Summary
Lymphopenia is a serious consequence of HIV infection and the administration of cancer chemotherapeutic agents. Although growth factors can be administered to patients to increase circulating neutrophils, there is no effective method to stimulate CD8+ lymphocyte production in humans, in vivo. This report is the first to describe the administration of recombinant interleukin-7 to humans and demonstrates the ability of this cytokine to mediate selective increases in CD4+ and CD8+ lymphocytes along with a decrease in the percentage of CD4+ T-regulatory cells. These studies suggest an important role for interleukin-7 in the treatment of patients with lymphopenia.
Keywords: interleukin-7, regulatory T cells, human lymphocytes, lymphopoiesis
Interleukin (IL) 7 is a 17.5 kd cytokine produced by a variety of stromal cells, as well as by keratinocytes, dendritic cells, neurons, and endothelial cells but is not produced by lymphocytes.1,2 The IL-7 receptor contains a unique 75 kd α chain and a γ chain shared with IL-2, 4, 7, 9, 15, and 21. Homeostatic control of lymphocyte levels in mice seems to be dependent on the cytokine, IL-7. IL-7 receptor knockout mice exhibit thymic atrophy, arrest of T-cell development at the double positive stage, and severe lymphopenia.3 Administration of IL-7 to mice results in an increase in thymic emigrants, increases in B and T cells, and increased recovery of T cells after cyclophosphamide administration or after bone marrow transplantation.4–7 Transgenic mice overexpressing IL-7 exhibit expansion of immature B cells and can develop lymphoproliferative disorders.8,9
In vitro studies have suggested a role for IL-7 in human immune function. The addition of IL-7 to neonatal human thymic cultures increases the number of immature and mature T cells.10 A mutation in the IL-7 receptor α chain in humans leads to an unusual form of combined immunodeficiency disease characterized by severe T-cell defects but normal B cells and natural killer cells.11 An inverse relationship exists between circulating numbers of CD4+ lymphocytes and IL-7 serum levels in humans.1,2 Thus, on the basis of these and other preclinical studies, IL-7 seems to be involved in the homeostasis of both B and T cells in the mouse but only T cells in the human.
Nonhuman primates administered IL-7 showed histologic evidence of hyperplasia in Peyer patches and lymph nodes with increased circulating levels of lymphocytes.12 We have now assessed the immunologic impact of IL-7 administration in humans and have found that IL-7 results in a rapid and selective increase in circulating CD4+ and CD8+ lymphocytes.
MATERIALS AND METHODS
Patients
All patients were treated in the Surgery Branch, National Cancer Institute in a protocol approved by the Institutional Review Board, NCI. Eleven patients had metastatic melanoma and one (patient 7, Table 1) had metastatic sarcoma. All patients were HLA-A*0201+ and had measurable disease. Eligibility criteria included creatinine <1.4 mg/dL, liver function enzymes <3 times the normal limit, absolute neutrophil count >1000/mm3, absolute lymphocyte count >200/mm3, platelet counts >100,000/mm3, and coagulation parameters <1.5 times the upper limit of normal. Patients were not eligible if they had any form of immunosuppressive disease or had resting blood pressure >140/90.
TABLE 1.
Patient Characteristics
|
Serum IL-7 Levels*(pg/mL) |
Serum Neutralizing Antibody |
|||||||
|---|---|---|---|---|---|---|---|---|
| Patient† | IL-7 Dose (μg/kg) | Age/Sex | Prior Treatment‡ | Pre | 24 h | 48 h | Pre | Day 28 |
| 1 | 3 | 42/F | S,I | — | — | — | 0 | 0 |
| 2 | 3 | 43/F | S,C,I | — | — | — | 0 | 0 |
| 3 | 3 | 20/F | S,I | — | — | — | 0 | 0 |
| 4 | 10 | 66/F | S,C,I | 0 | 64.5 | 0 | 0 | 0 |
| 5 | 10 | 67/M | S,I | 0 | 51.3 | 0 | 0 | 0 |
| 6 | 10 | 45/M | S,C,I | 0 | 53.9 | 0 | 0 | 0 |
| 7 | 30 | 58/M | S | 0 | 194 | 0 | 0 | 1:100 |
| 8 | 30 | 59/M | S | 0 | 809 | 275 | 0 | 1:100 |
| 9 | 30 | 49/F | S,I | 0 | 102 | 0 | 0 | 0 |
| 10 | 60 | 51/M | S | 0 | 622 | 14.3 | 0 | 1:200 |
| 11 | 60 | 57/M | S | 0 | 497 | 183 | 0 | 1:100 |
| 12 | 60 | 33/M | S | 0 | 946 | 52.6 | 0 | 1:50 |
The limit of detection in this assay was 12.5 pg/mL.
All patients had metastatic melanoma except for patient 7 with metastatic sarcoma.
S indicates surgery; C, chemotherapy; I, immunotherapy.
IL-7
IL-7 (CYT 99-007) was produced according to good manufacturing practice and supplied to the National Cancer Institute under a Cooperative Research and Development Agreement with Cytheris Corp (Issy les Moulineaux, France). IL-7 was purified from inclusion bodies of recombinant Escherichia coli containing DNA encoding the human protein. Purity levels were 100% by SE-HPLC and >98.5% by sodium dodecyle sulfate-polyacrlyamide gel electrophoresis. The IL-7 is a non-glycosylated 153 amino acid protein expressing a methio-nine at the N-terminal position of the natural 152 amino acid human sequence. It was supplied as a lyophilized powder in vials and reconstituted with water for subcutaneous injection.
Treatment
Patients were treated in 4 sequential cohorts of 3 patients each at 3, 10, 30, or 60 μg/kg of IL-7 administered subcutaneously every 3 days for 8 doses. Patients thus received subcutaneous injections on days 0, 3, 6, 9, 12, 15, 18, and 21. Starting on day 0 and repeated on day 7, 14, and 21, patients received the gp100:209–217(210 M) and MART-1:26–35(27 L) peptides emulsified separately in incomplete Freund adjuvant and injected subcutaneously in different extremities. Patients underwent lymphopheresis before the beginning of the protocol and on day 28. Before and on day 28 all patients underwent complete physical examination and radiologic studies to determine the extent of metastatic cancer.
Immunologic Studies
In vitro sensitization boost assays, Elispot assays, and tetramer assays were performed to detect reactivity to the administered peptides as previously described. Serum was obtained at varying intervals for measurements of IL-7 and anti–IL-7 antibody measured by enzyme-linked immunosorbent assay.
Assessment of the impact of IL-7 administration on hematopoietic and lymphoid cells was performed using fluorescence-activated cell analysis (FACS) using commercial antibodies reactive with CD3, CD4, CD8, CD19, CD56, CD127, and other hematopoietic markers as previously described.
Bone Marrow Analysis
Bone marrow aspirates were collected into sterile sodium heparin, prelysed with ammonium chloride, and stained for 30 minutes at room temperature with antibodies directed against CD13, CD33, and CD36 (Beckman-Coulter, Miami, FL); CD3, CD10, CD14, CD16, CD19, CD22, CD34, CD56, CD45, and CD71 (BD Biosciences, San Jose, CA); CD64 (Caltag Laboratories, Burlingame, CA); and CD20, Kappa, Lambda (Dako-Cytomation, Carpinteria, CA). All cells were fixed in 1.0% paraformaldehyde and stored at 4°C for up to 12 hours before acquisition. Four color cytometry was performed with a BD (Becton-Dickinson, San Jose, CA) FACSCalibur flow cytometer. The sensitivity of fluorescent detectors was set and monitored using Calibrite beads (Becton-Dickinson, San Jose, CA) according to the manufacturer’s recommendations. Data were analyzed with CellQuest software (BD). Granulocytes, monocytes, mature lymphocytes, nucleated red blood cells, and immature hematopoietic precursors were determined based upon levels of CD45 expression, side scatter, and pattern of antigen expression.
Reverse Transcriptase-polymerase Chain Reaction Measurement of Foxp3 Expression in CD4+ T Cells
Peripheral blood mononuclear cells obtained before and on day 28 after treatment were simultaneously thawed and CD4+ cells were isolated using a CD4 Negative Isolation kit as per the manufact urer’s instructions (Dynal Biotech, product no. 113.17). Total RNA was extracted using an RNeasy mini kit (Qiagen) and RNA was reverse transcribed using the ThermoScript reverse transcriptase-polymerase chain reaction (RT-PCR) system (In Vitrogen Life Technologies). cDNA was amplified using specific primers and probes for Foxp3 and β-actin. Foxp3 was performed using the predeveloped TaqMan Gene Expression Assays from Applied Biosystems. Primers and probes for β-actin were the following: β-actin TaqMan probe 5′FAM-CCAGCCATGTACGTTGCTATCCAGGC-TAMRA-3′, forward primer 5′-GCGAGAAGATGATGACCCAGATC-3′, and reverse primer 5′-CCAGTGGTACGGCCAGAGG-3′. Real-time RT-PCR was performed using the ABI PRISM 5700 Sequence Detection System (Applied Biosystems). The mRNA for Foxp3 and β-actin were determined by comparing the unknown samples to the standard curves that were generated by using a serial dilution of plasmids that carried Foxp3 and β-actin genes.
Intracellular Detection of Foxp3 Protein by FACS Analysis
Intracellular staining for Foxp3 protein was carried out by using fixation and permeabilization buffers provided by the Foxp3 kit (clone PCH101, eBioscience) according to the manufacturer’s instructions, followed by visualization with PE-conjugated or PerCP-conjugated streptavidin antibody.
RESULTS AND DISCUSSION
Twelve patients with metastatic cancer (11 with melanoma and 1 with sarcoma) were treated with 4 different doses of IL-7 in cohorts of 3 patients each along with immunization using 2 melanoma antigen peptides (Table 1). Patients ranged in age from 20 to 59 years. All patients received all 8 planned doses of IL-7 given every 3 days for 21 days.
Serum levels of IL-7 at 24 hours after the first subcutaneous injection showed a direct relationship to the dose of IL-7 injected (Table 1). The calculated half-life of disappearance of IL-7 from the serum in the 3 patients treated at 60 μg/kg were 12.7, 12.5, and 12.3 hours, respectively. IL-7 levels were undetectable in serum by 72 hours after injection.
The impact of IL-7 administration on changes in the absolute neutrophil and lymphocyte counts is shown in Figure 1A and on CD4+ and CD8+ lymphocyte subsets in Figure 1B. The absolute levels of CD3, CD4, CD8, CD19, and CD56 lymphocytes throughout treatment are shown in Table 2. Absolute neutrophil, eosinophil, and basophil counts were unaffected by IL-7 administration although absolute lymphocyte counts as well as the CD4 and CD8 lymphocyte subsets increased in a dose-dependent manner over the 21-day period. Both CD4+ and CD8+ lymphocytes increased at about the same rate and the CD4/CD8 ratio remained constant (Fig. 1C). No changes were seen at the 3 μg/kg dose, moderate increases in CD4 and CD8 lymphocytes were seen at the 10 μg/kg dose that increased at 30 and 60 μg/kg. At the 60 μg/kg dose, both CD4 and CD8 counts increased 3 to 7-fold by day 21. By day 28, 7 days after the last IL-7 injection at 60 μg/kg, lymphocyte counts were decreasing but still had not reached baseline levels. There was no consistent impact on B cells (CD19+) or NK cells (CD56+) (Table 2).
FIGURE 1.
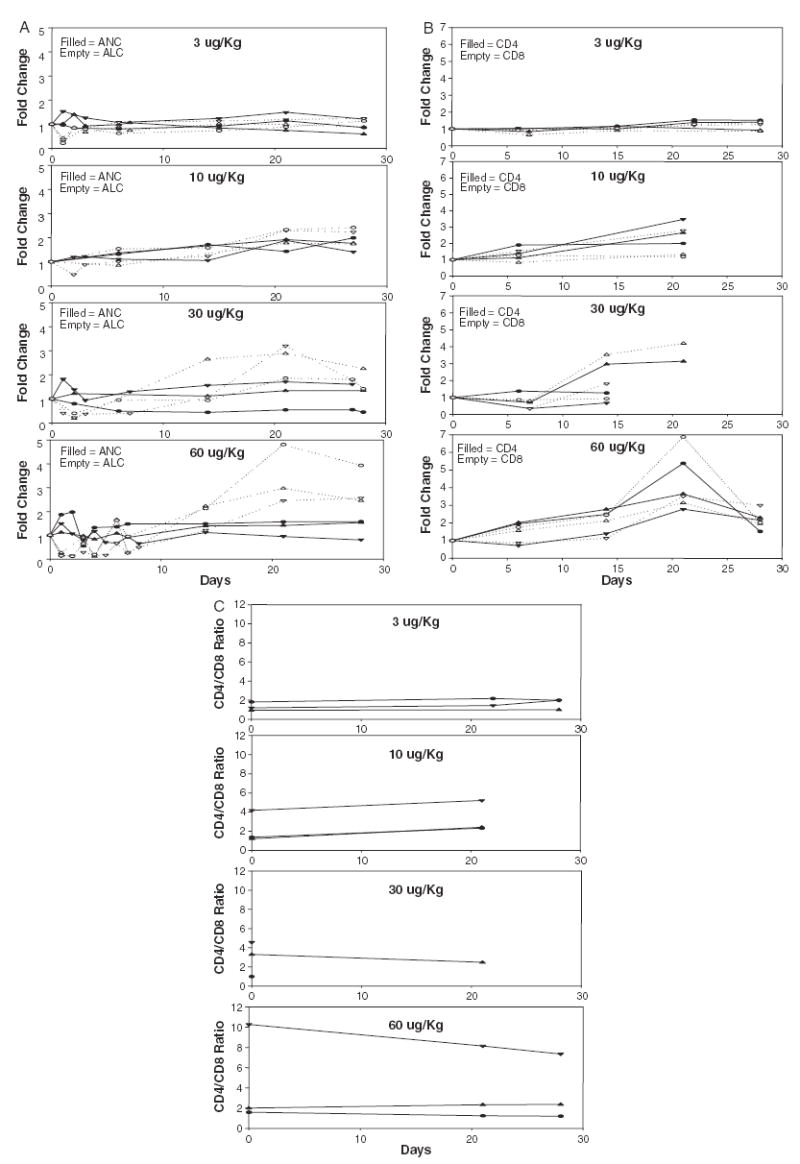
Changes in circulating levels of hematopoietic cells in patients receiving IL-7. Each line represents an individual patient. A, Absolute neutrophil (filled symbols) and absolute lymphocyte (empty symbols) counts. B, CD4+ (filled symbols) and CD8+ (empty symbols) cells. C, CD4/CD8 ratio for each patient.
TABLE 2.
Hematologic Effects of IL-7 Administration
|
Lymphocytes |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | CD3 | CD4 | CD8 | CD19 | CD56 | ||||
| Patient | IL-7 Dose (μg/kg) | Day | Neutrophils | (cells/μL) | |||||
| 1 | 3 | 0 | 7763 | 2473 | 2228 | 1029 | 1080 | 50 | 198 |
| 21 | 5715 | 2448 | ND | ND | ND | ND | ND | ||
| 28 | 4598 | 2166 | 2003 | 947 | 944 | 41 | 121 | ||
| 2 | 3 | 0 | 2247 | 2279 | 1876 | 999 | 800 | 245 | 193 |
| 21 | 3351 | 2718 | 2384 | 1393 | 969 | 141 | 200 | ||
| 28 | 2843 | 2610 | 2425 | 1394 | 1016 | 217 | 222 | ||
| 3 | 3 | 0 | 4103 | 2180 | 1549 | 978 | 537 | 344 | 345 |
| 22 | 6365 | 2798 | 2193 | 1494 | 685 | 325 | 317 | ||
| 28 | 4822 | 3569 | 2247 | 1480 | 745 | 487 | 367 | ||
| 4 | 10 | −1 | 2261 | 1931 | 1479 | 815 | 669 | 302 | 104 |
| 27 | 4332 | 3435 | 3055 | 2157 | 890 | 213 | 154 | ||
| 27 | 4000 | 3368 | ND | ||||||
| 5 | 10 | 2826 | 1814 | 1065 | 831 | 199 | 265 | 410 | |
| 21 | 5354 | 4178 | 3458 | 2885 | 554 | 143 | 616 | ||
| 27 | 3989 | 4038 | ND | ||||||
| 6 | 10 | −1 | 2531 | 1080 | 860 | 511 | 374 | 27 | 173 |
| 21 | 3614 | 2513 | 1499 | 1024 | 44 | 111 | 183 | ||
| 27 | 5040 | 2609 | ND | ||||||
| 7 | 30 | 0 | 3764 | 1525 | 1186 | 891 | 271 | 145 | 167 |
| 21 | 5058 | 4404 | 3981 | 2798 | 1138 | 172 | 280 | ||
| 28 | 5090 | 3450 | ND | ||||||
| 8 | 30 | 0 | 3342 | 1258 | 963 | 802 | 176 | 101 | 186 |
| 21 | 5544 | 3949 | ND | ||||||
| 27 | 5221 | 2203 | ND | ||||||
| 9 | 30 | 0 | 2696 | 2661 | 2155 | 1027 | 1051 | 48 | 99 |
| 21 | 1450 | 4949 | ND | ||||||
| 28 | 1215 | 3791 | ND | ||||||
| 10 | 60 | −1 | 3461 | 1179 | 887 | 587 | 293 | 164 | 126 |
| 21 | 4923 | 3519 | 3182 | 2144 | 921 | 82 | 237 | ||
| 28 | 5346 | 2905 | 1979 | 1352 | 572 | 105 | 124 | ||
| 11 | 60 | −1 | 4200 | 1757 | 1346 | 1211 | 118 | 305 | 78 |
| 21 | 4004 | 4301 | 3861 | 3366 | 414 | 127 | 246 | ||
| 28 | 3387 | 4503 | 2956 | 2600 | 354 | 109 | 159 | ||
| 12 | 60 | −1 | 3769 | 2053 | 1778 | 903 | 568 | 65 | 176 |
| 21 | 5881 | 9853 | 9545 | 4861 | 3910 | 44 | 240 | ||
| 28 | 5915 | 8076 | 2822 | 1369 | 1139 | 22 | 47 | ||
ND indicates not done.
Phenotypic analysis of CD4+ and CD8+ lymphocytes at day 28 from the 3 patients receiving 60 μg/kg IL-7 revealed a trend toward increase of CD45RA+ and decrease in CD45RO+ cells in both CD4+ and CD8+ lymphocytes thus suggesting an increase in naive relative to memory cells. These differences were not significant but may become more impressive as additional patients are evaluated. No consistent changes were seen in the percentage of cells expressing HLA-DR and CD25. There was a trend toward decreased expression of CD127 (the IL-7 receptor α chain) at the 60 μg/kg dose.
A subset of CD4+ lymphocytes, commonly expressing cell surface CD25, and expressing high levels of Foxp3, has been shown in murine models and in humans to be a potent suppressor of immune function and to play a role in the control of autoimmunity.13 These T-regulatory cells can suppress the ability of antitumor T cells to effectively treat established murine cancers.14 Compelling evidence for a role for Foxp3-expressing T-regulatory cells in humans comes from patients with a mutation in the Foxp3 gene that leads to an X-linked disease characterized by immunodysregulation, polyendocrinopathy, and enteropathy (IPEX syndrome).15 These patients suffer from autoimmune manifestations in early infancy including the development of auto-antibodies, severe enteropathy, type 1 diabetes mellitus, and hypothyroidism.
Because of the potentially negative impact of the generation of these immunosuppressive Foxp3 expressing T-regulatory cells in humans, we analyzed the expression of Foxp3 on the increased levels of CD4+ cells in patients after IL-7 administration (Table 3) (Fig. 2). A decrease in Foxp3 expression in CD4+ cells was seen using semi-quantitative RT-PCR measurements in 7 of 8 patients tested. At the 60 μg/kg dose, Foxp3/β-actin message in CD4+ cells was reduced by 29%, 73%, and 47% in the 3 patients, respectively (Table 3). Measurement of intracellular Foxp3 expression by FACS analysis also revealed a decrease in the percentage of CD4+ cells expressing Foxp3 that was more marked in patients with the highest levels of increase of CD4+ cells (patients treated at 60 μg/ kg) (Fig. 2A). After IL-7 administration, these 3 patients showed an increase in CD4+ cells but a decrease in Foxp3-expressing cells of 32%, 20%, and 70%, respectively (Fig. 2A and Table 3). Interestingly, Foxp3 expressing cells before IL-7 administration (about 3% to 10% of CD4+ cells) did not express CD127, the IL-7 receptor α chain, which helps explain the reduced ability of IL-7 to increase the level of these regulatory cells (Fig. 2B).
TABLE 3.
T-Regulatory Cell Markers in Patients Receiving IL-7
|
Semiquantative RT-PCR |
FACS Analysis |
||||
|---|---|---|---|---|---|
| Pre | Day 28 | Pre | Day 28 | ||
| Patient | Dose of IL-7 (μg/kg) | (Foxp3/106 β actin) | (% of CD4+cells Expressing Foxp3) | ||
| 1 | 3 | 2217 | 1332 (−40%) | 11.05 | 9.96 (−10%) |
| 3 | 3 | 2289 | 1391 (−39%) | 8.64 | 9.25 (+7%) |
| 5 | 10 | 2323 | 1418 (−39%) | 5.94 | 4.16 (−30%) |
| 6 | 10 | 1340 | 1527 (+14%) | 6.38 | 5.20 (−18%) |
| 7 | 30 | 582 | 253 (−57%) | 3.15 | 1.91 (−39%) |
| 10 | 60 | 1219 | 985 (−29%) | 8.68 | 6.05 (−32%) |
| 11 | 60 | 1337 | 365 (−73%) | 7.58 | 6.06 (−20%) |
| 12 | 60 | 1501 | 801 (−47%) | 5.31 | 1.57 (−70%) |
FIGURE 2.
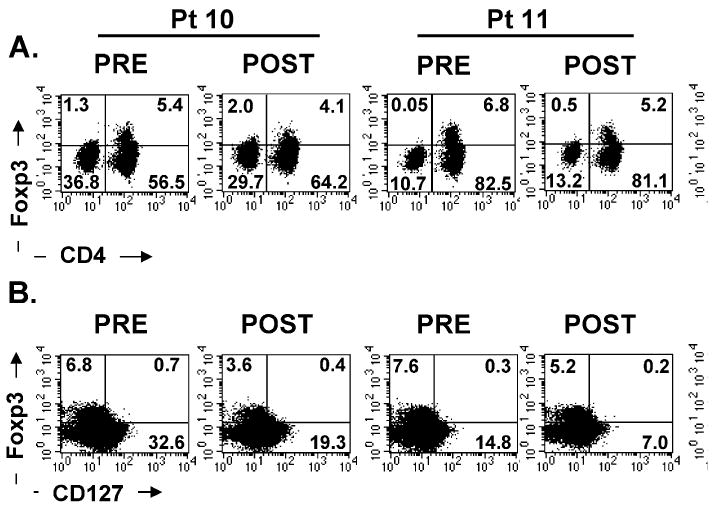
Peripheral blood mononuclear cells from 3 patients (60 λμg/kg dose) (A) were stained with fluorescein isothiocynate-conjugated CD4, PerCP-conjugated CD3, and APC-conjugated CD25 antibodies followed by intracellular staining with biotinylated Foxp3 antibody. The dot plots were gated on CD3+ T lymphocytes. B, Peripheral blood mononuclear cells from these patients were also stained with fluorescein isothiocynate-conjugated CD4, PE-conjugated CD25, and antigen presenting cells-conjugated CD127 (IL-7Rαchain) followed by intracellular staining for Foxp3 protein. The dot plots were gated on CD3+CD4+ T cells. The quadrants were set based on isotype control antibodies as well as negative control, and the numbers represent the percentage of cells in each quadrant.
These results are in marked contrast to results seen in patients receiving IL-2. IL-2 is a T cell growth factor that is predominantly involved in the generation and survival of T-regulatory cells.16 Administration of IL-2 leads to significant increases in T-regulatory cells in patients with cancer.17,18 Intermittent administration of IL-2 to HIV-positive patients has little, if any, impact on CD8+ lymphocytes and seems to increase CD4+ cells expressing Foxp3 suggestive of T-regulatory cells.19,20 In contrast, IL-7 in our study selectively increased CD8+ T cells and CD4+ T cells that lacked expression of Foxp3.
The administration of IL-7 was well tolerated in all patients. Except for mild and transient fevers and mild erythema at the injection site few toxicities were seen. One patient treated at 10 μg/kg had a transient grade 3 hypocalcemia probably related to the apheresis procedure and 2 patients (1 at 30 μg/kg and 1 at 60 μg/kg) had a transient grade 3 rise in liver function enzymes that rapidly returned to baseline levels. No grade 4 toxicities were seen. All side effects promptly returned to normal after the IL-7 administration ceased. No patient experienced an objective clinical cancer response.
In this trial, patients also received immunization with 2 melanoma peptides to determine whether immunization might be enhanced by the concomitant administration of IL-7. We have treated over 300 patients with these melanoma peptides alone and have not seen changes in overall levels of CD8+ or CD4+ cells.21,22 The 4 peptide injections administered over 21 days did not result in the generation of immune precursors against the peptides as measured using Elispot, tetramer, or in vitro sensitization assays, which is consistent with our prior experience with this limited immunization in the absence of IL-7 administration. The immunocompetence of these patients, however, was demonstrated by their ability to react against influenza peptide, a memory response that was not affected by IL-7 administration at any dose (data not shown). Thus, in this limited study, we could derive no evidence that the simultaneous administration of peptides plus IL-7 increased immune reactivity against class 1 restricted peptides.
In contrast to results in murine models, we saw little change in circulating B-cell levels in patients although there was a suggestion of a decrease at the 60 μg/kg IL-7 dose. However, because of the proliferative impact of IL-7 on B cells in mice and the lymphoproliferative disorders seen in IL-7 transgenic mice,8,9 we performed bone marrow biopsies before and after treatment in the 6 patients receiving 30 or 60 μg/kg of IL-7. In 2 of the 3 patients treated at 30 μg/kg, CD19+CD10+ progenitor B cells increased from 3.8% and 3.4% of marrow mononuclear cells at baseline to 30% and 15%, respectively. In 2 of the 3 patients receiving a dose of 60 μg/kg, the progenitor B cells increased from 8% and 3% to 44% and 8%, respectively. These B cells were normal maturing polyclonal B cells. In the other 3 patients, B-cell progenitor levels did not change. There was no evidence of hematopoietic neoplasms. Thus, in humans, IL-7 administration led to expansion of B-cell progenitors in the bone marrow of some patients that was not reflected in the circulation. Mature T cells were increased in the bone marrow of all 6 patients evaluated.
The IL-7 administered to these patients was produced in Escherichia coli and thus did not contain the normal glycosylation seen in eukaryotic IL-7. Because of the possibility that nonglycosylated IL-7 might be immunogenic in humans, we measured anti–IL-7 antibody titers before and after IL-7 administration (Table 1). All patients receiving 10 to 60 μg/kg of IL-7 developed low titer binding antibodies detected by enzyme-linked immunosorbent assay by day 28, seven days after the last injection of IL-7. However, when tested in a specific bioassay to measure neutralization potential, sera from several patients were positive but none of these sera reached the positive threshold defined within the protocol (>1/400 by day 28 or at the last measure at day 56). Furthermore, these antibodies did not seem to affect circulating lymphocyte levels because these levels remained normal with prolonged follow-up of patients as they proceed to other treatments for their cancer (data not shown). Nevertheless, a new formulation of fully glycosylated IL-7 is currently being produced in eukaryotic cells for future studies, that will enable us to explore the effect of higher doses and/or more prolonged treatments by repeated cycles of IL-7 injections.
The studies reported here demonstrate that IL-7 is a potent lymphopoietic factor in humans and has substantial potential for use in the treatment of patients developing lymphopenia from HIV infection or from chemotherapy used in cancer treatment. The selective increase in non–T-regulatory CD4+ T cells represents a significant advantage of the use of this cytokine.
Acknowledgments
The authors are grateful to the senior staff of the Surgery Branch for their helpful comments and care of these patients including Drs James C. Yang, Suzanne L. Topalian, Richard E. Royal, Marybeth S. Hughes, Udai S. Kammula, and Nicholas P. Restifo.
Footnotes
Michel Morre and Renaud Buffet work for the Cytheris Corp. None of the other authors have any conflicting financial interests.
References
- 1.Fry TJ, Mackall CL. Interleukin-7: from bench to clinic. Blood. 2002;99:3892–3904. doi: 10.1182/blood.v99.11.3892. [DOI] [PubMed] [Google Scholar]
- 2.Fry TJ, Connick E, Falloon J, et al. A potential role for interleukin-7 in T-cell homeostasis. Blood. 2001;97:2983–2990. doi: 10.1182/blood.v97.10.2983. [DOI] [PubMed] [Google Scholar]
- 3.Peschon JJ, Morrissey PJ, Grabstein KH, et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrissey PJ, Conlon P, Braddy S, et al. Administration of IL-7 to mice with cyclophosphamide-induced lymphopenia accelerates lymphocyte repopulation. J Immunol. 1991;146:1547–1552. [PubMed] [Google Scholar]
- 5.Talmadge JE, Jackson JD, Kelsey L, et al. T-cell reconstitution by molecular, phenotypic, and functional analysis in the thymus, bone marrow, spleen, and blood following split-dose polychemotherapy and therapeutic activity for metastatic breast cancer in mice. J Immunother. 1993;14:258–268. doi: 10.1097/00002371-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 6.El Kassar N, Lucas PJ, Klug D, et al. A dose effect of IL-7 on thymocyte development. Blood. 2004;104:1419–1427. doi: 10.1182/blood-2004-01-0201. [DOI] [PubMed] [Google Scholar]
- 7.Chu Y, Memon S, Sharrow S, et al. TCR Exogenous IL-7 increases recent thymic emigrants in peripheral lymphoid tissue without enhancing thymic function. Blood. 2004;104:1110–1119. doi: 10.1182/blood-2003-10-3635. [DOI] [PubMed] [Google Scholar]
- 8.Fisher AG, Burdet C, Bunce C, et al. Lymphoproliferative disorders in IL-7 transgenic mice: expansion of immature B cells which retain macrophage potential. Int Immunol. 1995;7:415–423. doi: 10.1093/intimm/7.3.415. [DOI] [PubMed] [Google Scholar]
- 9.Samaridis J, Casorati G, Traunecker A, et al. Development of lymphocytes in interleukin 7-transgenic mice. Eur J Immunol. 1991;21:453–460. doi: 10.1002/eji.1830210230. [DOI] [PubMed] [Google Scholar]
- 10.Yeoman H, Clark DR, DeLuca D. Development of CD4 and CD8 single positive T cells in human thymus organ culture: IL-7 promotes human T cell production by supporting immature T cells. Dev Comp Immunol. 1996;20:241–263. doi: 10.1016/0145-305x(96)00013-4. [DOI] [PubMed] [Google Scholar]
- 11.Puel A, Ziegler SF, Buckley RH, et al. Defective IL7R expression in T(−)B(+)NK(+) severe combined immunodeficiency. Nat Genet. 1998;20:394–397. doi: 10.1038/3877. [DOI] [PubMed] [Google Scholar]
- 12.Fry TJ, Moniuszko M, Creekmore S, et al. IL-7 therapy dramatically alters peripheral T-cell homeostasis in normal and SIV-infected nonhuman primates. Blood. 2003;101:2294–2299. doi: 10.1182/blood-2002-07-2297. [DOI] [PubMed] [Google Scholar]
- 13.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 14.Antony PA, Piccirillo CA, Akpinarli A, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bakke AC, Purtzer MZ, Wildin RS. Prospective immunological profiling in a case of immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) Clin Exp Immunol. 2004;137:373–378. doi: 10.1111/j.1365-2249.2004.02537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malek TR. The main function of IL-2 is to promote the development of T regulatory cells. J Leukoc Biol. 2003;74:961–965. doi: 10.1189/jlb.0603272. [DOI] [PubMed] [Google Scholar]
- 17.Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4 + CD25hiFoxp3 + regulatory T cells in cancer patients. Blood. 2006;107:2409–2414. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H, Chua K, Guimond M, et al. Lymphopenia and IL-2 therapy alter homeostasis of CD4+CD25+ regulatory T cells. Nat Med. 2005;11:1238–1243. doi: 10.1038/nm1312. [DOI] [PubMed] [Google Scholar]
- 19.Kovacs JA, Baseler M, Dewar RJ, et al. Increases in CD4 T lymphocytes with intermittent courses of interleukin-2 in patients with human immunodeficiency virus infection. N Engl J Med. 1995;332:567–575. doi: 10.1056/NEJM199503023320904. [DOI] [PubMed] [Google Scholar]
- 20.Sereti I, Imamichi H, Natarajan V, et al. In vivo expansion of CD4+CD45RO−CD25+ T cells expressing foxP3 in IL-2-treated HIV-infected patients. J Clin Invest. 2005;115:1839–1847. doi: 10.1172/JCI24307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]


