Abstract
Background
Cytotoxic T lymphocyte–associated antigen (CTLA)-4 can inhibit T-cell responses and is involved in tolerance against self antigens. We previously reported autoimmune manifestations and objective cancer regressions in patients with metastatic melanoma treated with CTLA-4 blockade. The possibility of activating tumor-reactive T cells while removing inhibitory activity with CTLA-4 blockade has stimulated interest in using anti–CTLA-4 antibodies in combination with other cancer immunotherapies to improve clinical outcomes. In this study, we assessed the antitumor activity and autoimmune toxicity of CTLA-4 blockade in combination with an immune-activating stimulus, interleukin (IL)-2, in patients with metastatic melanoma.
Methods
Thirty-six patients received anti–CTLA-4 antibody every 3 weeks. Three patients per cohort received doses of .1, .3, 1.0, and 2.0 mg/kg. Twenty-four patients received 3.0 mg/kg. All patients received IL-2 therapy (720,000 IU/kg every 8 hours to a maximum of 15 doses).
Results
Eight patients (22%) experienced objective tumor responses (three complete and five partial), including metastases in the lungs, lymph nodes, mediastinum, and subcutaneous tissues. Six of the eight patients have ongoing objective responses at 11 to 19 months. Five patients (14%) developed grade III/IV autoimmune toxicities secondary to anti–CTLA-4 administration, including four patients with enterocolitis and one with arthritis and uveitis.
Conclusions
There is not evidence to support a synergistic effect of CTLA-4 blockade plus IL-2 administration, because the 22% objective response rate is that expected from the sum of these two agents administered alone. Durable cancer regressions were seen in patients treated with this combination.
Keywords: Cytotoxic T lymphocyte-associated antigen 4, Interleukin 2, Melanoma, Autoimmunity
Understanding the regulation of T-cell activation has resulted in new strategies for cancer immunotherapy. In addition to major histocompatibility complex/peptide interaction with the T-cell receptor, further costimulatory signals are needed for induction of interleukin (IL)-2 production, proliferation, and differentiation of T cells from a naive to an effector state. An important second signal is provided through the interaction of T-cell surface CD28 with B7 on an antigen-presenting cell.1 In the absence of CD28/B7 interaction, clonal anergy may result.2
The complexity of this relationship has been further elucidated by the role of cytotoxic T lymphocyte–associated antigen (CTLA)-4, a close homologue of CD28 that binds to members of the B7 family with a higher affinity than does CD28.3 Whereas CD28 is constitutively expressed on the surface of naive T cells,4 CTLA-4 is expressed on the surface of most T cells only after activation.5 On binding to B7–1 or B7–2, CTLA-4 antagonizes T-cell activation, inhibits IL-2 production and receptor expression, and interferes with cell-cycle regulation.6–8 CTLA-4 downregulates T-cell responses9 and may play a role in tolerance against self tumor antigens.10 Therefore, manipulation of CTLA-4 interactions may enhance current cancer immunotherapies that involve T-cell recognition of established tumors.
CTLA-4 blockade in murine cancer models increased regression of immunogenic tumors11 and was associated with depigmentation, thus implying a role for CTLA-4 not only in tumor antigenicity, but also in suppression of autoimmunity.10 Furthermore, we have previously reported that the administration of anti–CTLA-4 antibody to metastatic melanoma patients resulted in objective cancer regression in 3 of 14 patients and autoimmune manifestations in 6 of 14 patients.12 This ability to block inhibitory signals to tumor-reactive T cells has stimulated interest in using anti–CTLA-4 antibodies in combination with other cancer therapies to improve clinical outcome.13
In our prior experience, 27 (14.8%) of 182 patients treated with IL-2 alone experienced objective cancer regressions.14 IL-2 stimulates T-cell growth but has also been implicated in the expansion of suppressive (regulatory) T cells that express cell-surface CTLA-4. We thus hypothesized that CTLA-4 blockade might enhance the antitumor effect of IL-2 administration. This study thus evaluated the treatment of patients with metastatic melanoma by using high-dose bolus IL-2 and escalating doses of anti–CTLA-4 antibody.
METHODS
Patients and Treatment
All patients were ≥16 years of age with a histological diagnosis of stage IV melanoma and at least one site of measurable disease. Patients had not received prior therapy with anti–CTLA-4 antibody or high-dose IL-2, defined as ≥600,000 IU/kg every 8 hours, and had not received systemic therapy for 3 weeks before this treatment. All patients had life expectancies >3 months and an Eastern Cooperative Oncology Group performance status of ≤2. Laboratory values required were as follows: white blood count ≤2500/mL, absolute neutrophil count ≥1500/mL, platelets ≥100,000/mL, hemoglobin ≥10 g/dL, creatinine ≤2.0 mg/dL, serum glutamic oxaloacetic transaminase ≤3 times the upper limit of normal, and a bilirubin level ≤1.0 times the upper limit of normal. Patients were excluded if they had any other prior invasive malignancy, autoimmune disease, or active infection; were pregnant or breast-feeding; had any medical condition requiring systemic or topical corticosteroids; or had any comorbidity that contraindicated the administration of IL-2. All patients signed an informed consent and were treated in an institutional review board–approved protocol in the Surgery Branch, National Cancer Institute.
Patients underwent apheresis before treatment and after every course. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque separation and cryopreserved at −180°C in heat-inactivated human AB serum with 10% dimethyl sulfoxide.
All patients received anti–CTLA-4 antibody (MDX-010; Medarex, Inc., Bloomsbury, NJ) as an intravenous bolus over 90 minutes and received IL-2 therapy as previously described (720,000 IU/kg every 8 hours to a maximum number of tolerated doses up to 15 doses). In the phase I portion of the study, three patients were treated at each anti–CTLA-4 dose level, up to a maximum dose of 2.0 mg/kg. Dose levels were .1, .3, 1.0, and 2.0 mg/kg. Subsequently, 24 patients received 3.0 mg/kg of anti–CTLA-4 antibody. Patients started treatment with an initial loading dose of anti–CTLA-4 antibody alone. Three weeks later, they received another dose of antibody, followed within 24 hours with IL-2 administration. On the basis of our previous experience, patients responding to IL-2 usually experienced tumor regression within two cycles of treatment; therefore, administration of antibody and IL-2 was repeated 3 weeks later. Thus, a treatment course consisted of three doses of antibody and two cycles of IL-2. Patients with stable disease or regressing lesions could receive up to three courses of treatment, or nine doses of MDX-010. Patients discontinued treatment for disease progression or severe toxicity attributable to anti–CTLA-4 therapy, defined as a grade III/IV infusion-related reaction, grade III/IV autoimmune toxicity, any significant ocular symptoms, or any grade III/IV toxicity not attributable to IL-2 therapy or disease progression.
Autoimmunity Screening and Clinical Response Evaluation
At the initial patient screening and after every treatment course, patients underwent a physical examination; assessment of performance status; ophthalmological examination; anti–nuclear antibody screening; anti-idiotype antibody testing; hematologic, biochemical, and thyroid function tests; and diagnostic imaging appropriate to tumor evaluation by using the same imaging modality, method of assessment, or technique used for baseline evaluation.
For each patient, response evaluation criteria in solid tumors (RECIST) were used to determine the radiographical response to treatment. The sum of the longest diameters of all tumors before and after therapy was calculated. A partial response was defined as a decrease of ≥30% (but not 100%) of the sum of the longest diameters of index lesions lasting at least 1 month, with no growth of lesions or appearance of new lesions. A complete response was a disappearance of all lesions for ≥1 month. Patients who did not reach either a partial or complete response were considered nonresponders.
Flow Cytometry
PBMCs were Fc receptor–blocked with mouse immunoglobulin G (Caltag, Burlingame, CA), stained with fluorochrome-labeled antibodies against the selected T-cell markers CD3, CD4, CD8, CD45RO, CD69, and HLA-DR (BD Biosciences, San Diego, CA), and analyzed with a FACSCalibur with CellQuest software (BD Biosciences).
Pharmacokinetics
Blood samples were drawn immediately before the first and second dose of anti–CTLA-4 antibody and at set time points during and after treatment. Quantitative enzyme-linked immunosorbent assay was used to determine plasma concentration, as previously reported, by using microtiter wells coated with recombinant human CTLA-4/Fc chimeric protein (R&D Systems, Minneapolis, MN). Anti–CTLA-4 was detected with an anti-human immunoglobulin G alkaline phosphatase probe.
RESULTS
Patient Characteristics
Thirty-six patients were treated. Three patients were treated in each of the .1, .3, 1.0, and 2.0 mg/kg dose groups, and 24 patients were treated at the maximum dose of 3.0 mg/kg (Table 1). Patients who completed a course at 3.0 mg/kg received an average of 11 doses of IL-2 (range, 7–19). Patients were heavily pretreated before this study; 22% had received chemotherapy, 19% had received radiotherapy, 64% had received immunotherapy, and 69% had received a combination of two or more treatment modalities. All patients had stage IV melanoma with excision of the primary lesion. Twenty-four (67%) had visceral metastases.
TABLE 1.
Patient characteristics, clinical response, and autoimmune toxicity
| Patient no. | Age (y) | Sex | Disease sites | Prior therapy | Dose (mg/kg) | No. cycles received | Response (mo)a | Autoimmune toxicity (grade III/IV) |
|---|---|---|---|---|---|---|---|---|
| 1 | 45 | M | Adrenal, ALN, kidney, lung hilum, mesenteric, RP, SQ, pleura | S | 1 | 3 | NR | |
| 2 | 61 | M | Brain, SQ | S, I | 1 | 3 | NR | |
| 3 | 42 | M | SQ | S, C, I | 1 | 3 | NR | |
| 4 | 43 | M | Mediastinum | S | 3 | 9 | PR (19+) | |
| 5 | 58 | F | Iliac LN | S, C, I | 3 | 3 | NR | |
| 6 | 54 | M | Lung, SQ, spleen | S, I | 3 | 6 | NR | |
| 7 | 31 | M | Liver, lung, mediastinum, mesentery, SQ | R, S, C, I | 1 | 3 | NR | |
| 8 | 51 | M | ALN, intramuscular | R, S, C | 1 | 6b | PR (7) | Colitis |
| 9 | 33 | M | ALN, intraperitoneal, liver, mediastinum, spleen | S | 1 | 3 | NR | |
| 10 | 49 | F | ALN, liver, SQ | S, I | 2 | 3 | NR | |
| 11 | 60 | F | SQ | S, I | 2 | 3 | NR | Gastritis, enterocolitis, colitis |
| 12 | 60 | M | Lung | S | 2 | 7 | CR (16+) | |
| 13 | 48 | M | Liver, lung | S | 3 | 3 | NR | |
| 14 | 44 | M | Subclavicular LN, submandibular | R, S, C, I | 3 | 9+ | PR (11) | |
| 15 | 47 | M | Abdominal wall, SQ | R, S | 3 | 9 | PR (14+) | |
| 16 | 42 | F | ALN, SQ | S, I | 3 | 3 | NR | |
| 17 | 45 | F | Lung | S, I | 3 | 3 | NR | |
| 18 | 50 | F | Adrenal, brain, lung RP, SQ | S, I | 3 | 3 | NR | |
| 19 | 50 | M | Inguinal LN, lung, mesentery, pulmonary hila, SQ | S, I | 3 | 3 | CR (13+) | Colitis |
| 20 | 67 | F | Inguinal LN, SQ | S, I | 3 | 2 | CR (13+) | |
| 21 | 45 | M | Liver, lung | S, I | 3 | 1 | NR | |
| 22 | 57 | M | Bone, liver, spleen | S, I | 3 | 2 | NR | |
| 23 | 39 | F | Lung | S, I | 3 | 5 | NR | |
| 24 | 28 | F | Iliac LN, liver, lung | S, C, I | 3 | 3 | NR | |
| 25 | 48 | M | Liver, parotid | S | 3 | 6 | NR | |
| 26 | 21 | M | Lung | S, I | 3 | 6 | NR | |
| 27 | 58 | M | ALN submandibular, skin, SQ | R, S, I | 3 | 3 | NR | |
| 28 | 51 | F | Lung, mediastinum, SQ | S | 3 | 9 | PR (11+) | |
| 29 | 40 | M | ALN, lung | S, I | 3 | 3 | NR | Colitis |
| 30 | 48 | F | Lung, SQ | S, I | 3 | 3 | NR | |
| 31 | 25 | M | Abdominopelvic, adrenal, ALN, heart, mediastinum, spleen, SQ | S | 3 | 3 | NR | |
| 32 | 49 | F | Iliac LN, inguinal LN, SQ | R, S, C, I | 3 | 3 | NR | Arthritis, uveitis |
| 33 | 56 | M | ALN, cervical LN, iliac LN, SQ, lung | R, S, C, I | 3 | 2 | NR | |
| 34 | 44 | M | Lung, lung hilum | S, I | 3 | 2 | NR | |
| 35 | 52 | F | Bone, cervical LN, SQ, liver, lung, spleen | S | 3 | 2 | NR | |
| 36 | 58 | F | Adrenal, liver, lung, spleen | S | 3 | 3 | NR |
ALN, axillary lymph node; RP, retroperitoneal; SQ, subcutaneous; S, surgery; NR, no response; I, immunotherapy; C, chemotherapy; PR, partial response; LN, lymph node; R, radiotherapy; CR, complete response.
Responses as of December 1, 2004; + indicates ongoing response.
Patient 8 had a 7 month PR after six doses at 1 mg/kg. He was later re-treated at 1 mg/kg with subsequent progression of disuse. Patient 14 had an 11-month PR after three doses at 3 mg/kg. He was later re-treated at 3 mg/kg without further response.
Clinical Responses and Durations
Eight patients (22%) experienced objective tumor regression (three complete and five partial), including metastases in the lungs, lymph nodes, mediastinum, and subcutaneous tissues (Table 1; Fig. 1). One of three patients in each of the .3, 1.0, and 2.0 mg/kg dose cohorts was an objective responder. Five (21%) of the 24 patients who received the 3.0 mg/kg dose achieved an objective response. Six (75%) of eight responding patients have ongoing objective responses at 11 to 19 months.
FIG. 1.
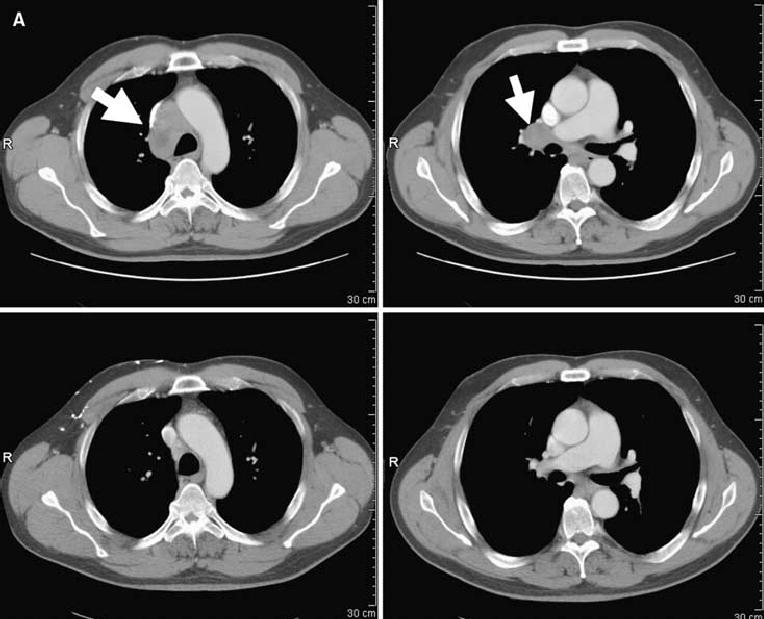
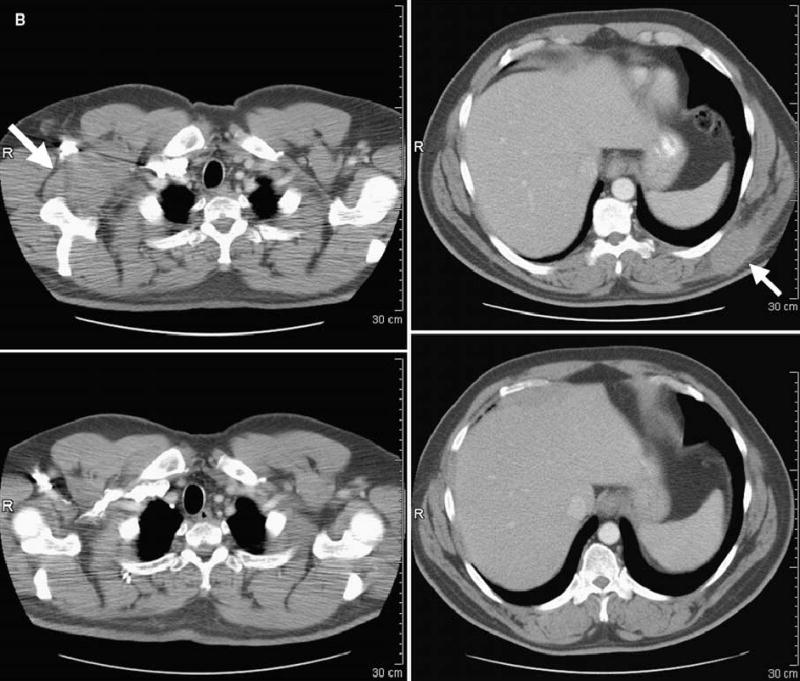
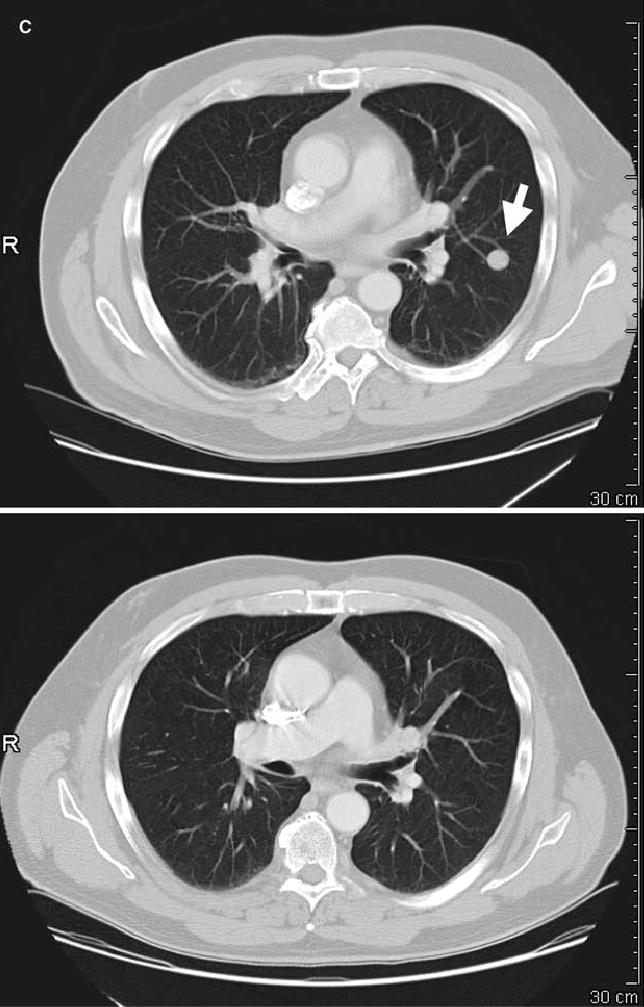
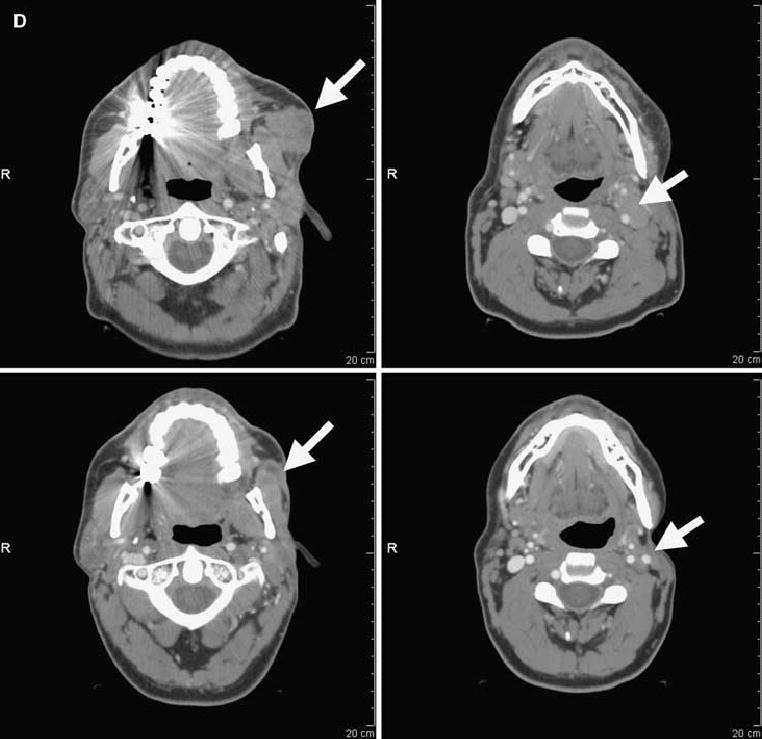
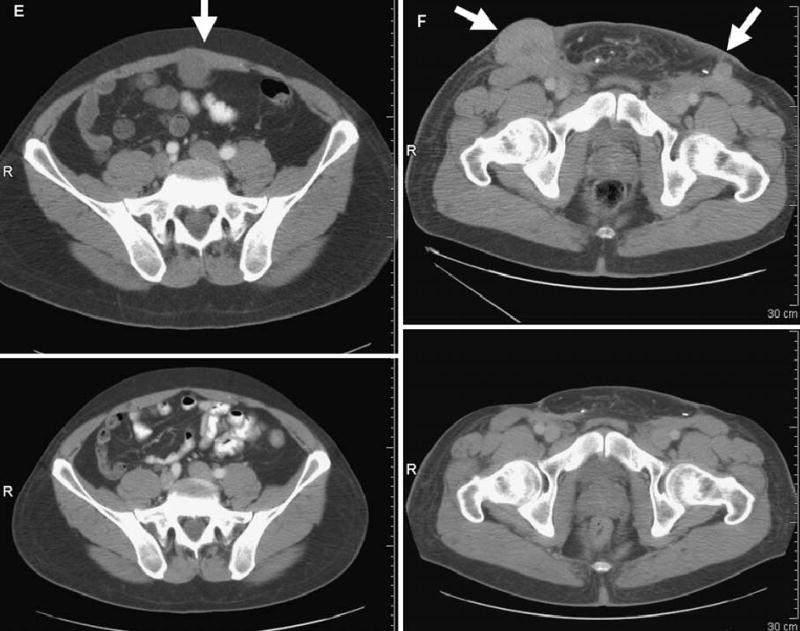
Computed tomography scans illustrating disease status before treatment (upper panels) and after treatment (lower panels) for patients treated with anti–CTLA-4 antibody and IL-2. Arrows on pretreatment scans denote sites of disease. (A) Patient 4: regression of disease in the mediastinum. (B) Patient 8: regression of disease in the right axilla and latissimus dorsi. (C) Patient 12: regression of disease in the left lung. (D) Patient 14: regression of disease in subcutaneous tissue of the face and submandibular lymph nodes. (E) Patient 15: regression of disease in the abdominal wall. (F) Patient 19: regression of disease in subcutaneous tissue and an inguinal lymph node. R, right.
Toxicity/Autoimmune Effects
All patients received high-dose bolus IL-2, and the expected reported side eDects were seen in these patients.15,16 However, five patients (14%) developed grade III/IV toxicities attributable to anti–CTLA-4 administration, including four patients with enterocolitis and one with arthritis and uveitis (Table 1). In all dose cohorts, two (25%) of eight responders experienced grade III/IV autoimmunity attributable to anti–CTLA-4 therapy, whereas in the 3.0 mg/kg dose group, one (20%) of five responders experienced grade III/IV autoimmunity attributable to anti–CTLA-4 therapy (no significant difference). All patients with grade III/IV autoimmune toxicity recovered without sequelae. Because the toxicities and management of anti–CTLA-4 induced autoimmunity in humans are still poorly understood, the detailed histories of these patients are given below.
Patient 8 tolerated his first two treatment courses with toxicities limited to those expected with IL-2. After the second course, the patient achieved a partial response but developed diarrhea and was unable to tolerate a diet. Colonoscopy revealed multiple small aphthous ulcers throughout the colon. Acute colitis with crypt abscesses and gland involution in the sigmoid colon and rectum were documented on biopsy. The patient received intravenous dexamethasone 4 mg every 4 hours for 3 days, with relief of symptoms. The patient started a regular diet the next day, was switched to oral corticosteroids for 4 days, and was discharged from the hospital. His response persisted for 7 months before the appearance of a new lesion.
Patient 11 tolerated her first treatment cycle (anti–CTLA-4 alone) without incident. During her second cycle, she tolerated nine doses of IL-2; dosing was discontinued for asymptomatic supra-ventricular tachycardia. During her third cycle, she developed renal dysfunction and a fever to 39.9°C. Twelve days after completing the cycle, she developed severe diarrhea, abdominal distention, and cramping. A flexible sigmoidoscopy was performed. Colon biopsy samples revealed mild chronic mucosal inflammation. A rectal ulcer showed reactive changes and focal erosion of the epithelium with a lymphoid infiltrate consisting predominantly of CD3 T cells (CD4+ greater than CD8+). The diarrhea continued; therefore, 4 days later, an upper endoscopy was performed. Biopsy samples revealed chronic inflammation in the stomach and duodenum. Duodenal immunohistochemistry confirmed a predominant CD3+ lymphoid infiltrate. Intravenous dexamethasone 4 mg every 4 hours was initiated, the patient was started on total parental nutrition, and oral feeding was withheld. She had immediate resolution of her diarrhea and was switched to oral dexamethasone. Eight days later, she was tolerating a regular diet. She received corticosteroids for 1 month. Radiographical evaluation 2.5 weeks after completion of corticosteroids revealed progressive disease. The patient was taken off study.
Patient 19 tolerated his first treatment course without incident, although 3 weeks later he reported having five to six liquid stools per day. Endoscopy revealed chronic active inflammation, scattered epithelial apoptotic bodies, and colonic mucosa with hyperplastic features. Immunohistochemistry revealed an inflammatory infiltrate consisting predominantly of CD3+ T lymphocytes (CD4+ greater than CD8+). The patient was treated with mesalamine, with temporary cessation of diarrhea. Two weeks later, the patient was seen in an outpatient clinic and reported diarrhea up to six times per day associated with cramping, nausea, and chills. Repeat endoscopy revealed focal active colitis in the proximal and distal sigmoid colon and in the rectum. Immunohistochemistry revealed B-cell aggregates in the lamina propria and intraepithelial lymphocytes, predominantly CD8+, with occasional crypts showing increased numbers of lymphocytes. The patient was started on intravenous dexamethasone 4 mg every 6 hours and converted to oral corticosteroids, with subsequent resolution of his symptoms. He received corticosteroids for 3 months and remains a complete responder at 13 months.
Patient 29 completed his third cycle of treatment, stopping IL-2 therapy secondary to an increased creatinine level and hypotension. Four days later, the patient had an increasing creatinine level, mental status changes, increased potassium, and multiple loose stools. The patient was intubated to protect his airway and underwent veno-veno hemodialysis for renal failure. Diarrhea persisted in the setting of negative stool cultures. Two days later, broad-spectrum antibiotics—dexamethasone 2 mg every 6 hours and hydrocortisone 50 mg every 6 hours—were initiated. The diarrhea completely resolved within 2 days; therefore, dexamethasone and hydrocortisone were discontinued. The following day, the patient was started on a diet, at which time the diarrhea returned. He was instructed to take nothing by mouth, and intravenous dexamethasone was reinitiated. Three days later, a flexible sigmoidoscopy revealed colitis (Fig. 2). Corticosteroids were changed to an oral formulation and tapered after 3 weeks. The patient had normal renal function and was having regular bowel movements and tolerating a regular diet at his 4-week follow-up, 1 week after completing his course of corticosteroids. He experienced minor regression of his axillary tumor burden, followed by disease progression.
FIG. 2.
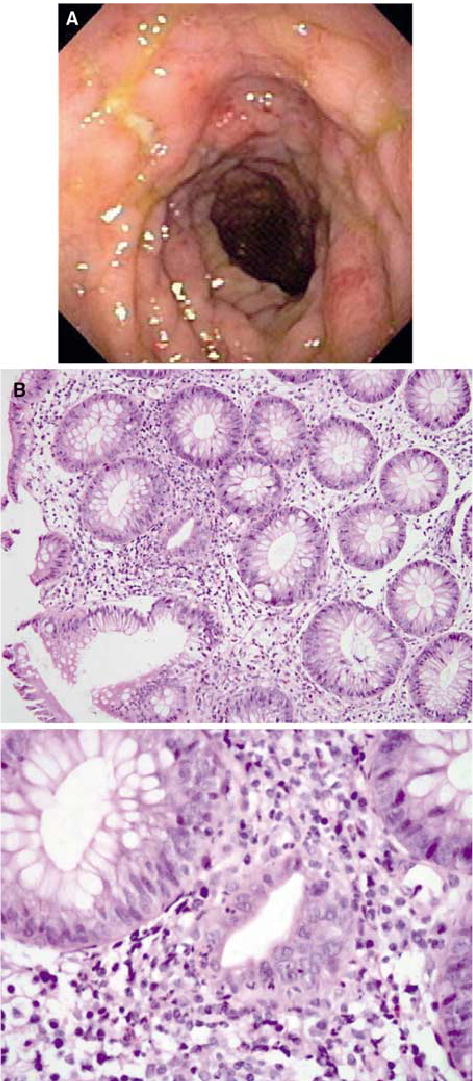
(A) Colonoscopic view of bowel edema and ulceration in the descending colon of patient 29, who experienced autoimmune colitis. (B) Histopathologic analyses revealed focal active colitis (upper panel) with crypt destruction, loss of goblet cells, and neutrophilic infiltrates in the crypt epithelium (lower panel) (original magnification: upper panel, ×20; lower panel, ×60).
Patient 32 tolerated three courses of anti–CTLA-4 therapy well; her course was notable only for hypotension after five doses of IL-2 administration on two occasions. At her evaluation at the end of course 3, she complained of blurry vision and pain in her hands and ankles. Slit-lamp examination showed moderate anterior uveitis in both eyes, vitritis, and mild bilateral disc swelling. Visual acuity was 20/25 in the right eye and 20/40 in the left eye. The patient had a normal baseline eye examination before anti–CTLA-4 administration. Physical examination revealed joint swelling in her fingers, wrists, ankles, and knees. The patient was started on prednisolone acetate 1% eyedrops every 4 hours, which were tapered over the next month, and a cyclooxygenase 2 inhibitor for joint pain and inflammation. At the 1-month follow-up, her visual acuity had improved to 20/20 in both eyes, the anterior uveitis had dissipated, the papillitis had improved, and she had improvement in her musculoskeletal symptoms. Tumors progressed, and the patient was classified as a nonresponder.
Pharmacokinetics
The mean peaks of anti–CTLA-4 30 minutes after the first and second doses were measured for all patients in the .1, .3, 1.0 and 2.0 mg/kg groups. Fifteen patients were evaluated in the 3.0 mg/kg group. Similarly, troughs before the second doses were determined (Table 2). Peaks and troughs were proportionally higher with escalating doses of drug administration. Serum values decreased proportionally at 24, 48, and 72 hours and up to 7 days after dosing. Patients on a protocol receiving only anti–CTLA-4 without IL-2 revealed serum concentrations comparable to the serum concentrations seen in these patients at the same drug doses.12
TABLE 2.
Pharmacokinetics of anti–CTLA-4 antibody
| Dose cohort (mg/kg) | After dose 1 (μg/mL)a | Before dose 2 (μg/mL) | After dose 2 (μg/mL)a | 24 h after dose 2 (μg/mL) | 48 h after dose 2 (μg/mL) | 72 h after dose 2 (μg/mL) |
|---|---|---|---|---|---|---|
| .1 (n = 3) | 1.2 ± 1.1 | .0 ± .0 | 1.9 ± .7 | 1.4 ± .15 | .4 ± .7 | .0 ± .0 |
| .3 (n = 3) | 6.1 ± .41 | .6 ± .9 | 8.8 ± 3.3 | 6.4 ± 1.08 | 3.2 ± .71 | 2.8 ± .54 |
| 1.0 (n = 3) | 16.3 ± 5.0 | 2.0 ± .8 | 13.7 ± 11.6 | 21.2 ± 11.4 | 9.8 ± 4.3 | 8.0 ± 5.13 |
| 2.0 (n = 3) | 41.9 ± 7.9 | 7.1 ± 1.4 | 48.9 ± 19.9 | 30.8 ± 10.6 | 18.7 ± 3.79 | 15.3 ± 6.04 |
| 3.0 (n = 15) | 88.8 ± 20.1 | 16.7 ± 4.0 | 124.9 ± 41.72 | 72.4 ± 15.4 | 49.7 ± 11.1 | 37.6 ± 7.62 |
Values are expressed as mean concentrations ± SD.
CTLA, cytotoxic T lymphocyte–associated antigen.
Peaks drawn 30 minutes after completion of infusion.
Analysis of Circulating Lymphocytes
Flow cytometry was used to compare surface marker expression on PBMCs before treatment and after two or three cycles of treatment. Expression of CD3, CD4, HLA-DR, CD45RO, CD69, and CD25 was evaluated in 28 total patients from the .3, 1.0, 2.0, and 3.0 mg/kg dose cohorts, including the 8 clinical responders. HLA-DR expression, a marker of T-cell activation, was significantly increased on posttherapy CD3+CD4+ and CD3+CD4− cells (P = .0003 and .003, respectively; two-tailed t-test). The expression of CD45RO, a memory cell marker, also increased after therapy in CD3+CD4+ cells (P = .0004) but did not change significantly in CD3+CD4− cells. CD25+ expression, a marker of T-regulatory cells and activated T cells, increased after treatment in CD4+ cells (P = .004). The percentage of cell populations that expressed CD69 did not change significantly (Table 3).
TABLE 3.
Flow cytometric analysis of selected T-cell surface markers (after minus before) after anti-CTLA-4 and IL-2 treatment in selected patients
| Patient no. | HLA-DR+ (CD3+CD4+) | HLA-DR+ (CD3+CD4−) | CD45RO+ (CD3+CD4+) | CD45RO+ (CD3+CD4−) | CD69+ (CD3+CD4+) | CD69+ (CD3+ CD4−) | CD25+ (CD4+) |
|---|---|---|---|---|---|---|---|
| 4a | 7.3% | 7.2% | 7.4% | 5.5% | 5.2% | 7.5% | 7.9% |
| 5 | −15.4% | −26.7% | 2.8% | −1.0% | −14.3% | −5.1% | −5.1% |
| 6 | 11.4% | −5.6% | 1.8% | 6.3% | 34.2% | 27.9% | 12.7% |
| 8a | 12.1% | 5.1% | 4.4% | 5.9% | 7.8% | 7.0% | 5.0% |
| 9 | 7.5% | 11.1% | −.7% | −15.2% | 41.1% | 39.1% | 10.1% |
| 10 | 11.1% | 11.5% | .9% | −5.9% | −2.4% | −9.4% | .9% |
| 11 | −6.3% | 6.3% | −11.7% | −5.5% | −9.4% | −7.6% | −1.1% |
| 12a | 9.8% | 11.9% | 10.7% | −19.7% | −18.7% | −10.3% | 14.6% |
| 13 | 7.4% | 9.8% | 17.5% | −2.2% | .9% | −2.8% | 18.4% |
| 14a | 28.1% | 13.0% | 2.3% | 1.4% | −11.4% | 1.1% | .8% |
| 15a | 13.6% | 16.4% | 4.8% | 2.1% | −9.6% | −10.1% | 16.3% |
| 16 | 8.2% | 7.9% | 2.4% | −1.1% | 8.9% | −2.5% | 1.1% |
| 17 | 5.9% | −2.5% | 1.4% | −.2% | 13.1% | 14.9% | 1.6% |
| 18 | 7.3% | 15.8% | 9.3% | −1.3% | 1.3% | 2.6% | 6.4% |
| 19a | −6.5% | −16.2% | 6.2% | −9.5% | −30.2% | −10.2% | −.3% |
| 20a | −.1% | 15.3% | .2% | −17.5% | −48.0% | −33.8% | −2.4% |
| 22 | 4.7% | 12.5% | −3.0% | −4.3% | 2.7% | 2.8% | −4.6% |
| 23 | −1.1% | 10.1% | 3.6% | 7.9% | .1% | .8% | 1.2% |
| 24 | 11.8% | 15.0% | 2.0% | 16.1% | .3% | 2.5% | 4.8% |
| 25 | 10.3% | 7.3% | −.7% | 2.3% | 1.0% | 11.9% | 11.4% |
| 26 | −2.0% | −3.7% | 11.5% | 2.6% | 14.4% | 8.5% | 7.5% |
| 27 | 11.0% | 10.9% | 1.4% | −2.3% | −6.6% | −7.3% | 5.6% |
| 28a | 21.2% | 24.5% | 8.3% | −2.6% | 12.2% | −2.2% | 6.6% |
| 29 | 8.6% | 17.1% | 11.3% | 21.4% | −.2% | 2.5% | 1.0% |
| 30 | 1.0% | 1.5% | 3.5% | −5.3% | 0.5% | 3.9% | 2.3% |
| 32 | 13.4% | 28.1% | 6.5% | 8.1% | 21.6% | 16.8% | −3.4% |
| 34 | 10.1% | 7.0% | 4.8% | −6.3% | 3.2% | 2.6% | −5.8% |
| 35 | 1.4% | −11.0% | 7.5% | 1.1% | −2.2% | −4.8% | −3.7% |
| Mean change | 6.9% | 7.1% | 4.2% | −.7% | .6% | 1.7% | 3.9% |
| P valueb | .0003 | .0033 | .0004 | .6871 | .8672 | .5184 | .0041 |
CTLA, cytotoxic T-lymphocyte–associated antigen; IL, interleukin.
Objective clinical responders.
Two-tailed P value using the paired t-test.
DISCUSSION
Thymic selection of cytotoxic T lymphocytes can delete self-reactive CD8 cells. However, circulating T lymphocytes that have escaped central deletion can be tolerized to self antigens by peripheral suppression mechanisms. One of these suppressive regulatory elements is CTLA-4. Multiple animal models have suggested enhanced antitumor immunity with CTLA-4 blockade,10,11,17 and we have demonstrated antitumor activity in humans treated with anti–CTLA-4 antibody in conjunction with peptide vaccination.12 IL-2 administration may also mediate antitumor effects. However, IL-2 also stimulates T-regulatory cells that constitutively express CTLA-4 and can suppress immune reactions.18,19 We thus hypothesized that the administration of IL-2 in the presence of CTLA-4 blockade might enhance antitumor reactivity. Alternatively, anti–CTLA-4 may release inhibitory influences on activated CD25-expressing CD4 and CD8 effector cells and, thus, increase their antitumor response in an IL-2–supported milieu.
In this study, we have shown a 22% objective response rate to CTLA-4 blockade plus IL-2 administration in 36 patients. In our other studies using anti–CTLA-4 antibody with peptide vaccination in 56 patients with metastatic melanoma, the objective response rate was 12.5%.12,20 Given a 15% historical response rate to IL-2 in patients with metastatic melanoma,14,15 there does not seem to be evidence to support a synergistic effect of the addition of IL-2 to anti–CTLA-4 administration.
In our previous report, 6 of 14 patients developed grade III/IV autoimmune dermatitis, enterocolitis, hepatitis, and hypophysitis attributable to CTLA-4 blockade.12 Furthermore, CTLA-4–deficient mice develop lymphoproliferative disease with migration of T-cell blasts to visceral organs,21 and other animal models of CTLA-4 blockade have demonstrated T cell–associated autoimmune toxicities, including demyelinating lesions, encephalomyelitis, colitis, and diabetes.22–25 In this study, there was a 14% incidence (5 of 36 patients) of grade III/IV autoimmunity attributable to anti–CTLA-4 antibody administration across all dose cohorts and a 12.5% incidence in the 3 mg/kg dose cohort. This was compared with a 25% incidence (14 of 56 patients) when patients were treated with anti–CTLA-4 without the addition of IL-2. Although not significant (P = .29), the incidence of autoimmunity may have decreased between the two studies, possibly secondary to the supportive effect of IL-2 on CD4+CD25+ T-regulatory cell activation and proliferation.
Our extensive prior experience with IL-2 therapy allowed prompt treatment and documentation of the numerous cytokine-induced toxicities and determination of those toxicities attributable to CTLA-4 blockade. Two (25%) of 8 responders developed grade III/IV autoimmune toxicity, versus 3 (11%) of 28 nonresponders (Fisher’s exact test; 2 tailed; P = .3). In 56 prior patients treated with anti--CTLA-4 antibody plus peptide vaccination alone, however, we did see a significant association of response with grade III/IV autoimmunity (P = .008). Some of the responses in the current series may have been due to IL-2 alone and thus may have obscured any association of response with autoimmune side effects. The use of corticosteroids to treat severe autoimmunity did not seem to affect the antitumor effect of CTLA-4 blockade; however, we do not know whether it had any effect on the IL-2--induced tumor response. The correlation of autoimmunity with clinical tumor regression is the subject of ongoing studies.
Significant increases in markers of T-cell activation after treatment (HLA-DR and CD45RO) in CD4+ cells were seen. HLA-DR was also increased in CD8+ populations. These data correlate with our phenotypical analysis of PBMCs from patients treated with anti–CTLA-4 plus peptide vaccine. CD25+ cells in CD3+ and CD4+ populations mildly increased after treatment; however, in the presence of IL-2, which upregulates CD25, it is difficult to determine activated phenotypes from regulatory phenotypes.18 In murine models, CD4+CD25+ T-regulatory cells have been reported to control inflammatory responses in the intestine. It has also been suggested that CD25+ cells may be functionally dependent on CTLA-4, because CTLA-4 blockade resulted in an inability of populations of T-regulatory cells to inhibit intestinal inflammation.25 Furthermore, CD4+CD25+ cells constitutively express CTLA-4 on their cell surface26; therefore, it is possible that blocking these cells with an anti–CTLA-4 antibody may liberate autoreactive T cells. These investigations are the subject of current investigations in our laboratory.
Clinical responses seem to be durable. In our original published series of patients treated with anti–CTLA-4 antibody, all three responders continue to have ongoing responses at 29 to 32 months,12 and we present here 6 (75%) of 8 responders, including the 2 complete responders, with ongoing tumor regressions at 11 to 19 months.
This clinical trial suggests a role for CTLA-4 blockade in breaking tolerance to both human cancer antigens and self antigens. The role of peptide vaccinations and higher doses of anti–CTLA-4 remains to be elucidated.
References
- 1.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–58. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz RH. A cell culture model for T lymphocyte clonal anergy. Science. 1990;248:1349–56. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- 3.Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter JA. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174:561–9. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gross JA, Callas E, Allison JP. Identification and distribution of the costimulatory receptor CD28 in the mouse. J Immunol. 1992;149:380–8. [PubMed] [Google Scholar]
- 5.Lindsten T, Lee KP, Harris ES, et al. Characterization of CTLA-4 structure and expression on human T cells. J Immunol. 1993;151:3489–99. [PubMed] [Google Scholar]
- 6.Brunner MC, Chambers CA, Chan FK, Hanke J, Winoto A, Allison JP. CTLA-4-mediated inhibition of early events of T cell proliferation. J Immunol. 1999;162:5813–20. [PubMed] [Google Scholar]
- 7.Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med. 1996;183:2533–40. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walunas TL, Bakker CY, Bluestone JA. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med. 1996;183:2541–50. doi: 10.1084/jem.183.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson CB, Allison JP. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997;7:445–50. doi: 10.1016/s1074-7613(00)80366-0. [DOI] [PubMed] [Google Scholar]
- 10.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–66. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–6. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 12.Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100:8372–7. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol. 2002;3:611–8. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg SA, Yang JC, White DE, Steinberg SM. Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2: identification of the antigens mediating response. Ann Surg. 1998;228:307–19. doi: 10.1097/00000658-199809000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–16. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 16.Schwartzentruber DJ. Guidelines for the safe administration of high-dose interleukin-2. J Immunother. 2001;24:287–93. doi: 10.1097/00002371-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Hurwitz AA, Foster BA, Kwon ED, et al. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60:2444–8. [PubMed] [Google Scholar]
- 18.Jian-Xin L, Leonard WJ. Interleukin-2. In: Thompson AW, Lotze MT, eds. The Cytokine Handbook. San Diego: Elsevier Press, 2003:167–99.
- 19.Birebent B, Lorho R, Lechartier H, et al. Suppressive properties of human CD4+CD25+ regulatory T cells are dependent on CTLA-4 expression. Eur J Immunol. 2004;34:3485–96. doi: 10.1002/eji.200324632. [DOI] [PubMed] [Google Scholar]
- 20.Attia P, Phan GQ, Marker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–53. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–8. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 22.Hurwitz AA, Sullivan TJ, Krummel MF, Sobel RA, Allison JP. Specific blockade of CTLA-4/B7 interactions results in exacerbated clinical and histologic disease in an actively-induced model of experimental allergic encephalomyelitis. J Neuroimmunol. 1997;73:57–62. doi: 10.1016/s0165-5728(96)00168-3. [DOI] [PubMed] [Google Scholar]
- 23.Perrin PJ, Maldonado JH, Davis TA, June CH, Racke MK. CTLA-4 blockade enhances clinical disease and cytokine production during experimental allergic encephalomyelitis. J Immunol. 1996;157:1333–6. [PubMed] [Google Scholar]
- 24.Luhder F, Hoglund P, Allison JP, Benoist C, Mathis D. Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) regulates the unfolding of autoimmune diabetes. J Exp Med. 1998;187:427–32. doi: 10.1084/jem.187.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–10. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]


