Abstract
Purpose
Previously, we reported our experience treating 14 patients with metastatic melanoma using a fully human antibody to cytotoxic T-lymphocyte antigen-4 (anti–CTLA-4) in conjunction with peptide vaccination. We have now treated 56 patients to evaluate two different dose schedules of anti–CTLA-4 and to explore the relationship between autoimmunity and tumor regression.
Patients and Methods
A total of 56 patients with progressive stage IV melanoma were enrolled onto the study. All had Karnofsky performance status ≥60% with no prior history of autoimmunity. Twenty-nine patients received 3 mg/kg anti–CTLA-4 every 3 weeks, whereas 27 received 3 mg/kg as their initial dose with subsequent doses reduced to 1 mg/kg every 3 weeks. In both cohorts patients received concomitant vaccination with two modified HLA-A*0201-restricted peptides from the gp100 melanoma-associated antigen, gp100:209-217(210M) and gp100:280-288(288V).
Results
Two patients achieved a complete response (ongoing at 30 and 31 months, respectively) and five patients achieved a partial response (durations of 4, 6, 25+, 26+, and 34+ months, respectively), for an overall objective response rate of 13%. Tumor regression was seen in lung, liver, brain, lymph nodes, and subcutaneous sites. Of 14 patients with grade 3/4 autoimmune toxicity, five (36%) experienced a clinical response compared with only two responses in the 42 patients (5%) with no autoimmune toxicity (P = .008). There were no significant differences in response rate or toxicity between the two dose schedules.
Conclusion
Administration of anti–CTLA-4 monoclonal antibody plus peptide vaccination can cause durable objective responses, which correlate with the induction of autoimmunity, in patients with metastatic melanoma.
INTRODUCTION
T-cell activation requires at least two signals. The interaction between the T-cell receptor and the specific antigen presented by a major histocompatibility complex molecule on an antigen-presenting cell (APC), such as a dendritic cell, conveys the first signal to the T cell.1 Engagement of additional receptors on the surface of the T cell can either enhance or inhibit the T cell response. Failure of the T cell to receive a second signal can lead to anergy.2,3 Thus second signals fine-tune the immune response, and the overall T-cell response is determined by the integration of all signals, both stimulatory and inhibitory.
The most well-studied T-cell receptors for costimulatory molecules are CD28 and cytotoxic T-lymphocyte antigen-4 (CTLA-4; also called CD152) that react with ligands on the APC, CD80 and CD86 (also named B7-1 and B7-2, respectively).4–6 Although closely related within the immunoglobulin superfamily,7,8 CD28 and CTLA-4 molecules function antagonistically. Engagement by CD28 enhances T-cell activation, proliferation, and interleukin-2 (IL-2) production.4,5,9 CTLA-4 also binds to CD80 and CD86, but with greater affinity than it binds to CD28,10 and antagonizes T-cell activation by interfering with IL-2 secretion and IL-2 receptor expression, and by inhibiting the expression of critical cell cycle components.11–14 CTLA-4 is not found on the surface of most resting T cells, but is upregulated transiently after T-cell activation.15,16 Naturally occurring immunosuppressive regulatory T cells (identifiable by their concurrent expression of CD4, CD25, and foxp3, and by their in vitro capacity to inhibit the proliferation of other T cells17–19) constitutively express surface CTLA-4.
CTLA-4 blockade in select murine tumor models enhanced immune-mediated tumor rejection.20–23 Evidence of autoimmunity such as depigmentation in a melanoma model23 and prostatitis in a prostate cancer model22 was also seen. We previously described 14 patients with stage IV metastatic melanoma who were administered a humanized anti–CTLA-4 antibody (MDX-010; Medarex Inc, Princeton, NJ) at 3 mg/kg every 3 weeks with concomitant vaccination with two gp100 peptides.24 Three of these patients experienced objective cancer regressions including two complete responses. Six of 14 patients experienced grade 3/4 autoimmunities including dermatitis, enterocolitis, hepatitis, and hypophysitis (all of which were successfully managed with supportive care and/or corticosteroid therapy). Because the administration of gp100 peptides alone to melanoma patients did not result in tumor regression or grade 3/4 autoimmunity in prior studies,25,26 these results suggested that CTLA-4 blockade played a role in the maintenance of tolerance to self-antigens in humans, as well as in tumor regression.
In this report we have now extended these observations to the treatment of 56 patients and have evaluated two dose schedules of anti–CTLA-4 administration. Durable objective tumor regressions were seen that correlated with the induction of autoimmunity in these patients.
PATIENTS AND METHODS
Patients and Treatment
All patients expressed HLA-A*0201, had stage IV metastatic melanoma, Karnofsky performance status ≥ 60%, no previous gp100 vaccination, and no evidence of autoimmune or immunodeficiency disease, and ≥ 3 weeks had elapsed since any previous systemic cancer therapy. All patients signed an informed consent before protocol enrollment and were treated in the Surgery Branch, National Institutes of Health (Bethesda, MD). This study was approved by the Institutional Review Board of the National Cancer Institute and consisted of two cohorts. Because of the development of grade 3/4 autoimmune toxicity in the initial 14 patients reported previously who received all doses of antibody at 3 mg/kg,24 the next 27 patients received a decreased maintenance dose (ie, the doses subsequent to the first 3 mg/kg dose) of 1 mg/kg; these patients constitute cohort 2. After observing that the autoimmunity was reversible and seemed to be associated with clinical response, we then treated 15 additional patients with the original 3 mg/kg doses; these 29 patients constitute cohort 1.
In cohort 1, patients received the same therapy as previously published24; anti–CTLA-4 antibody (Ab; MDX-010) every 3 weeks at 3 mg/kg intravenously during 90 minutes, and 1 mg of gp100:209-217(210M) peptide (IMDQVPFSV) emulsified in incomplete Freund’s adjuvant injected in one extremity and 1 mg of gp100:280-288(288V) peptide (YLEPGPVTV) emulsified in incomplete Freund’s adjuvant injected in another extremity. In cohort 2, patients were given the Ab and gp100 peptides in a similar manner, except that after the initial dose of the Ab (which was 3 mg/kg as in cohort 1) subsequent doses were 1 mg/kg. MDX-010 was provided by the manufacturer; peptides were provided by the Cancer Therapy Evaluation Program, National Cancer Institute.
When possible, patients underwent apheresis to collect peripheral-blood mononuclear cells (PBMCs) before treatment and 3 weeks after every two treatment cycles. PBMCs were isolated by Ficoll-Hypaque separation, cryopreserved at 108 cells/vial in heat-inactivated human AB serum with 10% dimethyl sulfoxide, and stored at −180°C until further use.
Clinical Response Evaluation and Autoimmunity Screening
All patients underwent computed axial tomography of the chest, abdomen, and pelvis, and magnetic resonance imaging of the brain within 4 weeks before treatment and subsequently after every two cycles of therapy; other radiologic modalities were used as needed to evaluate disease sites. For every patient, the sum of the longest diameters of all tumors before and after treatment was calculated, in accordance with Response Evaluation Criteria in Solid Tumors Group criteria.27 A partial response was defined as the reduction of ≥ 30% (but < 100%) of the sum of the longest diameters of all assessable metastases lasting at least 1 month with no new or enlarging tumors; a complete response was the disappearance of all assessable tumors for at least 1 month. Patients not having either a partial or a complete response were deemed non-responders. To screen for potential autoimmunity, all patients at baseline received an ophthalmologic examination, which was repeated after 3 months on study. At baseline all patients were negative for serum antithyroglobulin Ab, rheumatoid factor, and antinuclear antibody. Human antihuman (anti-idiotypic) Ab, antinuclear antibody, thyroid-stimulating hormone, and free T4 were routinely evaluated every 3 to 12 weeks while patients were enrolled onto the study.
Pharmacokinetics
Plasma concentrations of MDX-010 were detected as reported previously24 using enzyme-linked immunosorbent assays (ELISA). Briefly, dilutions of plasma samples were incubated in wells coated with CTLA-4-Ig (R&D Systems, Minneapolis, MN). Bound anti–CTLA-4 Ab was detected with alkaline phosphatase–labeled goat antihuman immunoglobulin G (IgG) F(ab)-specific probe developed with para-nitrophenylphosphate substrate.
Flow Cytometry
Flow cytometry was used to assess for the surface expression of selected T-cell markers and was performed as previously described in patients for whom adequate samples existed.24 Briefly, cryopreserved PBMCs were thawed in ice-cold buffer, washed, and Fc-receptor blocked with mouse IgG (Caltag Laboratories, Burlingame, CA). Cells were then incubated with appropriate fluorochrome-labeled antibodies (BD Biosciences, San Diego, CA) and relevant isotype controls, washed twice subsequently, and fixed with 1% formaldehyde. FACSCalibur and CellQuest software (BD Biosciences) was used for acquisition and analysis.
In Vitro Sensitization Assay
In vitro sensitization assay (IVS) assay was used to assess immunologic reactivity as previously described in patients for whom adequate samples existed.24,25 Briefly, cryopreserved PBMCs were thawed in complete media with 10% heat-inactivated human AB serum, grown in culture with 1 μmol/L of native gp100:209-217 or gp100:280-288 peptide and 300 U/mL of IL-2. Cells were collected 11 to 13 days after initiation of the culture and then coincubated for 18 to 24 hours with peptide-pulsed T2 cells in 96-well plates. Interferon gamma (IFN-γ) release in the supernatant was measured using commercial ELISA assays (Pierce-Endogen, Rockford, IL). A positive assay was defined by three criteria: IFN-γ ≥ 100 pg/mL, ≥ 2 times greater when compared with an irrelevant control peptide, and ≥ 2 times greater than preimmunization samples (all tested on cryopreserved cells within the same assay).
RESULTS
Patient Characteristics
All patients had stage IV melanoma (Table 1). Twenty-seven patients (48%) had visceral metastases and 40 patients (71%) had intrathoracic metastases. All patients had undergone resection of their primary lesion before enrollment onto this study. Twenty-two patients (39%) had previously undergone chemotherapy and 41 patients (73%) had received prior immunotherapy, including high-dose bolus IL-2, low-dose bolus IL-2, subcutaneously injected IL-2, or IFN-α. Patients 1 to 14 and 42 to 56 were in cohort 1 (3 mg/kg each dose), and patients 15 to 41 were in cohort 2 (all but the first dose at 1 mg/kg).
Table 1.
Patient Characteristics, Clinical Response, and Toxicity
| Patient | Age (years) | Sex | Disease Sites | Prior Therapy | No. Cycles Received* | Response (months)† | Autoimmune Toxicity (grade III/IV) |
|---|---|---|---|---|---|---|---|
| Cohort 1 | |||||||
| 01 | 52 | M | Lung | I, S | 2 | PR (34+) | Enterocolitis; dermatitis |
| 02 | 40 | F | Supraclavicular LN | C, I, S | 1 | NR | Dermatitis |
| 03 | 39 | M | Lung, mediastinum, SQ | S | 6 | NR | |
| 04 | 55 | F | Skin, SQ | I, S | 1 | NR | |
| 05 | 67 | M | Liver, RP, SQ | C, I, R, S | 4 | NR | |
| 06 | 59 | M | Lung, SQ | I, S | 4 | NR | |
| 07 | 48 | M | Lung, brain, adrenal, SQ | I, S | 2 | NR | |
| 08 | 48 | M | Lung, liver, adrenal, mesentery, SQ | C, I, S | 2 | NR | |
| 09 | 53 | M | Mediastinum, mesentery, skin | I, R, S | 2 | NR | Colitis |
| 10 | 62 | M | Lung, hilum | C, I, S | 2 | NR | |
| 11 | 54 | M | Lung, brain, SQ | S | 5 | CR (31+) | Hypophysitis |
| 12 | 43 | M | Subdiaphragm, muscle, SQ | I, S | 3 | NR | Hepatitis |
| 13 | 49 | F | Lung, SQ | C, I, S | 4 | CR (30+) | Dermatitis |
| 14 | 63 | M | Lung, pelvic LN | S | 4 | NR | |
| 42 | 55 | M | Axilla, SQ | S | 2 | NR | |
| 43 | 58 | F | Lung, liver, adrenal, spleen | S | 6 | PR (4) | |
| 44 | 47 | M | Bone, supraclavicular | I, S | 2 | NR | |
| 45 | 46 | M | Parotid, cervical LN, SQ | C, I, S | 4 | NR | |
| 46 | 30 | M | Axilla, retrosternal | I, S | 2 | NR | |
| 47 | 63 | M | Periaortic LN, inguinal LN, iliac LN | I, S | 2 | NR | Colitis |
| 48 | 42 | M | Lung, liver, mediastinal, hilar, periportal | S | 2 | NR | Colitis |
| 49 | 30 | F | Liver, spleen | I, S | 1 | NR | Colitis |
| 50 | 53 | M | Lung | I, R, S | 4 | NR | |
| 51 | 23 | F | Lung, liver, brain, bone, iliac LN, inguinal LN, SQ | C, I, R, S | 1 | NR | |
| 52 | 44 | F | Abdominal wall, RP | S | 3 | NR | |
| 53 | 53 | M | Adrenal, axilla, iliac, inguinal LN, bone, kidney, liver, RP | C, I, S | 2 | NR | |
| 54 | 54 | F | Lung, paracaval, periaortic | C, I, S | 8 | NR | |
| 55 | 43 | F | RP, Iliac LN, SQ | C, I, R, S | 3 | NR | |
| 56 | 41 | M | Lung, adrenal, axilla, bone, brain, mediastinum, RP, SQ | C, I, R, S | 4 | NR | |
| Cohort 2 | |||||||
| 15 | 54 | F | Lung, mediastinum | C, S | 4 | PR (26+) | Colitis |
| 16 | 39 | M | Lung, liver, SQ | I, R, S | 4 | NR | |
| 17 | 48 | M | Lung, adrenal, SQ | S | 2 | NR | Colitis |
| 18 | 32 | M | Lung, liver, mediastinum, RP | C, I, R, S | 2 | NR | |
| 19 | 60 | F | Lung, mediastinum, GB, RP, SQ | S | 12 | PR (25+) | Dermatitis |
| 20 | 62 | M | Mediastinum, adrenal, mesentery, spleen, bone | S, I | 4 | NR | |
| 21 | 50 | M | Lung | S | 4 | NR | |
| 22 | 50 | M | Lung, supraclavicular LN, bone | S | 2 | NR | |
| 23 | 64 | M | Lung, liver, mesentery, omentum | C, I, R, S | 4 | NR | |
| 24 | 62 | M | Lung, buttock, SQ | I, S | 8 | PR (6) | |
| 25 | 61 | F | Axilla, supraclavicular LN, paraaortic LN, mesentery | I, S | 6 | NR | |
| 26 | 61 | F | Mediastinal/paratracheal LN | S | 8 | NR | |
| 27 | 21 | M | Lung, hilum | C, I, S | 12 | NR | |
| 28 | 45 | F | Lung, liver, bone, SQ | C, I, R, S | 4 | NR | |
| 29 | 63 | F | Mediastinum, axilla, RP, bladder, SQ | R, I, S | 2 | NR | |
| 30 | 59 | F | Lung, mediastinum, SQ | I, S | 4 | NR | |
| 31 | 56 | M | Lung, liver, axilla, SQ | C, I, S | 2 | NR | |
| 32 | 57 | M | Psoas, periaortic, SQ | I, S | 2 | NR | |
| 33 | 65 | F | Lung | S | 4 | NR | |
| 34 | 49 | M | Lung, inguinal LN, SQ | C, I, S | 4 | NR | |
| 35 | 66 | M | Lung, liver, mediastinum, axilla | R, I, S | 4 | NR | |
| 36 | 57 | M | RP | S | 3 | NR | Uveitis |
| 37 | 47 | F | Adrenal, axilla, adrenal, mediastinum, supraclavicular LN | C, I, S | 2 | NR | |
| 38 | 33 | F | Lung, mediastinum, SQ breast | C, I, R, S | 2 | NR | |
| 39 | 61 | M | Lung, axilla, mesentery, pelvis, retrocrural, SQ | C, I, R, S | 2 | NR | |
| 40 | 42 | M | Femur, SQ | C, I, R, S | 9 | NR | |
| 41 | 53 | M | Lung, subcarinal, hilum, liver, substernum, SQ | I, S | 1 | NR | Colitis, episcleritis‡ |
NOTE. Patients 01–14 and 42–56 were in the 3 → 3 mg/kg cohort (cohort 1); Patients 01–14 were previously reported.24 Patients 15–41 were in the 3 → 1 mg/kg cohort (cohort 2).
Abbreviations: LN, lymph node; SQ, subcutaneous; RP, retroperitoneal; I, immunotherapy; C, chemotherapy; S, surgery; R, radiation therapy; PR, partial response; CR, complete response; +, ongoing response; NR, no response.
One treatment cycle consisted of one infusion of anti–CTLA-4 Ab and one vaccination with gp100:209-217(210M) and gp100:280-288(288V) peptides.
Responses as of January 31, 2005.
Grade II toxicity.
Clinical Response
Two patients achieved a complete response, and five patients experienced a partial response (Table 1) for an overall objective response rate of 13%. Both complete responses (patients 11 and 13) are ongoing at 31 and 30 months, respectively. Of the five partial responders, two (patients 24 and 43) experienced disease recurrence at 6 and 4 months, respectively, whereas three (patients 1, 15, and 19) have ongoing responses at 34, 26, and 25 months, respectively. Patient 15 experienced slight shrinkage of tumor at the end of her first course (four cycles); however, at the completion of her second course, she had experienced almost the complete disappearance of a left lung parenchymal lesion (Figs 1A and 1B) and a bulky mediastinal mass (Figs 1C and 1D). Patient 19 experienced the disappearance of many subcutaneous lesions after the first course, with stable disease in the remainder of her disease locations. After her second course, she experienced significant shrinkage in lung (Figs 1E and 1F), mediastinal, gallbladder, and retroperitoneal lesions, in addition to complete disappearance of many subcutaneous lesions (Figs 1G and 1H).
Fig 1.
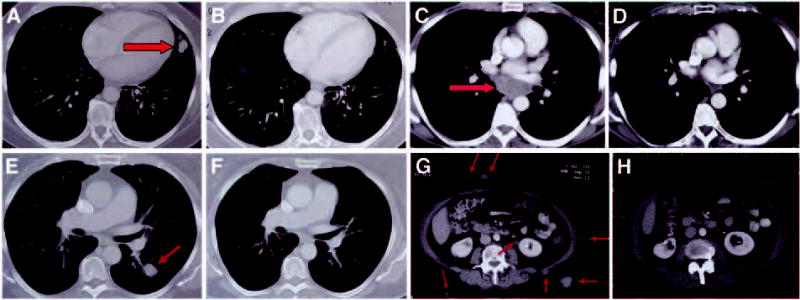
Computed axial tomography scans illustrating pretreatment disease status (left) and post-treatment (right) for patient 15 [(A) and (B) lung; (C) and (D) mediastinum)], and patient 19 [(E) and (F) lung; and abdomen (G) and (H)]. Arrows on pretreatment scans denote sites of disease.
The response rate in the group of patients receiving 3 mg/kg anti–CTLA-4 for each dose was 14% (four of 29), which did not differ statistically from the response rate of 11% (three of 27) seen in the group of patients receiving 3 mg/kg as a first dose, followed by a 1 mg/kg maintenance dose (P = .54, Fisher’s exact test). Table 1 also lists the prior therapies for each patient. There were no correlations found between any previous treatments and clinical response (data not shown). In particular, of the seven patients who experienced a clinical response to anti–CTLA-4, three had received prior immune-based therapy (patients 1, 13, and 24). Patients 1 and 13 had progressive disease before beginning anti–CTLA-4, and patient 24 had received an adjuvant Bacille Calmette-Guérin vaccine 25 years earlier. In addition, two of the seven responders had received prior chemotherapy (compared with 20 of 49 nonresponders). Previous exposure to this systemic treatment did not correlate with response (P = .692, two-tailed Fisher’s exact test). The times from previous chemotherapy administration to clinical response to anti–CTLA-4 in patients 13 and 15 were 18 and 7 months, respectively. In each of these instances, the patient had demonstrated progressive disease after their final administration of chemotherapy before initiating treatment with anti–CTLA-4.
Autoimmune Effects
Fourteen patients (25%) experienced grade 3/4 autoimmune toxicity (Table 1), which included colitis (n = 7), dermatitis (n = 4), uveitis (n = 1), enterocolitis (n = 1), hepatitis (n = 1), and hypophysitis (n = 1). Overall, the rate of autoimmunity in cohort 1 was 31% (nine of 29), compared with 19% (five of 27) in cohort 2 (P = .22, Fisher’s exact test). Of the 14 patients who suffered grade 3/4 autoimmune toxicity, five (36%) showed evidence of tumor regression, whereas of the 43 patients who did not suffer grade 3/4 autoimmune toxicity, only two (5%) responded. This difference was statistically significant (P = .008, Fisher’s exact test; Table 2). In addition, five patients experienced grade 1/2 autoimmune toxicity, including vitiligo (n = 2), positive antinuclear antibodies (n = 2), and pulmonary infiltrates (n = 1; reported previously24). Including these five patients with grade 1/2 autoimmune toxicity into the analysis results in 29% (five of 17) of responding patients experiencing autoimmunity and 5% (two of 39) of responding patients not experiencing autoimmunity (P = .022, Fisher’s exact test).
Table 2.
Relationship Between Autoimmune Toxicity and Clinical Response
| Cohort | Grade III/IV Toxicity in Responders | Grade III/IV Toxicity in Nonresponders | No Grade III/IV Toxicity in Responders | No Grade III/IV Toxicity in Nonresponders | P * |
|---|---|---|---|---|---|
| 3 → 3 | 3 of 9 | 6 of 9 | 1 of 20 | 19 of 20 | .076 |
| 3 → 1 | 2 of 5 | 3 of 5 | 1 of 22 | 21 of 22 | .079 |
| Total | 5 of 14 | 9 of 14 | 2 of 42 | 40 of 42 | .008 |
Fisher’s exact test comparing responders with toxicity to responders without toxicity (within each cohort).
GI effects
Eight patients (patients 1, 9, 15, 17, 41, 47, 48, and 49) experienced grade 3/4 autoimmune enterocolitis. All eight patients presented with significant diarrhea; however, the timing of their presentations varied from days to weeks after their previous dose of anti–CTLA-4. The diarrhea required hospitalization and intravenous hydration. Oral intake was reduced or stopped until symptoms cleared. Colonoscopies were performed and findings ranged from multiple areas of inflammation and mucosal ulceration to pan-colitis. Patient 1 also had upper endoscopy revealing duodenal ulcerations, as well. Biopsies were taken during the colonoscopies and revealed a spectrum of histopathologic changes. Some biopsies showed lymphocytes and neutrophils infiltrating into the lamina propria, sometimes with crypt abscesses (patient 15; Fig 2A). Others revealed a lymphocytic-like colitis with significant increase in intraepithelial lymphocytes, positive status for CD3 and CD8 T-cell markers (patient 47; Fig 2B) in addition to CD3+CD4+ lymphocytes and eosinophils (not shown). After pathologic confirmation of autoimmune enterocolitis was obtained, patients were treated with intravenous and ultimately oral corticosteroids, often dexamethasone, at doses up to 4 mg every 6 to 8 hours, tapered over several weeks. Two patients (9 and 41) required multiple hospitalizations, both with and without corticosteroid therapy, before symptom resolution. All patients returned to baseline without additional intervention.
Fig 2.
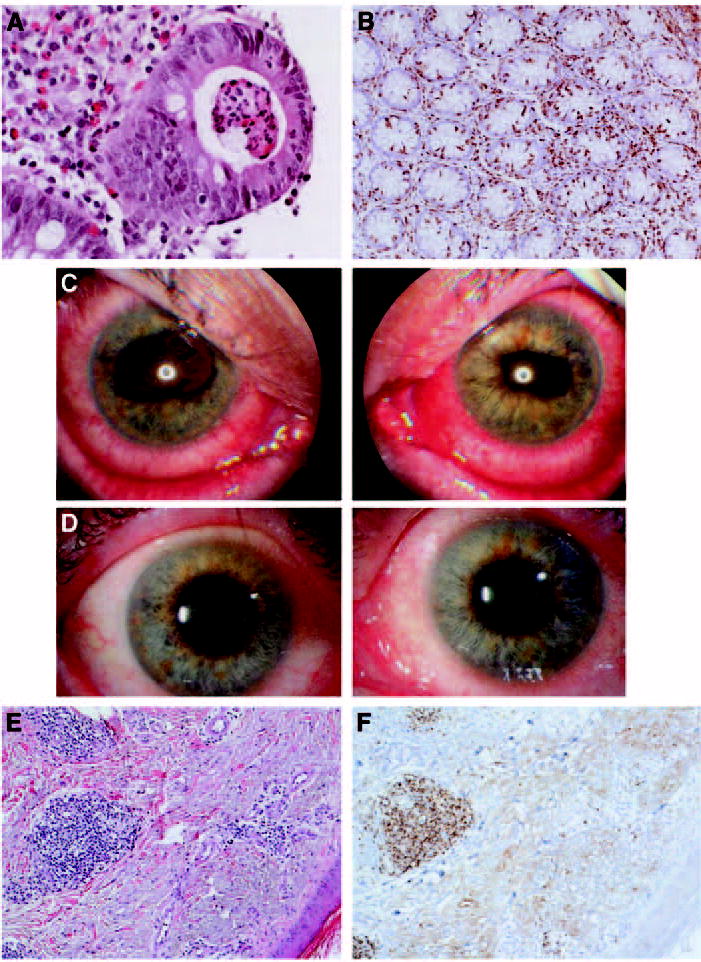
Selected histopathologic analyses from patients experiencing grade 3/4 autoimmune events. (A) Colon biopsy (patient 15) showing mononuclear cells and neutrophils filling the lamina propria and extending into the crypts with crypt abscesses. (B) Immunohistochemistry of colon biopsy (patient 47) revealing significant infiltrate of CD3+CD8+ intraepithelial lymphocytes into the lamina propria. (C) Ophthalmologic photographs (patient 36) of both eyes at the time of presentation with uveitis with posterior synechiae (iris adhesions to the lens) causing irregular pupils. (D) The same patient, 4 days later, after treatment with topical corticosteroids. (E) Punch biopsy of papule (patient 19) showing superficial perivascular lymphocytic infiltrate, which immunohistochemistry revealed to be CD3+CD8+ cells (F) (Magnifications: (A) × 400, (B) ×100, (E) ×100, (F) ×100).
Ocular effects
Patient 36, 1 week after being discharged from the hospital with autoimmune colitis, presented with decreased visual acuity, photophobia, and painful tearing from both eyes. Ophthalmologic examination confirmed the diagnosis of autoimmune uveitis (Fig 2C). The patient was administered periocular corticosteroid injections and corticosteroid eye drops. Within 4 days, his visual acuity had returned to normal and he was free of symptoms (Fig 2D).28
Patient 41, in the midst of being evaluated for severe diarrhea, complained of blurry vision and irritated, watery eyes. Ophthalmologic examination revealed episcleritis, and treatment with prednisolone acetate 1% eye drops was initiated. Because of the severity of what was diagnosed as autoimmune colitis, he was administered intravenous dexamethasone, which was switched to oral prednisone after 1 week, with a tapered schedule for discharge. Both the colitis and episcleritis resolved during the next 3 weeks.
Dermatologic effects
Four patients (1, 2, 13, and 19) experienced grade 3/4 autoimmune dermatitis. The patients all presented with significant generalized pruritis and maculopapular rash, often within 1 week of their last dose of anti–CTLA-4. In patients with subcutaneous disease (13 and 19) this coincided with the regression of their subcutaneous disease sites. Biopsies of their macular lesions demonstrated severe dermatitis with papillary dermal edema, at times with perivascular lymphocytic infiltrate (patient 19; Fig 2E), which immunohistochemistry demonstrated to be CD3+CD8+ (patient 19; Fig 2F) and CD3+CD4+ (not shown). Symptomatic relief for these lesions was provided by hydroxyzine or diphenhydramine and all patients were free of symptoms within weeks, although patient 2 developed vitiligo on both upper extremities.
Hepatic effects
On routine screening blood chemistry, patient 12 was noted to have increasing hepatic enzymes after his third treatment. Liver biopsy was performed and revealed acute hepatitis with a predominantly lymphocytic infiltrate in a lobular pattern of inflammation. Immunohistochemistry demonstrated predominantly CD4+ cells in the periportal regions and CD8+ cells in hepatic lobules. During the next 2 weeks, the AST level peaked at 1,193 U/L (normal, 9 to 34 U/L) and the ALT level peaked at 2,860 U/L (normal, 6 to 41 U/L). Oral prednisone was initiated and during the ensuing 4 months, his liver function tests returned to baseline without adverse event. Patient 52, while being treated with a quinolone antibiotic for a presumed infection, developed a rash and an abrupt elevation of her hepatic enzymes. The AST level peaked at 510 U/L (normal, 9 to 34 U/L) and the ALT level peaked at 900 U/L (normal, 6 to 41 U/L). The reaction was attributed to the antibiotic, which was discontinued and a hepatic biopsy was not performed. Without intervention her hepatic enzymes normalized during the next month.
Pituitary effects
Patient 11, after his fourth treatment cycle, began to develop progressive personality changes and memory loss. Brain MRI demonstrated disappearance of a left temporal intracranial metastasis, with no other noted abnormality. However, laboratory testing revealed undetectable levels of thyroid-stimulating hormone, free T4, adrenocorticotropic hormone, cortisol, growth hormone, prolactin, and testosterone, findings consistent with pan-hypopituitarism. Frozen samples of pretreatment sera were analyzed for the same hormones and revealed a completely normal pituitary profile before anti–CTLA-4 administration. Because of this patient’s marked clinical response, high-dose corticosteroids were withheld; however, replacement doses of deficient hormones were administered. Over the following month his personality and memory problems abated and 2 years later he remains cancer free. He is currently taking replacement doses of cortisol, thyroid hormone, and testosterone; however, his prolactin level has returned to normal.
Pharmacokinetics
In our previous report24 of patients treated with 3 mg/kg MDX-010, we observed a mean peak serum concentration after the first dose of 72 ± 33 μg/mL, with a trough before the second dose of 12 ± 7 μg/mL. With repeated dosing, the mean peak plasma level was 99 ± 41 μg/mL, which decreased to 17 ± 10 μg/mL before the subsequent dose. In this study we performed a similar analysis for the cohort of patients treated with 3 mg/kg MDX-010 initially, followed by 1 mg/kg. The mean peak following the first dose (3 mg/kg) was 81 ± 14 μg/mL and the trough 3 weeks later was 15 ± 5 μg/mL. The mean peak for the subsequent (1 mg/kg) doses was 43 ± 10 μg/kg with a trough of 7 ± 3 μg/mL (Table 3).
Table 3.
Pharmacokinetic Differences Between Two Dose Schedules
| First Peak (μg/mL)* |
First Trough (μg/mL) † |
Subsequent Peaks (μg/mL)* |
Subsequent Troughs (μg/mL) † |
|||||
|---|---|---|---|---|---|---|---|---|
| Cohort | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| 3 → 3 mg/kg‡ | 72 | 33 | 12 | 7 | 99 | 41 | 17 | 10 |
| 3 → 1 mg/kg§ | 81 | 14 | 15 | 5 | 43 | 10 | 7 | 3 |
Abbreviation: SD, standard deviation.
Peaks drawn 2.5 hours after completion of infusion.
Troughs drawn immediately before dose (3 weeks after previous dose).
Cohort 1.
Cohort 2.
Immunologic Response
Previously,24 tetramer and enzyme-linked immuno-spot (ELISPOT) analysis did not detect any differences between pre- and post-treatment samples. The frequency of tetramer-reactive CD8+ cells in all samples tested was less than 1%. The ex vivo ELISA after an 11 day coculture detected specific T-cell response. These data are shown in Tables 4 and 5. For the first cohort of 14 patients receiving 3 mg/kg of anti–CTLA-4 Ab, it was shown using IVS (Table 4) that all 11 patients from whom samples were obtainable developed specific immunity against the native gp100:209-217 peptide. In that same cohort, six patients (55%) developed specific immunity against the native gp100:280-288 peptide. In this study, similar pre- and post-treatment samples were available in 12 patients in the 3 mg/kg followed by 1 mg/kg group (cohort 2). Among these patients, seven of 12 (58%) achieved specific immunity against the native gp100:209-217 peptide, whereas three of 12 (25%) did so against the native gp100:280-288 peptide (Table 5). These results are similar to those obtained in patients previously vaccinated with peptide immunization alone.25,26,29 It thus appears that administration of anti–CTLA-4 Ab did not influence the immunization with peptides in either cohort. Furthermore, the magnitude of T-cell response did not correlate with clinical response (data not shown).
Table 4.
Example of Reactivity Developed by Patient 121 After One Course of Vaccination and Anti-CTLA-4
| Stimulator (pg/mL IFN-γ)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Peptide* 1μmol/L | v T2 | v T2(280) 1 μmol/L | v T2(209) 1 μmol/L | v T2(flu) 1μmol/L | v 526 (A2,3) | v 624 (A2,3) | v 888 (A1,24) | v 938 (A1,24) | |
| 121 | Pretreatment | 280 | 354 | 284 | 234 | 279 | 44 | 51 | 43 | 72 |
| 209 | 158 | 128 | 173 | 170 | 40 | 39 | 32 | 45 | ||
| flu | 2,708 | 3,152 | 1,380 | 9,519 | 694 | 823 | 754 | 809 | ||
| 121 | After 1 course | 280 | 1,968 | 6,501 | 1,604 | 1,500 | 319 | 323 | 269 | 365 |
| 209 | 748 | 607 | 20,520 | 571 | 2,377 | 810 | 169 | 168 | ||
| flu | 3,087 | 3,000 | 2,249 | 23,650 | 683 | 764 | 826 | 783 | ||
NOTE. Values are in bold if they are at least twice the control value and greater than 100 pg/mL IFN-γ. Abbreviations: CTLA-4, cytotoxic T-lymphocyte antigen-4; IFN-γ, interferon gamma; flu, influenza peptide. Tumor lines 526 and 624 are known to express A2, whereas lines 888 and 938 do not.
Day 11 after coculture with 1 mmol/L peptide; tested for IFN-γ release after coculture with T2 cells pulsed with peptide.
Table 5.
Resulting Immunity in Each Cohort After Peptide Vaccination
| gp100 Peptide Immunity* |
gp100 Peptide Immunity* |
||||
|---|---|---|---|---|---|
| Cohort | Evaluated | No. | % | No. | % |
| 3 → 3 mg/kg† | 11 | 11 of 11 | 100 | 6 of 11 | 55 |
| 3 → 1 mg/kg‡ | 12 | 7 of 12 | 58 | 3 of 12 | 25 |
| P = .037† | P = .214§ | ||||
Did not differ significantly from previous data of patients immunized with respective peptides alone.
Cohort 1.
Cohort 2.
P value for Fisher’s exact test comparing 3 → 3 to 3 → 1 for a given peptide.
Phenotypic Changes
Using flow cytometry, pre- and post-treatment peripheral blood lymphocyte samples were examined for changes in surface expression of various makers (Table 6). In our previous report of 14 patients receiving an initial dose of 3 mg/kg anti–CTLA-4 Ab with equal maintenance doses, increases in expression of HLA-DR (a marker of T-cell activation) were observed in both CD4+ and CD4− (CD8+) lymphocytes.24 In addition, an increase in the expression of CD45RO (a memory cell marker) was noted in CD4+ lymphocytes. In the present study, we performed a similar analysis on 16 patients in the second cohort (3 mg/kg initial dose, followed by 1 mg/kg anti–CTLA-4 Ab maintenance dose). Again, there was a significant increase in the expression of HLA-DR on the surface of post-treatment CD4+ (P < .0001, paired t test) and CD4− (CD8+) cells (P = .015), and an increase in the expression of CD45RO on the surface of CD4+ cells (P = .009), though not among the CD4− (CD8+) cells. In addition, there was a significant decrease in expression of CD25 (IL-2 receptor α, also a marker of suppressor T cells) on CD4+ cells (P = .047), although not a significant decrease in overall CD4CD25 expression among all CD3+ cells. There was no correlation found between clinical response and surface markers. Of the 25 patients evaluated by a fluorescence-activated cell sorter (16 in this report and 11 in our previous report24), five had an increase in CD4CD25 expression (none of these patients were responders). Five of the seven responders were included in this analysis. Although all five of the responders had a decrease in CD4CD25 expression, 15 of the 20 nonresponders also had a decrease (P = .292, two-tailed Fisher’s exact test).
Table 6.
Flow Cytometric Analysis of Selected T-Cell Surface Markers (post-treatment–pretreatment) After Two Treatment Cycles for Selected Patients in Cohort 2 (3 → 1 mg/kg)
| Patient | HLA-DR+ (%CD3+CD4+) | HLA-DR+ (%CD3+CD4−) | CD45RO+ (%CD3+CD4+) | CD45RO+ (%CD3+CD4−) | CD4+ CD25+ (%CD3+) | CD25+ (%CD3+CD4+) |
|---|---|---|---|---|---|---|
| 15 | 5.8 | −1.7 | −1.4 | −6.1 | −2.4 | 5.9 |
| 17 | 16.3 | −0.8 | 20.8 | 9.8 | −18.3 | 28.5 |
| 19 | 6.0 | 2.0 | 6.6 | 5.6 | −1.8 | −1.2 |
| 20 | 9.2 | 4.0 | 4.1 | −0.1 | −5.7 | −9.1 |
| 21 | 6.6 | 11.5 | 3.0 | 5.7 | −2.9 | −5.1 |
| 23 | 4.7 | 1.5 | 0.5 | 5.7 | −0.5 | −1.3 |
| 24 | 9.9 | −1.9 | 12.6 | −2.9 | −4.1 | −4.9 |
| 25 | 11.0 | 5.6 | 18.9 | −6.4 | −7.9 | −9.8 |
| 27 | 17.9 | 2.5 | 9.2 | 2.0 | 0.5 | −1.3 |
| 31 | 3.9 | 4.9 | 1.5 | −0.4 | −2.9 | −8.9 |
| 32 | 15.9 | 1.6 | 20.1 | 3.1 | 3.5 | 4.4 |
| 33 | 23.7 | −1.0 | −2.1 | −3.1 | −8.4 | −14.3 |
| 34 | 2.2 | 0.4 | 8.1 | 0.2 | −0.1 | −0.3 |
| 36 | 3.0 | 3.2 | 2.2 | 6.6 | 7.2 | 8.5 |
| 39 | 13.0 | 1.0 | 51.6 | 27.4 | −1.6 | −0.3 |
| 40 | 7.1 | 4.2 | 3.6 | −5.2 | 3.4 | 3.0 |
| Mean change | +9.8 | +2.3 | +10.0 | +2.6 | −2.6 | −4.7 |
| P * | .00001 | .015 | .009 | .222 | .092 | .047 |
Two-tailed P value using paired t-test.
DISCUSSION
Current approaches to cancer immunotherapy such as vaccine administration, adoptive cell therapies, and cytokine administration are aimed at stimulating T cells to recognize cancer antigens and develop effector mechanisms that can destroy cancer cells. Given that many cancer antigens are derived from nonmutated proteins,30 it may be necessary to break tolerance of T cells to self-antigens in an effort to unleash their antitumor properties. Recent studies have indicated that CTLA-4, a molecule expressed on the surface of T cells, plays an important role in maintaining peripheral tolerance to these self-antigens.31–33
CTLA-4 binds the costimulatory ligands B7-1 and B7-2 on APCs with greater affinity than the costimulatory molecule CD28, and has an inhibitory effect on T-cell activation. The net immune response results from the integration of signals generated by the total T-cell/APC interaction. In mouse models, it has been shown that blockade of CTLA-4 can enhance antitumor immune actions, but can also lead to lymphoproliferative disease and autoimmunity in visceral organs.34–36 Blockade of CTLA-4 in selected animal models has been shown to induce autoimmune encephalitis, pancreatitis, thyroiditis, collagen-induced arthritis, and systemic lupus erythematosus.37,38 CTLA-4 knockout mice uniformly develop fatal systemic autoimmunity resulting from unopposed T-cell activation and reaction to self-antigens.39–41
In our previous report,24 14 patients with metastatic melanoma were treated with a human antibody to CTLA-4 in conjunction with peptide vaccination. Three patients experienced objective clinical regression of their tumors and six patients experienced grade 3/4 autoimmune toxicity, including the three responders. These results suggested a possible correlation between tumor regression and autoimmunity. On the basis of our previous clinical trials, in which peptide vaccines alone did not cause objective tumor regression or autoimmunity,25,26,29 it appeared that CTLA-4 blockade played a significant role in both the tumor regression and autoimmunity.
In the current study, we have treated an additional 15 patients with the 3 mg/kg dose regimen of anti–CTLA-4 originally used, and have treated a second cohort of 27 patients to evaluate whether decreasing the maintenance dose from 3 to 1 mg/kg would reduce the incidence of significant autoimmune toxicity, and if so, would this dose reduction influence the regression of established tumors? Similar results were obtained in the two patient cohorts. Four of 29 (14%) patients in the 3 mg/kg cohort experienced an objective regression compared with three of 27 (11%) of patients in the reduced-dose cohort. Grade 3/4 autoimmune toxicities were seen in nine of 29 (31%) and five of 27 (19%) patients in the high- and low-dose cohorts, respectively.
There was a strong correlation between the induction of tumor regression and grade 3/4 autoimmune toxicity. Histopathologic analysis of the sites involved in the toxicities of treatment often showed inflammatory changes that were difficult to distinguish from autoimmune changes. In many cases, such as pituitary gland involvement, biopsy was not possible. Of 14 patients who experienced autoimmune toxicity, five (36%) experienced objective cancer regression, compared with only two responders in 42 patients (5%) who had no autoimmunity (P = .008; Table 2). Most of the non–cancer-bearing organs targeted by T cells (colon, duodenum, liver, eye, and pituitary) do not express the gp100 antigen, suggesting that the autoimmunity was not a result of the peptide vaccination, but rather the result of broken self-tolerance induced by CTLA-4 blockade. A recent publication by Sanderson et al42 suggested a link between autoimmunity and disease recurrence in a series of 19 patients who were treated in the adjuvant setting with anti–CTLA-4 and peptide vaccination. They suggested an increased incidence of disease progression and a decreased incidence of autoimmunity in patients with the AA allele (high CTLA-4 surface expression), compared with those with the AG or GG allele. We are currently investigating the relationship between polymorphisms in the CTLA-4 gene and response to CTLA-4 blockade in our patients with metastatic melanoma.
To further assess the role of vaccination and address the question of whether anti–CTLA-4 can be used to augment vaccination, we currently are enrolling HLA-A*0201 patients in a prospective trial with randomization to receive either anti–CTLA-4 alone or with the HLA-A*0201-restricted peptides used in this trial. In addition, this trial is using escalating doses of anti–CTLA-4 (up to 9 mg/kg) to further delineate the optimal dose for patients with metastatic melanoma.
Regressions of established tumors were seen at multiple organs, including lung, mediastinum, brain, gallbladder, retroperitoneum, spleen, liver, adrenal gland, and subcutaneous sites. The majority of objective cancer regressions in these patients have been durable. In cohort 1, three of four objective responses are ongoing for more than 2 years. In cohort 2, two of the three responses are ongoing at 25 and 26 months.
The management of the autoimmune toxicities required strict attention to patient complaints and prompt diagnosis because the toxicities were potentially lethal if not treated vigorously. Autoimmune toxicity occurred after the first or second treatment cycle in six of the 13 patients, suggesting that patients are susceptible from the time they receive their first dose. With aggressive medical management, often involving high doses of corticosteroids, all of the autoimmune toxicities could be controlled or reversed. It is both surprising and important to note that abrogating the autoimmune adverse effects of anti–CTLA-4 with corticosteroids did not seem to interfere with antitumor activity. The two patients with objective cancer regressions (patients 1 and 15) who received high-dose corticosteroids for the treatment of autoimmune colitis continue to have ongoing responses at 34 and 26 months, respectively.
This study emphasizes the similarity of the peripheral tolerance mechanisms that limit immune responses to cancer antigens and the natural tolerance that exists to normal self-antigens. It also suggests the possible involvement of CTLA-4 interactions in the etiology of several human autoimmune diseases. Because of the strong correlation of autoimmunity with objective cancer regression, we have recently begun a clinical trial in which patients are receiving escalating doses of anti–CTLA-4 antibody to determine whether increased autoimmunity, and consequently increased antitumor responses, will occur.
Authors’ Disclosures of Potential Conflicts of Interest
Although all authors completed the disclosure declaration, the following authors or their immediate family members indicated a financial interest. No conflict exists for drugs or devices used in a study if they are not being evaluated as part of the investigation. For a detailed description of the disclosure categories, or for more information about ASCO’s conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
| Authors | Employment | Leadership | Consultant | Stock | Honoraria | Research Funds | Testimony | Other |
|---|---|---|---|---|---|---|---|---|
| Geoff Nichol | Medarex Inc | Medarex Inc (C) | Medarex Inc (C) | |||||
| Michael J. Yellin | Medarex Inc | Medarex Inc (C) | ||||||
| Dollar Amount Codes (A) 3 $10,000 (B) $10,000-99,999 (C) ≥ $100,000 (N/R) Not Required | ||||||||
Footnotes
Authors’ disclosures of potential conflicts of interest are found at the end of this article.
References
- 1.Bretscher P, Cohn M. A theory of self-nonself discrimination. Science. 1970;169:1042–1049. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- 2.Jenkins MK, Ashwell JD, Schwartz RH. Allogeneic non-T spleen cells restore the responsiveness of normal T cell clones stimulated with antigen and chemically modified antigen-presenting cells. J Immunol. 1988;140:3324–3330. [PubMed] [Google Scholar]
- 3.Schwartz RH. A cell culture model for T lymphocyte clonal anergy. Science. 1990;248:1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- 4.Linsley PS, Brady W, Grosmaire L, et al. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991;173:721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koulova L, Clark EA, Shu G, et al. The CD28 ligand B7/BB1 provides costimulatory signal for alloactivation of CD4+ T cells. J Exp Med. 1991;173:759–761. doi: 10.1084/jem.173.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linsley PS, Brady W, Urnes M, et al. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174:561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunet JF, Denizot F, Luciani MF, et al. A new member of the immunoglobulin superfamily—CTLA-4. Nature. 1987;328:267–270. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 8.Gross JA, Callas E, Allison JP. Identification and distribution of the costimulatory receptor CD28 in the mouse. J Immunol. 1992;149:380–388. [PubMed] [Google Scholar]
- 9.Alegre ML, Frauwirth KA, Thompson CB. T-cell regulation by CD28 and CTLA-4. Nat Rev Immunol. 2002;1:220–228. doi: 10.1038/35105024. [DOI] [PubMed] [Google Scholar]
- 10.Linsley PS, Greene JL, Brady W, et al. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. 1994;1:793–801. doi: 10.1016/s1074-7613(94)80021-9. [DOI] [PubMed] [Google Scholar]
- 11.Walunas TL, Bakker CY, Bluestone JA, et al. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med. 1996;183:2541–2550. doi: 10.1084/jem.183.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med. 1996;183:2533–2540. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunner MC, Chambers CA, Chan FK, et al. CTLA-4-mediated inhibition of early events of T cell proliferation. J Immunol. 1999;162:5813–5820. [PubMed] [Google Scholar]
- 14.Greenwald RJ, Oosterwegel MA, van der Woude D, et al. CTLA-4 regulates cell cycle progression during a primary immune response. Eur J Immunol. 2002;32:366–373. doi: 10.1002/1521-4141(200202)32:2<366::AID-IMMU366>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 15.Lindsten T, Lee KP, Harris ES, et al. Characterization of CTLA-4 structure and expression on human T cells. J Immunol. 1993;151:3489–3499. [PubMed] [Google Scholar]
- 16.Walunas TL, Lenschow DJ, Bakker CY, et al. CTLA-4 can function as a negative regulator of cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 17.Shevach E. CD4+CD25+ suppressor T cells: More questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 18.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor, foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 19.Khattri R, Cox T, Yasayko SA, et al. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 20.Egen JG, Kuhns MS, Allison JP. CTLA-4: New insights into its biological function and use in tumor immunotherapy. Nat Immunol. 2002;3:611–618. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- 21.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 22.Hurwitz AA, Yu TF, Leach DR, Allison JP. CTLA-4 blockade synergizes with tumor-derived granulocyte-macrophage colony-stimulating factor for treatment of an experimental mammary carcinoma. Proc Natl Acad Sci U S A. 1998;95:10067–10071. doi: 10.1073/pnas.95.17.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phan GQ, Touloukian CE, Yang JC, et al. Immunization of patients with metastatic melanoma using both class I- and class II-restricted peptides from melanoma-associated antigens. J Immunother. 2003;26:349–356. doi: 10.1097/00002371-200307000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 28.Robinson MR, Chan CC, Yang JC, et al. Cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma: A new cause of uveitis. J Immunother. 2004;27:478–479. doi: 10.1097/00002371-200411000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Pass HA, Schwarz SL, Wunderlich JR, et al. Immunization of patients with melanoma peptide vaccines: Immunologic assessment using the ELISPOT assay. Cancer J Sci Am. 1998;4:316–323. [PubMed] [Google Scholar]
- 30.Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3:666–675. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vasu C, Prabhakar BS, Holterman MJ. Targeted CTLA-4 engagement induces CD4+ CD25+CTLA-4high T regulatory cells with target (allo)antigen specificity. J Immunol. 2004;173:2866–2876. doi: 10.4049/jimmunol.173.4.2866. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor PA, Noelle RJ, Blazar BR. CD4(+) CD25(+) immune regulatory cells are required for induction of tolerance to alloantigen via co-stimulatory blockade. J Exp Med. 2001;193:1311–1318. doi: 10.1084/jem.193.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in CTLA-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 35.Chambers CA, Sullivan TJ, Allison JP. Lymphoproliferation in CTLA-4-deficient mice is mediated by costimulation-dependent activation of CD4+ T cells. Immunity. 1997;7:885–895. doi: 10.1016/s1074-7613(00)80406-9. [DOI] [PubMed] [Google Scholar]
- 36.Tivol EA, Gorski J. Re-establishing peripheral tolerance in the absence of CTLA-4: Complementation by wild-type T cells points to an indirect role for CTLA-4. J Immunol. 2002;169:1852–1858. doi: 10.4049/jimmunol.169.4.1852. [DOI] [PubMed] [Google Scholar]
- 37.Uchikoshi F, Yang ZD, Rostami S, et al. Prevention of autoimmune recurrence and rejection by adenovirus-mediated CTLA4Ig gene transfer to the pancreatic graft in BB rat. Diabetes. 1999;48:652–657. doi: 10.2337/diabetes.48.3.652. [DOI] [PubMed] [Google Scholar]
- 38.Kristiansen OP, Larsen ZM, Pociot F. CTLA-4 in autoimmune diseases: A general susceptibility gene to autoimmunity? Genes Immun. 2000;1:170–184. doi: 10.1038/sj.gene.6363655. [DOI] [PubMed] [Google Scholar]
- 39.Ariyan C, Salvalaggio P, Fecteau S, et al. Cutting edge: Transplantation tolerance through enhanced CTLA-4 expression. J Immunol. 2003;171:5673–5677. doi: 10.4049/jimmunol.171.11.5673. [DOI] [PubMed] [Google Scholar]
- 40.Greenwald RJ, Latchman YE, Sharpe AH. Negative co-receptors on lymphocytes. Curr Opin Immunol. 2002;14:391–396. doi: 10.1016/s0952-7915(02)00341-2. [DOI] [PubMed] [Google Scholar]
- 41.Chambers CA, Kuhns MS, Egen JG, et al. CTLA-4-mediated inhibition in regulation of T cell responses: Mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol. 2001;19:565–594. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- 42.Sanderson K, Scotland R, Lee R, et al. Autoimmunity in a phase I Trial of a fully human anti-cytotoxic T-lymphocyte antigen-4 monoclonal antibody with multiple melanoma peptides and montanide ISA 51 for patients with resected stage III and IV melanoma. J Clin Oncol. 2005;23:741–750. doi: 10.1200/JCO.2005.01.128. [DOI] [PubMed] [Google Scholar]


