated in colorectal cancer identified by differential display. Cancer Res. 1999;59:4083–4089. [PubMed] [Google Scholar]
- 17.Williams SJ, Wreschner DH, Tran M, Eyre HJ, Sutherland GR, McGuckin MA. Muc13, a novel human cell surface mucin expressed by epithelial and hemopoietic cells. J Biol Chem. 2001;276:18327–18336. doi: 10.1074/jbc.M008850200. [DOI] [PubMed] [Google Scholar]
- 18.Pallesen LT, Berglund L, Rasmussen LK, Petersen TE, Rasmussen JT. Isolation and characterization of MUC15, a novel cell membrane-associated mucin. Eur J Biochem. 2002;269:2755–2763. doi: 10.1046/j.1432-1033.2002.02949.x. [DOI] [PubMed] [Google Scholar]
- 19.Yin BW, Lloyd KO. Molecular cloning of the CA125 ovarian cancer antigen: Identification as a new mucin, MUC16. J Biol Chem. 2001;276:27371–27375. doi: 10.1074/jbc.M103554200. [DOI] [PubMed] [Google Scholar]
- 20.Gum JR, Jr., Crawley SC, Hicks JW, Szymkowski DE, Kim YS. MUC17, a novel membrane-tethered mucin. Biochem Biophys Res Commun. 2002;291:466–475. doi: 10.1006/bbrc.2002.6475. [DOI] [PubMed] [Google Scholar]
- 21.Higuchi T, Orita T, Nakanishi S, et al. Molecular cloning, genomic structure, and expression analysis of MUC20, a novel mucin protein, up-regulated in injured kidney. J Biol Chem. 2004;279:1968–1979. doi: 10.1074/jbc.M304558200. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Zhao YH, Kalaslavadi TB, et al. Genome-wide search and identification of a novel gel-forming mucin MUC19/Muc19 in glandular tissues. Am J Respir Cell Mol Biol. 2003;30:155–165. doi: 10.1165/rcmb.2003-0103OC. [DOI] [PubMed] [Google Scholar]
- 23.Lapensee L, Paquette Y, Bleau G. Allelic polymorphism and chromosomal localization of the human oviductin gene (MUC9) Fertil Steril. 1997;68:702–708. doi: 10.1016/s0015-0282(97)00317-8. [DOI] [PubMed] [Google Scholar]
- 24.Inatomi T, Spurr-Michaud S, Tisdale AS, Zhan Q, Feldman ST, Gipson IK. Expression of secretory mucin genes by human conjunctival epithelia. Invest Ophthalmol Vis Sci. 1996;37:1684–1692. [PubMed] [Google Scholar]
- 25.Pflugfelder SC, Liu Z, Monroy D, et al. Detection of sialomucin complex (MUC4) in human ocular surface epithelium and tear fluid. Invest Ophthalmol Vis Sci. 2000;41:1316–1326. [PubMed] [Google Scholar]
- 26.Argueso P, Spurr-Michaud S, Russo CL, Tisdale A, Gipson IK. MUC16 mucin is expressed by the human ocular surface epithelia and carries the H185 carbohydrate epitope. Invest Ophthalmol Vis Sci. 2003;44:2487–2495. doi: 10.1167/iovs.02-0862. [DOI] [PubMed] [Google Scholar]
- 27.Jumblatt MM, McKenzie RW, Steele PS, Emberts CG, Jumblatt JE. MUC7 Expression in the human lacrimal gland and conjunctiva. Cornea. 2003;22:41–45. doi: 10.1097/00003226-200301000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Gipson IK, Spurr-Michaud S, Argueso P, Tisdale A, Ng TF, Russo CL. Mucin gene expression in immortalized human corneal-limbal and conjunctival epithelial cell lines. Invest Ophthalmol Vis Sci. 2003;44:2496–2506. doi: 10.1167/iovs.02-0851. [DOI] [PubMed] [Google Scholar]
- 29.Marom Z, Shelhamer JH, Sun F, Kaliner M. Human airway monohydroxyeicosatetraenoic acid generation and mucus release. J Clin Invest. 1983;72:122–127. doi: 10.1172/JCI110949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lundgren JD, Shelhamer JH, Kaliner MA. The role of eicosanoids in respiratory mucus hypersecretion. Ann Allergy. 1985;55 [PubMed] [Google Scholar]
- 31.Jacks T, Weinberg RA. The expanding role of cell cycle regulators. Science. 1998;280:1035–1036. doi: 10.1126/science.280.5366.1035. [DOI] [PubMed] [Google Scholar]
- 32.Jumblatt JE, Cunningham LT, Li Y, Jumblatt MM. Characterization of human ocular mucin secretion mediated by 15(S)-HETE. Cornea. 2002;21:818–824. doi: 10.1097/00003226-200211000-00018. [DOI] [PubMed] [Google Scholar]
- 33.Jumblatt JE, Cunningham L, Jumblatt MM. Effects of 15(S)-HETE on human conjunctival mucin secretion. Adv Exp Med Biol. 2002;506:323–327. doi: 10.1007/978-1-4615-0717-8_46. [DOI] [PubMed] [Google Scholar]
- 34.Bossenbroek NM, Sulahian TH, Ubels JL. Expression of nuclear retinoic acid receptor and retinoid X receptor mRNA in the cornea and conjunctiva. Curr Eye Res. 1998;17:462–469. doi: 10.1076/ceyr.17.5.462.5189. [DOI] [PubMed] [Google Scholar]
- 35.Hori Y, Spurr-Michaud S, Russo C, Argueso P, Gipson I. Differential regulation of membrane-associated mucins in the human ocular surface epithelium. Invest Ophthalmol Vis Sci. 2004;45:114–122. doi: 10.1167/iovs.03-0903. [DOI] [PubMed] [Google Scholar]
- 36.Lipshutz RJ, Fodor SP, Gingeras TR, Lockhart DJ. High density synthetic oligonucleotide arrays. Nat Genet. 1999;21:20–24. doi: 10.1038/4447. [DOI] [PubMed] [Google Scholar]
- 37.Young RA. Biomedical discovery with DNA arrays. Cell. 2000;102:9–15. doi: 10.1016/s0092-8674(00)00005-2. [DOI] [PubMed] [Google Scholar]
- 38.Mahajan VB, Wei C, McDonnell PJ. 3rd. Microarray analysis of corneal fibroblast gene expression after interleukin-1 treatment. Invest Ophthalmol Vis Sci. 2002;43:2143–2151. [PubMed] [Google Scholar]
- 39.Varela JC, Goldstein MH, Baker HV, Schultz GS. Microarray analysis of gene expression patterns during healing of rat corneas after excimer laser photorefractive keratectomy. Invest Ophthalmol Vis Sci. 2002;43:1772–1782. [PubMed] [Google Scholar]
- 40.Jun AS, Liu SH, Koo EH, Do DV, Stark WJ, Gottsch JD. Microarray analysis of gene expression in human donor corneas. Arch Ophthalmol. 2001;119:1629–1634. doi: 10.1001/archopht.119.11.1629. [DOI] [PubMed] [Google Scholar]
- 41.Cao Z, Wu HK, Bruce A, Wollenberg K, Panjwani N. Detection of differentially expressed genes in healing mouse corneas, using cDNA microarrays. Invest Ophthalmol Vis Sci. 2002;43:2897–2904. [PubMed] [Google Scholar]
- 42.Rheinwald JG, Hahn WC, Ramsey MR, et al. A two-stage, p16INK4A- and p53-dependent keratinocyte senescence mechanism that limits replicative potential indepedent of telomere status. Molec Cell Biol. 2002;22:5157–5172. doi: 10.1128/MCB.22.14.5157-5172.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Argüeso P, Balaram M, Spurr-Michaud S, Keutmann HT, Dana MR, Gipson IK. Decreased levels of the goblet cell mucin MUC5AC in tears of patients with Sjögren's syndrome. Invest Ophthalmol Vis Sci. 2002;43:1004–1011. [PubMed] [Google Scholar]
- 44.Danjo Y, Hazlett LD, Gipson IK. C57BL/6 mice lacking Muc1 show no ocular surface phenotype. Invest Ophthalmol Vis Sci. 2000;41:4080–4084. [PubMed] [Google Scholar]
- 45.de Cremoux P, Extra JM, Denis MG, et al. Detection of MUC1-expressing mammary carcinoma cells in the peripheral blood of breast cancer patients by real-time polymerase chain reaction. Clin Cancer Res. 2000;6:3117–3122. [PubMed] [Google Scholar]
- 46.Caro AA, Cederbaum AI. Role of phospholipase A2 activation and calcium in CYP2E1-dependent toxicity in HepG2 cells. J Biol Chem. 2003;278:33866–33877. doi: 10.1074/jbc.M300408200. [DOI] [PubMed] [Google Scholar]
- 47.Snyder DW, Bach NJ, Dillard RD, et al. Pharmacology of LY315920/S-5920,1 [[3-(Aminooxoacetyl)-2-ethyl-1-(phenylmethyl)-1H-indol-4-yl]oxy]acetate, a potent and selective secretory phospholipase A2 inhibitor: A new class of anti-inflammatory drugs, SPI. J Pharmacol Exper Therapeut. 1999;288:1117–1124. [PubMed] [Google Scholar]
- 48.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 49.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song CH, Choi JS, Kim DK, Kim JC. Enhanced secretory group II PLA2 activity in the tears of chronic blepharitis patients. Invest Ophthalmol Vis Sci. 1999;40:2744–2748. [PubMed] [Google Scholar]
- 51.Gerritsen ME, Tomlinson JE, Zlot C, Ziman M, Hwang S. Using gene expression profiling to identify the molecular basis of the synergistic actions of hepatocyte growth factor and vascular endothelial growth factor in human endothelial cells. Br J Pharmacol. 2003;140:595–610. doi: 10.1038/sj.bjp.0705494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gipson I, Argueso P. The role of mucins in the function of the corneal and conjunctival epithelia. Int Rev Cytol. 2003;231:1–49. doi: 10.1016/s0074-7696(03)31001-0. [DOI] [PubMed] [Google Scholar]
- 53.Fleiszig SM, Zaidi TS, Ramphal R, Pier GB. Modulation of Pseudomonas aeruginosa adherence to the corneal surface by mucus. Infect Immun. 1994;62:1799–1804. doi: 10.1128/iai.62.5.1799-1804.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boilard E, Bourgoin SG, Bernatchez C, Poubelle PE, Surette ME. Interaction of low molecular weight group IIA phospholipase A2 with apoptotic human T cells: role of heparan sulfate proteoglycans. Faseb J. 2003;17:1068–1080. doi: 10.1096/fj.02-0938com. [DOI] [PubMed] [Google Scholar]
- 55.Valentin E, Lambeau G. Increasing molecular diversity of secreted phospholipases A2 and their receptors and binding proteins. Biochim Biophys Acta. 2000;1488:59–70. doi: 10.1016/s1388-1981(00)00110-4. [DOI] [PubMed] [Google Scholar]
- 56.Diaz BL, Arm JP. Phospholipase A(2) Prostaglandins Leukot Essent Fatty Acids. 2003;69:87–97. doi: 10.1016/s0952-3278(03)00069-3. [DOI] [PubMed] [Google Scholar]
- 57.Six DA, Dennis EA. The expanding superfamily of phospholipase A2 enzymes: classification and characterization. Biochim Biophys Acta. 2000;1488:1–19. doi: 10.1016/s1388-1981(00)00105-0. [DOI] [PubMed] [Google Scholar]
- 58.Nevalainen TJ, Aho HJ, Peuravuori H. Secretion of group 2 phospholipase A2 by lacrimal glands. Invest Ophthalmol Vis Sci. 1994;35:417–421. [PubMed] [Google Scholar]
- 59.Qu XD, Lehrer RI. Secretory phospholipase A2 is the principal bactericide for staphylococci and other gram-positive bacteria in human tears. Infect Immun. 1998;66:2791–2797. doi: 10.1128/iai.66.6.2791-2797.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moreau JM, Girgis DO, Hume EB, Dajcs JJ, Austin MS, O'Callaghan RJ. Phospholipase A(2) in rabbit tears: a host defense against Staphylococcus aureus. Invest Ophthalmol Vis Sci. 2001;42:2347–2354. [PubMed] [Google Scholar]
- 61.Landreville S, Coluombe S, Carrier P, Gelb MH, Guerin SL. C S. Expression of phospholipases A2 and C in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:3997–4003. doi: 10.1167/iovs.04-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rajeevan MS, Ranamukhaarachchi DG, Vernon SD, Unger ER. Use of real-time quantitative PCR to validate the results of cDNA array and differential display PCR technologies. Methods. 2001;25:443–451. doi: 10.1006/meth.2001.1266. [DOI] [PubMed] [Google Scholar]
- 63.Gipson IK, Spurr-Michaud S, Moccia R, et al. MUC4 and MUC5B transcripts are the prevalent mucin messenger ribonucleic acids of the human endocervix. Biol Reprod. 1999;60:58–64. doi: 10.1095/biolreprod60.1.58. [DOI] [PubMed] [Google Scholar]
- 64.Moniaux N, Nollet S, Porchet N, Degand P, Laine A, Aubert J-P. Complete sequence of the human mucin MUC4: a putative cell membrane-associated mucin. Biochem J. 1999;338:325–333. [PMC free article] [PubMed] [Google Scholar]
- 65.Nakamura T, Nishida K, Dota A, Matsuki M, Yamanishi K, Kinoshita S. Elevated expression of transglutaminase 1 and keratinization-related proteins in conjunctiva in severe ocular surface disease. Invest Ophthalmol vis Sci. 2001;42:549–556. [PubMed] [Google Scholar]
- 66.Adhikary G, Crish J, Lass J, Eckert RL. Regulation of involucrin expression in normal human corneal epithelial cells: a role for activator protein one. Invest Ophthalmol Vis Sci. 2004;45:1080–1087. doi: 10.1167/iovs.03-1180. [DOI] [PubMed] [Google Scholar]