Abstract
Objective: To investigate the role of simultaneous blockade of CD40/CD40L and B7/CD28 pathways in the immune tolerance via co-expression of sCD40LIg and CTLA4Ig mediated by replication-defective adenovirus. Methods: Ad-sCD40LIg-IRES2-CTLA4Ig, replication-defective adenovirus co-expressing sCD40LIg and CTLA4Ig, was constructed and identified. The co-expression of sCD40LIg and CTLA4Ig was evaluated with confocal laser scanning microscope and Western blotting. Skin transplantations of C57BL/6 to BALB/c mice were performed. PBS, Ad-Shuttle-CMV and Ad-sCD40LIg-IRES2-CTLA4Ig were administered. Skin graft survival was monitored and the mRNA expression of both genes was evaluated in the skin allografts. Results: Ad-sCD40LIg-IRES2-CTLA4Ig was constructed successfully and identified. The co-expression of sCD40LIg and CTLA4Ig was identified with confocal laser scanning microscopy and Western blotting. Compared to the skin graft mean survival time (MST) of non-treated group ((5.75±0.71) d) or Ad-Shuttle-CMV-treated group ((5.50±0.53) d), the skin graft MST was dramatically prolonged in the Ad-sCD40LIg-IRES2-CTLA4Ig-treated group ((16.38±1.19) d, P<0.001). The mRNA expression of both genes was detected. Conclusion: Ad-sCD40LIg-IRES2-CTLA4Ig, a replication-defective adenovirus carrying genes encoding sCD40LIg and CTLA4Ig, was constructed. Simultaneous blockade of CD40/CD40L and B7/CD28 costimulatory pathway mediated by replication-defective adenovirus significantly prolonged skin allograft survival in mice.
Keywords: Immune tolerance, CTLA4Ig, sCD40LIg, Replication-defective recombinant adenovirus, Skin graft
INTRODUCTION
Costimulatory signals play a vital role in T cell activation. B7/CD28 and CD40/CD40L have been well-established as important costimulatory pathways, with the blockade of either resulting in suppression or anergy of T cells associated with prolongation of graft survival (Akalin et al., 1996; Bolling et al., 1996; Kita et al., 1999; Reddy et al., 2001; Laumonier et al., 2003; Thiel et al., 2005). Cytotoxic T lymphocyte associated antigen-4 (CTLA4), a ligand for B7, delivers negative signals to antigen presenting cells (APCs) to down-regulate proinflammatory responses, and competitively inhibits the binding of B7 and CD28 (Li et al., 2005). CTLA4Ig is a soluble recombinant fusion protein consisting of the extracellular domain of CTLA4 and the human IgG Fc region (Gao et al., 1999). A study indicated that CTLA4Ig alone or in combination with low-dose cyclosporine could not reverse acute rejection of renal allografts in rats (Perico et al., 1996). However, the combination of CTLA4Ig with T cell depletion (Rehman et al., 1996; Tu et al., 1996) or FTY720 (Ohba et al., 2000; Kita et al., 2002), or anti-LFA-1 monoclonal antibody (Corbascio et al., 2002) prolonged allograft survival. Signals from the CD40/CD40L costimulatory pathway also play an important role in acute rejection of organ grafts such as kidneys and hearts (Kawai et al., 2004; Pearson et al., 2002; Guillot et al., 2002).
To study the effect of blockade of both B7/CD28 and CD40/CD40L pathways on allograft rejection and immune tolerance, we constructed replication-defective recombinant adenovirus to co-express sCD40LIg (a soluble recombinant fusion protein consisting of the extracellular domain of CD40L and the human IgG Fc region) and CTLA4Ig. The co-expression and role of sCD40LIg and CTLA4Ig in mouse skin allograft survival were observed in the present study.
MATERIALS AND METHODS
Construction of Ad-sCD40LIg-IRES2-CTLA4Ig
Total RNA from peripheral blood lymphocytes was extracted using Trizol (Invitrogen, San Diego, CA, USA) to synthesize cDNA encoding extracellular domain of CD40L. Briefly, 2.5 μg of RNA were heated at 85 °C for 3 min and then reverse-transcribed into cDNA in a 25-μl solution containing 200 units of Superscript II RNase H-RT (Invitrogen), 50 ng random hexamers (Invitrogen), 160 μmol/L dNTP, and 10 mmol/L dithiothreitol. The reaction consisted of 10 min at 25 °C, 60 min at 42 °C, and 5 min at 99 °C. Polymerization reactions were performed in a 50-μl reaction volume containing 1 ng of cDNA, 5 units of AmpliTaq Gold DNA polymerase (PerkinElmer, USA), 1.5 mmol/L MgCl2, 160 μmol/L dNTPs, and 10−5 nmol oligonucleotide primers. The oligonucleotide primers were as follows: Forward: 5′-TTT AGA TCT ACC ATG GGT CTA CTG CTC ACA CA-3′, Reverse: 5′-ACA GGT ACC AGT TTG AGT ACG CC-3′. All above-mentioned primers were from TaKaRa (BioTech, Liaoning, Dalian, China). The thermal cycle profile used a 5-min denaturing step at 94 °C followed by 30 cycles (1 min of denaturation at 94 °C, 45 s of annealing at 53 °C, and 1 min of extension at 72 °C) and an extension step of 10 min at 72 °C. The products were then separated by agarose gel electrophoresis. Secretory CD40L (sCD4L) cDNA was produced by ligation of the signal peptide and extracellular domain of CD40L. Ligation of sCD40L encoding and human IgG Fc was performed to produce sCD40LIg and sCD40LIg cDNA was separated by agarose gel electrophoresis. Then, sCD40LIg cDNA encoding sCD40LIg was inserted into the plasmid pAdShuttle-IRES2-CTLA4Ig (internal ribosome entry site, IRES), which was a gift from Dr. HE Wei-feng, to produce the pAd-sCD40LIg-IRES2-CTLA4Ig. Homologous recombination of the linearized pAd-sCD40LIg-IRES2-CTLA4Ig and pAdEasy-1 vector (Stratagene, La Jolla, CA, USA) was performed in electrocompetent E. coli BJ5183 cells (Stratagene) by electroporation (2500 V, 200 Ohms, 25 μFD). The resultant plasmids were linearized with PacI and then transfected into the adenovirus packaging cell line AD-293 using Dosper liposome (Clontech, Palo Alto, CA, USA) to observe the cytopathic effect (CPE). AD-293 cells are human embryonic kidney cells, in which E1a proteins are provided in trans, allowing the production of infectious virus particles when cells are transfected with E1-deleted adenovirus vectors such as the pAdEasy-1 vector. CPE is characterized by cells rounding up and detaching from the plate, with the nucleus occupying a major part of the cell due to the high level of virus production.
Western blotting
Whole-cell extracts obtained from Ad-sCD40LIg-IRES2-CTLA4Ig-infected and Ad-Shuttle-CMV-infected 293 cells were fractionated by 8% SDS-PAGE and transferred to cellulose nitrate membrane (Ad-Shuttle-CMV is a gift from Dr. HE Wei-feng). After blocking, the membranes were incubated at 4 °C overnight in Tris-buffered saline (TBS: 50 mmol/L Tris-HCl, 150 mmol/L NaCl) containing a 1:1000 dilution of rabbit-anti-human CD40L antibody (Santa Cruz, California, CA, USA) and rabbit-anti-human CTLA4 antibody (Santa Cruz) and then incubated for 1 h at room temperature in TBS containing a 1:2000 anti-rabbit IgG antibody conjugated horseradish peroxidase (Santa Cruz). Immunoreactive bands were visualized by incubation with LumiGLO (Cell Signaling Tech, Beverly, MA, USA) and exposure to light-sensitive film.
Confocal laser scanning microscopy
As the kidney is one of the most important organs associated with transplantation in clinical therapy and the adenovirus constructed in the present study may be applied to renal transplantation, Human kidney-2 cells (HK-2 cells), which are immortalized human proximal tubular cells, were transfected (ATCC, Rockville, MA, USA) with Ad-sCD40LIg-IRES2-CTLA4Ig in Dulbecco’s modified Eagle’s medium (DMEM, Gibco) with 10% fetal bovine serum (Hyclone, Logan, UT, USA) supplemented with 100 IU/ml penicillin (Gibco, Grand Island, NY, USA) and 100 μg/ml streptomycin (Gibco). HK-2 cells transfected by Ad-Shuttle-CMV were used as the vehicle control. The third day, HK-2 cells were fixed with 4% formalin for 10 min, labelled with phycoerythrin (PE) labelled goat-anti-human CD40L and fluorescent isothiocyanate (FITC) labelled goat-anti-human CTLA4Ig antibody (Invitrogen), then mounted with 60% glycerin. The co-expression of sCD40LIg and CTLA4Ig was surveyed with confocal laser scanning microscopy (Leica, TCS 4 D, Wetzlar, Germany) under conditions of 488 nm wavelength for FITC and 568 nm for PE.
Skin grafting
Inbred C57BL/6 and BALB/c mice (Chinese Academy of Medical Sciences) weighing 20~25 g were used as donors and recipients, respectively. All animals were housed under special pathogen-free conditions. There were three groups (eight mice in each group): Group 1, non-treated; Group 2, receiving Ad-Shuttle-CMV; Group 3, Ad-sCD40LIg-IRES2-CTLA4Ig. Skin grafting was performed using a method described previously (Hashimoto et al., 2002) with modification. Briefly, donors and recipients were anesthetized with intraperitoneal barbanylum (10 g/L). Full thickness donor skin (10 mm×20 mm) was harvested from the dorsal skin of C57BL/6 mice and immersed immediately into ice-cold PBS (Group 1) or 5×109 pfu adenovirus in 5 ml of DMEM with 10% fetal bovine serum (FBS) supplemented with 100 IU/ml penicillin and 100 μg/ml streptomycin (Groups 2 and 3). Four hours later, skin was transplanted into BALB/c mice with graft beds (20 mm×20 mm). Grafts were covered by bandages, which were removed on the postoperative 5th day. The condition of the skin allografts was evaluated from day 5 to rejection. Grafts were considered rejected when 80% or more of the graft was altered in color and consistency.
RT-PCR analysis in the skin allografts
Total RNA was extracted from frozen skin allografts at 1st day, 3rd day, 5th day and 8th day after skin grafting and RT-PCR was performed to assess the sCD40LIg and CTLA4Ig mRNA expression according to the method described above. RNA from the skin of untransfected BALB/c mice was used as a control. The oligonucleotide primers were as follows: sCD40LIg: forward: 5′-TTT AGA TCT ACC ATG GGT CTA CTG CTC ACA CA-3′, reverse: 5′-CTA GAA GCA TCC TCG AGC GAC CG-3′; CTLA4: forward: 5′-AAC ATA TGC AGT GGC CAG CCT GCT GTG-3′, reverse: 5′-GCG GAT CCT TAG TCA GAA TCT GGG CAC GGT TCT GG-3′. All above-mentioned primers were from TaKaRa (BioTech, Liaoning, Dalian, China). The RT-PCR products were then separated by agarose gel electrophoresis.
Statistical analysis
Data were expressed as the mean±SE. Skin graft survival was evaluated by Kaplan-Meier analysis and log-rank tests. P-values less than 0.05 were considered significant (SPSS statistical software, Version 10, USA).
RESULTS
Identification of Ad-sCD40LIg-IRES2-CTLA4Ig
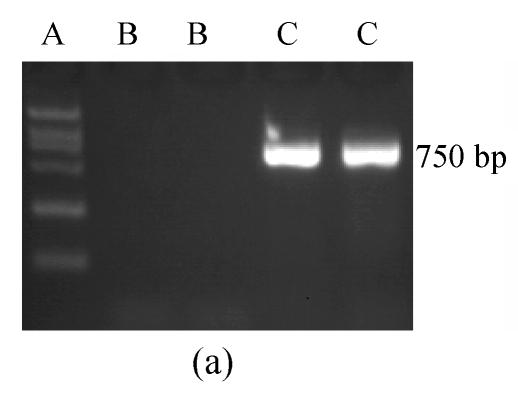
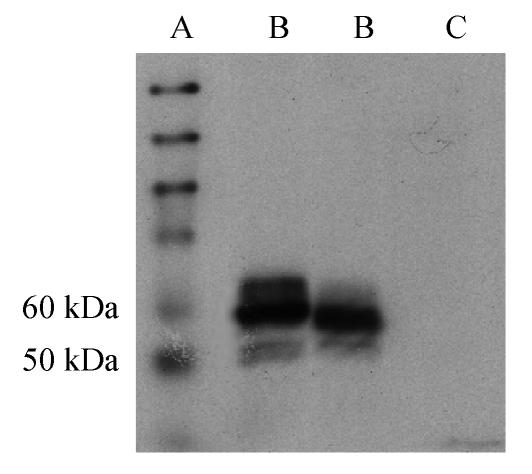
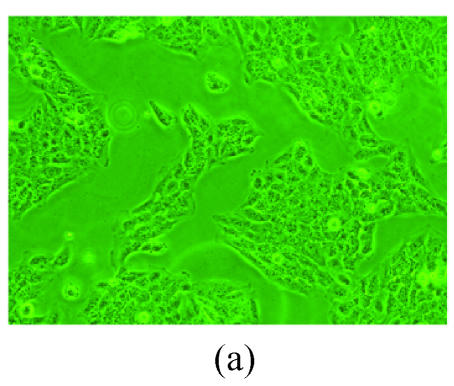
The RT-PCR amplification product of CD40L of 750 bp and the ligation product sCD40LIg of the sCD40L and IgG Fc were separated by agarose gel electrophoresis. There was no band in the controls without mRNA or ligation product (Fig.1). The sCD40LIg product of 60 kDa and CTLA4Ig product of 50 kDa were separated by 8% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (Fig.2) in the Western blotting analysis. The construction of Ad-sCD40LIg-IRES2-CTLA4Ig was confirmed via identification with agarose gel electrophoresis and Western blotting analysis. The CPE was observed in the 7th day after the 293 cells were transfected with Ad-sCD40LIg-IRES2-CTLA4Ig. Compared to the untreated 293 cells, the 293 cells transfected by replication-defective adenovirus became larger in the nucleus, more round in shape, and detached from the plate (Fig.3).
Fig. 1.
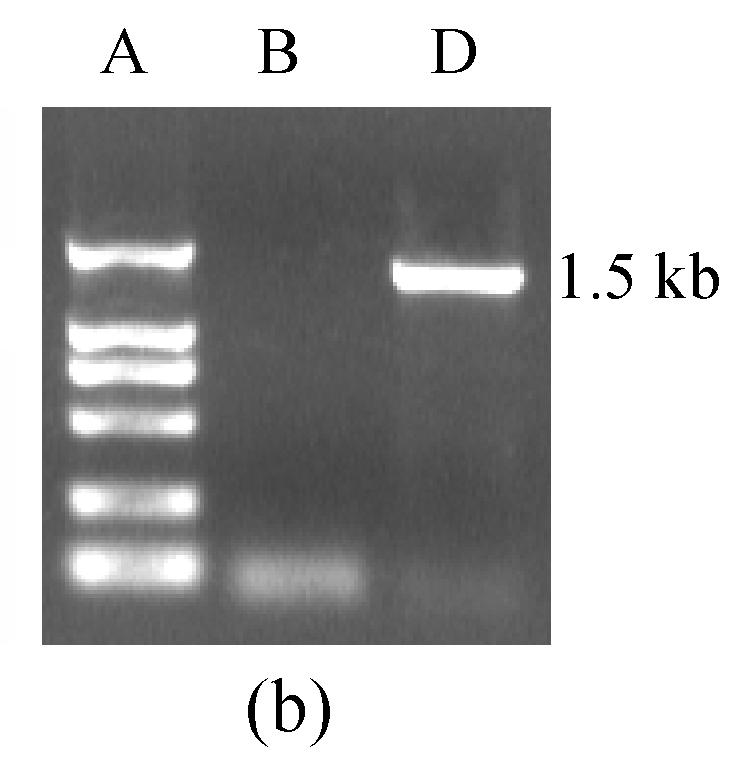
(a) The RT-PCR amplification products of CD40L of 750 bp, and (b) the ligation product sCD40LIg of the sCD40L and IgG Fc
A: DL2000 marker; B: Control; C: CD40L; D: sCD40LIg
Fig. 2.
Western blotting showing expression of sCD40LIg (60 kDa, upper bands in Lanes 2 and 3) and CTLA4Ig (50 kDa, lower bands in Lanes 2 and 3). No band was observed in the vehicle control (Lane 4)
A: Protein marker; B: Ad-sCD40LIg-IRES2-CTLA4Ig; C: Vehicle control
Fig. 3.
The CPE of the 293 cells transfected by adenovirus. Compared to the untreated 293 cells (a), the 293 cells transfected by Ad-sCD40LIg-IRES2-CTLA4Ig (b) became larger in the nucleus, more round in shape, and detached from the plate (200×). Scale bar=20 μm
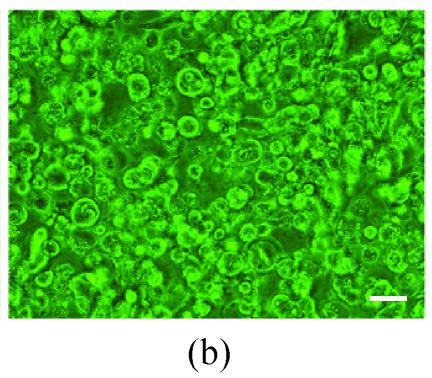
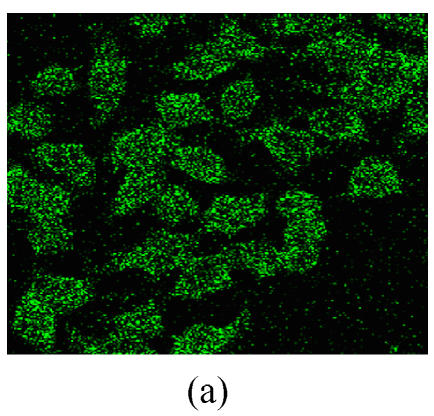
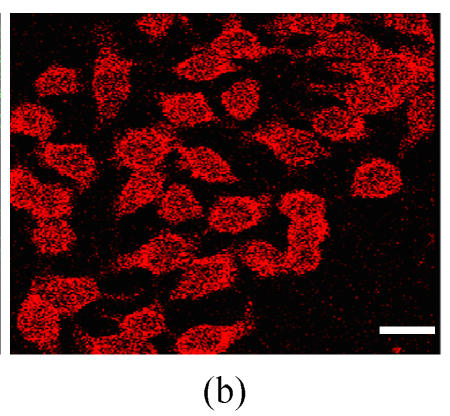
Co-expression of sCD40LIg and CTLA4Ig
The co-expression of the sCD40LIg and CTLA4Ig was examined in HK-2 cells by detecting the green and red fluorescence. The expression of sCD40LIg and CTLA4Ig was mainly in the cell membrane and cytoplasm. The expression locations of the two proteins were similar (Fig.4). There was no expression of the sCD40LIg or CTLA4Ig in the HK-2 cells transfected by Ad-Shuttle-CMV (data not shown).
Fig. 4.
The co-expression of sCD40LIg and CTLA4Ig in HK-2 cells. Co-expression of sCD40LIg (a) and CTLA4Ig (b) was detected with confocal laser scanning microscope (400×). Scale bar=20 μm
Skin allograft survival and mRNA expression of both genes
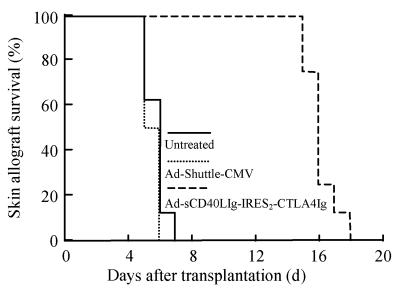
Compared to the skin graft MST of Group 1 or Group 2 ((5.75±0.71) and (5.50±0.53) d, respectively), simultaneous blockade of both costimulatory signals by Ad-sCD40LIg-IRES2-CTLA4Ig dramatically prolonged the skin allograft MST up to (16.38±1.19) d (P<0.001) (Fig.5). Analysis of sCD40LIg and CTLA4Ig mRNA in the skin allograft showed that adenovirus-mediated gene expression was optimal in the 3rd~5th day after grafting. However, the level of CTLA4Ig gene expression decreased 8 d after transplantation and expression of sCD40LIg was not detected after day 5 (Fig.6).
Fig. 5.
Kaplan-Meier curve of skin allograft survival in mice. Skin graft MST was prolonged by Ad-sCD40LIg-IRES2-CTLA4Ig to (16.38±1.19) d as compared to control, P<0.001
Fig. 6.
The mRNA expression of sCD40LIg and CTLA4Ig in the skin allograft. The sCD40LIg (upper panel) and CTLA4Ig (lower panel) mRNA expression was examined on the 1st, 3rd, 5th, 8th day after grafting. The mRNA expression also was examined in the skin of the untransfected BALB/c mouse as control
Lane 1: 1st day; Lane 2: 3rd day; Lane 3: 5th day; Lane 4: 8th day; Lane 5: Untransfected
DISCUSSION
The co-expression of two proteins coded by different genes via IRES (internal ribosome entry site) was identified in our study. When the IRES is located between two different genes, the first gene initiates its translation depending on catabolite gene activator protein (CAP), whereas the translation initiation of the second gene is IRES-dependent (Molla et al., 1992; Mountford and Smith, 1995). In some cases, the co-expression of two (Gurtu et al., 1996) or three genes (Li and Zhang, 2004) was simultaneously successful. In our study, the sCD40LIg and CTLA4Ig proteins were expressed simultaneously, but a different study (Yu et al., 2003) indicated that when each transgene was under the independent control of one internal promoter, the co-expression of the two separate genes is more efficient than that mediated by IRES. Therefore, promotion of the co-expression efficiency of two genes ligated by IRES is a problem to be addressed in future studies.
Increasing recent years evidence showed that replication-defective adenovirus without E1a and E3 domains may serve as a useful vector for gene transduction. The advantages of this vector may include the following: the procedure and techniques for construction of this viral vector are straightforward; the vector can carry long exogenous DNA fragments; infect more cell types at higher infectious rate. In addition, there is less risk of inducing alteration of the host DNA since the infection cannot interfere with the host genome (Kanaya et al., 2003). However, the application of the gene expression mediated by replication-defective adenovirus is hindered by the short expression period. The susceptibility of different cell types to adenoviral infection is often decided by the availability of the coxsackievirus and adenovirus receptor (CAR) (Walters et al., 1999), as well as αvβ3 and αvβ5 integrins, cellular receptors for adenovirus internalization (Chen et al., 2002). Adenovirus vectors can efficiently infect a wide range of cell types with the exception of some celltypes with low expression level of CAR, such as T cells (Chen et al., 2002), and human dendritic cells (DC) (Tillman et al., 1999) and murine DC (Okada et al., 2003). Further experiments will be necessary to confirm the distribution of the CAR and αvβ3 and αvβ5 integrins in the skin allograft.
In addition to receptors, the immune response also plays a crucial role in the gene expression mediated by adenovirus. Specific immune responses directed against viral proteins and some transgene products cause the complete elimination of the transduced cells within a few weeks after vector administration (Harvey et al., 1999). The immunities induced by adenovirus-mediated gene expression are both cellular and humoral. Cellular immunity results in the elimination of transduced cells or in depletion of vector, and humoral immunity prevents successful readministration of vector. Control of adenoviral antigen specific cellular immune responses will be critical to successful gene therapy if long-term transgene expression is required (Lochmüller et al., 1996; Guibinga et al., 1998; Jiang et al., 2001). The macrophages in the liver and spleen constitute the first barrier of defense against blood-borne pathogens (Worgall et al., 1997). A study showed that depletion of macrophages from spleen and liver decreases hepatic inflammation, significantly prolongs transgene expression, and delays the onset of humoral immune responses after systemic administration of an adenovirus vector (Kuzmin et al., 2001). In our study, the non-vascular administration of vector may avoid the phagocytosis by macrophages mainly located in the liver and the spleen. Therefore, the gene expression period should be prolonged to benefit the allograft survival. However, our study showed that the mRNA expression of sCD40LIg gene just lasted for about 5 d and the mRNA expression of CTLA4Ig gene got weaker 5 d later, which may be the result of the low efficiency of infection due to the non-vascular administration way of vector under the low temperature condition.
During antigen presentation, one of the most important costimulatory pathways is the interaction of CD40L on activated T lymphocytes and CD40 on macrophages, dendritic cells (DCs), and B cells. Interfering with CD40/CD40L binding prevents the activation and maturation of macrophages and DCs (Mackey et al., 1998), and prevents isotype switching and generation of memory B cells (Cerutti et al., 1998). CTLA4, another important costimulatory molecule, inhibits T-cell activation by downregulating the interaction between CD28 on T cells and B7 on antigen presenting cells. CTLA4Ig results in systemic immunosuppression by downregulating CD28-dependent T-cell activation and proliferation, and IL-2 production (Walunas et al., 1996; Krummel and Allison, 1996). The potential benefit of the blockade of the B7/CD28 and CD40/CD40L interactions includes nontoxic, transient immune tolerance specific to those adenoviral antigens required for successful gene transfer. Many studies revealed that the blockade of CD40 signaling caused the elimination of activated B cells (Sakata et al., 2000; Kuss et al., 1999). A study has reported that antiviral cytolytic T lymphocyte (CTL) responses against recombinant adenovirus were strongly reduced in CD40L-deficient mice or in mice treated with anti-CD40L antibodies (Whitmire et al., 2000; Andreasen et al., 2000). Macrophage depletion in combination with temporary blockade of CD40/CD40L completely stabilized transgene expression and inhibited the production of neutralizing anti-adenovirus antibodies, permitted successful vector readministration via reducing the pool of proinflammatory cytokines and the immediate inflammatory responses induced by adenovirus vectors. This in turn prevented the rapid destruction of highly transduced cells, thereby permitting higher initial levels of transgene production (Kuzmin et al., 2001). Another report showed that a high-capacity adenovirus expressing CTLA4Ig prolonged transgene expression in skeletal muscle and showed low levels of infiltrating CD8+ and CD4+ T cells. In contrast, a control vector without CTLA4Ig administration to skeletal muscle resulted in the accumulations of both CD4+ and CD8+ T cells 30 d posttreatment (Jiang et al., 2002). Guo et al.(2003) demonstrated that AdCTLA4Ig combined with anti-ICOS antibody potently induced a stable immune tolerance after heart allografting in rat, which is mediated by the induction of CD4+CD25+ regulatory T cells. Blockade of the CD40/CD40L and B7/CD28 costimulatory pathways by anti-CD40L antibody and soluble CTLA4Ig protein, respectively, has been used to abolish adenovirus-specific B-cell functions and severely compromise T-cell responses, prolong transgene expression, and allow efficient readministration in liver, lung, brain, and muscle (Chirmule et al., 2000; Guerette et al., 1996; Guibinga et al., 1998; Ideguchi et al., 1999; Jooss et al., 1998; Kay et al., 1995; 1997; Wilson et al., 1998; Yang et al., 1996). A bicistronic vector expressing both CTLA4Ig and CD40Ig has been observed to provide better immune suppression than vectors expressing either protein alone (Jiang et al., 2001). In the present study, CTLA4Ig and sCD40LIg were simultaneously expressed via replication-defective with IRES to block the B7/CD28 and CD40/CD40L pathways. We can speculate that the adenovirus-mediated CTL and immune responses against viral proteins or transgene products are reduced in this study. In turn, the expression of the CTLA4Ig and sCD40LIg can prolong the corresponding gene expression mediated by adenovirus. One interpretation of this difference between the theoretical period and the short time (about 8 d) of the mRNA expression is that the non-vascular administration of the adenovirus vector affected the infection efficacy.
Additional blockade of CD40/CD40L facilitates the ability of CTLA4Ig-modified dendritic cells to induce immune tolerance (Sun et al., 2003). When the B7/CD28 and CD40/CD40L pathways were blocked simultaneously with other techniques, the survival of grafts was significantly prolonged (Saito et al., 1998; Safley et al., 2005; Yamashita et al., 2003; Benda et al., 2002). Consistent with that finding, our results showed that simultaneous blockade of both pathways via co-expression of sCD40LIg and CTLA4Ig mediated by replication-defective adenovirus dramatically prolonged the skin graft survival in mice. We think that the prolongation of the skin allograft in mice is due to the immune suppression by sCD40LIg and CTLA4Ig. However, we did not evaluate the effect of the adenovirus on the normal tissue or organ function. The safety of the replication-defective adenovirus will be investigated in future.
CONCLUSION
In the present study, we successfully constructed adenovirus-carrying genes encoding both sCD40LIg and CTLA4Ig. This provides an important tool for using adenovirus for gene therapy in vivo. Compared to the vehicle control, our study indicated the simultaneous blockade of both costimulatory signals significantly prolonged the graft survival. It suggests that simultaneous blockade of the CD40/CD40L and B7/CD28 costimulatory pathways via co-expression of sCD40LIg and CTLA4Ig mediated by replication-defective adenovirus is an acceptable method to induce immune tolerance.
Acknowledgments
The authors gratefully acknowledge invaluable support of Dr. HE Wei-feng for constructing the adenovirus vector.
Footnotes
Project (No. 30371416) supported by the National Natural Science Foundation of China
References
- 1.Akalin E, Chandraker A, Russell ME, Turka LA, Hancock WW, Sayegh MH. CD28-B7 T cell costimulatory blockade by CTLA4Ig in the rat renal allograft model: inhibition of cell-mediated and humoral immune responses in vivo. Transplantation. 1996;62(12):1942–1945. doi: 10.1097/00007890-199612270-00047. [DOI] [PubMed] [Google Scholar]
- 2.Andreasen SO, Christensen JE, Marker O, Thomsen AR. Role of CD40 ligand and CD28 in induction and maintenance of antiviral CD8+ effector T cell responses. J Immunol. 2000;164(7):3689–3697. doi: 10.4049/jimmunol.164.7.3689. [DOI] [PubMed] [Google Scholar]
- 3.Benda B, Ljunggren HG, Peach R, Sandberg JO, Korsgren O. Co-stimulatory molecules in islet xenotransplantation: CTLA4Ig treatment in CD40 ligand-deficient mice. Cell Transplant. 2002;11(7):715–720. doi: 10.3727/000000002783985440. [DOI] [PubMed] [Google Scholar]
- 4.Bolling SF, Lin H, Wei RQ, Turka LA. Preventing allograft rejection with CTLA4Ig: effect of donor-specific transfusion route or timing. J Heart Lung Transplant. 1996;15(9):928–935. [PubMed] [Google Scholar]
- 5.Cerutti A, Zan H, Schaffer A, Bergsagel L, Harindranath N, Max EE, Casali P. CD40 ligand and appropriate cytokines induce switching to IgG, IgA, and IgE and coordinated germinal center and plasmacytoid phenotypic differentiation in a human monoclonal IgM+ IgD+B cell line. J Immunol. 1998;160(5):2145–2157. [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Z, Ahonen M, Hämäläinen H, Bergelson JM, Kähäri VM, Lahesmaa R. High-efficiency gene transfer to primary T lymphocytes by recombinant adenovirus vectors. J Immunol Methods. 2002;260(1-2):79–89. doi: 10.1016/S0022-1759(01)00521-X. [DOI] [PubMed] [Google Scholar]
- 7.Chirmule N, Raper SE, Burkly L, Thomas D, Tazelaar J, Hughes JV, Wilson JM. Readministration of adenovirus vector in nonhuman primate lungs by blockade of CD40-CD40 ligand interactions. J Virol. 2000;74(7):3345–3352. doi: 10.1128/JVI.74.7.3345-3352.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corbascio M, Ekstrand H, Osterholm C, Qi Z, Simanaitis M, Larsen CP, Pearson TC, Riesbeck K, Ekberg H. CTLA4Ig combined with anti-LFA-1 prolongs cardiac allograft survival indefinitely. Transpl Immunol. 2002;10(1):55–61. doi: 10.1016/S0966-3274(02)00014-X. [DOI] [PubMed] [Google Scholar]
- 9.Gao YH, Wang P, Takagi K, Shimozato O, Yagita H, Okigaki T, Matasumura M. Expression of a soluble form of CTLA4 on macrophage and its biological activity. Cell Research. 1999;9(3):189–199. doi: 10.1038/sj.cr.7290017. [DOI] [PubMed] [Google Scholar]
- 10.Guerette B, Vilquin JT, Gingras M, Gravel C, Wood KJ, Tremblay JP. Prevention of immune reactions triggered by first-generation adenoviral vectors by monoclonal antibodies and CTLA4Ig. Hum Gene Ther. 1996;7(12):1455–1463. doi: 10.1089/hum.1996.7.12-1455. [DOI] [PubMed] [Google Scholar]
- 11.Guibinga GH, Lochmuller H, Massie B, Nalbantoglu J, Karpati G, Petrof BJ. Combinatorial blockade of calcineurin and CD28 signaling facilitates primary and secondary therapeutic gene transfer by adenovirus vectors in dystrophic (mdx) mouse muscles. J Virol. 1998;72(6):4601–4609. doi: 10.1128/jvi.72.6.4601-4609.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guillot C, Guillonneau C, Mathieu P, Gerdes CA, Menoret S, Braudeau C, Tesson L, Renaudin K, Castro MG, Lowenstein PR, et al. Prolonged blockade of CD40-CD40 ligand interactions by gene transfer of CD40Ig results in long-term heart allograft survival and donor-specific hyporesponsiveness, but does not prevent chronic rejection. J Immunol. 2002;168(4):1600–1609. doi: 10.4049/jimmunol.168.4.1600. [DOI] [PubMed] [Google Scholar]
- 13.Guo L, Fujino M, Kimura H, Funeshima N, Kitazawa Y, Harihara Y, Tezuka K, Makuuchi M, Suzuki S, Li XK. Simultaneous blockade of co-stimulatory signals, CD28 and ICOS, induced a stable tolerance in rat heart transplantation. Transpl Immunol. 2003;12(1):41–48. doi: 10.1016/S0966-3274(03)00016-9. [DOI] [PubMed] [Google Scholar]
- 14.Gurtu V, Yan GC, Zhang GH. IRES bicistronic expression vectors for efficient creation of stable mammalian cell lines. Biochem Biophys Res Commun. 1996;229(1):295–298. doi: 10.1006/bbrc.1996.1795. [DOI] [PubMed] [Google Scholar]
- 15.Harvey BG, Hackett NR, El-Sawy T, Rosengart TK, Hirschowitz EA, Lieberman MD, Lesser ML, Crystal RG. Variability of human systemic humoral immune responses to adenovirus gene transfer vectors administered to different organs. J Virol. 1999;73(8):6729–6742. doi: 10.1128/jvi.73.8.6729-6742.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashimoto N, Narumi S, Itabashi Y, Hakamada K, Sasaki M. Efficacy of donor splenocytes mixed with bone marrow cells for induction of tolerance in sublethally irradiated mice. Transpl Immunol. 2002;10(1):37–41. doi: 10.1016/S0966-3274(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 17.Ideguchi M, Kajiwara K, Yoshikawa K, Uchida T, Ito H. Local adenovirus-mediated CTLA4-immunoglo-bulin expression suppresses the immune responses to adenovirus vectors in the brain. Neuroscience. 1999;95(1):217–226. doi: 10.1016/S0306-4522(99)00402-9. [DOI] [PubMed] [Google Scholar]
- 18.Jiang ZL, Reay D, Kreppel F, Gambotto A, Feingold E, Kochanek S, McCarthy SA, Clemens PR. Local high-capacity adenovirus-mediated mCTLA4Ig and mCD40Ig expression prolongs recombinant gene expression in skeletal muscle. Mol Ther. 2001;3(6):892–900. doi: 10.1006/mthe.2001.0331. [DOI] [PubMed] [Google Scholar]
- 19.Jiang Z, Feingold E, Kochanek S, Clemens PR. Systemic delivery of a high-capacity adenoviral vector expressing mouse CTLA4Ig improves skeletal muscle gene therapy. Mol Ther. 2002;6(3):369–376. doi: 10.1006/mthe.2002.0676. [DOI] [PubMed] [Google Scholar]
- 20.Jooss K, Turka LA, Wilson JM. Blunting of immune responses to adenoviral vectors in mouse liver and lung with CTLA4Ig. Gene Ther. 1998;5(3):309–319. doi: 10.1038/sj.gt.3300595. [DOI] [PubMed] [Google Scholar]
- 21.Kanaya K, Tsuchida Y, Inobe M, Murakami M, Hirose T, Kon S, Kawaguchi S, Wada T, Yamashita T, Ishii S, et al. Combined gene therapy with adenovirus vectors containing CTLA4Ig and CD40Ig prolongs survival of composite tissue allografts in rat model. Transplantation. 2003;75(3):275–281. doi: 10.1097/01.TP.0000046966.35399.75. [DOI] [PubMed] [Google Scholar]
- 22.Kawai T, Sogawa H, Boskovic S, Abrahamian G, Smith RN, Wee SL, Andrews D, Nadazdin O, Koyama I, Sykes M, et al. CD154 blockade for induction of mixed chimerism and prolonged renal allograft survival in nonhuman primates. Am J Transplant. 2004;4(9):1391–1398. doi: 10.1111/j.1600-6143.2004.00523.x. [DOI] [PubMed] [Google Scholar]
- 23.Kay MA, Holterman AX, Meuse L, Gown A, Ochs HD, Linsley PS, Wilson CB. Long-term hepatic adenovirus-mediated gene expression in mice following CTLA4Ig administration. Nat Genet. 1995;11(2):191–197. doi: 10.1038/ng1095-191. [DOI] [PubMed] [Google Scholar]
- 24.Kay MA, Meuse L, Gown AM, Linsley P, Hollenbaugh D, Aruffo A, Ochs HD, Wilson CB. Transient immunomodulation with anti-CD40 ligand antibody and CTLA4Ig enhances persistence and secondary adenovirus-mediated gene transfer into mouse liver. Proc Natl Acad Sci USA. 1997;94(9):4686–4691. doi: 10.1073/pnas.94.9.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kita Y, Li XK, Ohba M, Funeshima N, Enosawa S, Tamura A, Suzuki K, Amemiya H, Hayashi S, Kazui T, et al. Prolonged cardiac allograft survival in rats systemically injected adenoviral vectors containing CTLA4Ig-gene. Transplantation. 1999;68(6):758–766. doi: 10.1097/00007890-199909270-00007. [DOI] [PubMed] [Google Scholar]
- 26.Kita Y, Nogimura H, Ida M, Kageyama Y, Ohi S, Ito Y, Matsushita K, Takahashi T, Suzuki K, Kazui T, et al. Combined therapy of CTLA4Ig-gene transfection with FTY720 administration in rat lung allografts. Transplant Proc. 2002;34(5):1437–1440. doi: 10.1016/S0041-1345(02)02918-4. [DOI] [PubMed] [Google Scholar]
- 27.Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med. 1996;183(6):2533–2540. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuss AW, Knodel M, Berberich-Siebelt F, Lindemann D, Schimpl A, Berberich I. A1 expression is stimulated by CD40 in B cells and rescues WEHI 231 cells from anti-IgM-induced cell death. Eur J Immunol. 1999;29(10):3077–3088. doi: 10.1002/(SICI)1521-4141(199910)29:10<3077::AID-IMMU3077>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 29.Kuzmin AI, Galenko O, Eisensmith RC. An immunomodulatory procedure that stabilizes transgene expression and permits readministration of E1-deleted adenovirus vectors. Mol Ther. 2001;3(3):293–301. doi: 10.1006/mthe.2000.0258. [DOI] [PubMed] [Google Scholar]
- 30.Laumonier T, Potiron N, Boeffard F, Chagneau C, Brouard S, Guillot C, Soulillou JP, Anegon I, Le Mauff B. CTLA4Ig adenoviral gene transfer induces long-term islet rat allograft survival, without tolerance, after systemic but not local intragraft expression. Hum Gene Ther. 2003;14(6):561–575. doi: 10.1089/104303403764539341. [DOI] [PubMed] [Google Scholar]
- 31.Li T, Zhang J. Stable expression of three genes from a tricistronic retroviral vector containing a picornavirus and 9-nt cellular internal ribosome entry site elements. J Virol Methods. 2004;115(2):137–144. doi: 10.1016/j.jviromet.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 32.Li W, Zheng XX, Kuhr CS, Perkins JD. CTLA4 engagement is required for induction of murine liver transplant spontaneous tolerance. Am J Transplant. 2005;5(5):978–986. doi: 10.1111/j.1600-6143.2005.00823.x. [DOI] [PubMed] [Google Scholar]
- 33.Lochmüller H, Petrof BJ, Pari G, Larochelle N, Dodelet V, Wang Q, Allen C, Prescott S, Massie B, Nalbantoglu J, et al. Transient immunosuppression by FK506 permits a sustained high-level dystrophin expression after adenovirus-mediated dystrophin minigene transfer to skeletal muscles of adult dystrophic (mdx) mice. Gene Ther. 1996;3(8):706–716. [PubMed] [Google Scholar]
- 34.Mackey MF, Barth RJJr, Noelle RJ. The role of CD40/CD154 interactions in the priming, differentiation, and effector function of helper and cytotoxic T cells. J Leukoc Biol. 1998;63(4):418–428. doi: 10.1002/jlb.63.4.418. [DOI] [PubMed] [Google Scholar]
- 35.Molla A, Jang SK, Paul AV, Reuer Q, Wimmer E. Cardioviral internal ribosomal entry site is functional in a genetically engineered dicistronic poliovirus. Nature. 1992;356(6366):255–257. doi: 10.1038/356255a0. [DOI] [PubMed] [Google Scholar]
- 36.Mountford PS, Smith AG. Internal ribosome entry sites and dicistronic RNAs in mammalian transgenesis. Trends in Genetics. 1995;11(5):179–184. doi: 10.1016/S0168-9525(00)89040-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohba M, Li XK, Kita Y, Tamura A, Enosawa S, Sasakuri S, Ogoshi S, Amemiya H, Suzuki S. Perioperative administration of FTY720 and CTLA4IG in rat heart transplantation. Transplant Proc. 2000;32(7):2024–2025. doi: 10.1016/S0041-1345(00)01541-4. [DOI] [PubMed] [Google Scholar]
- 38.Okada N, Masunaga Y, Okada Y, Iiyama S, Mori N, Tsuda T, Matsubara A, Mizuguchi H, Hayakawa T, Fujita T, et al. Gene transduction efficiency and maturation status in mouse bone marrow-derived dendritic cells infected with conventional or RGD fiber-mutant adenovirus vectors. Cancer Gene Ther. 2003;10(5):421–431. doi: 10.1038/sj.cgt.7700586. [DOI] [PubMed] [Google Scholar]
- 39.Pearson TC, Trambley J, Odom K, Anderson DC, Cowan S, Bray R, Lin A, Hollenbaugh D, Aruffo A, Siadak AW, et al. Anti-CD40 therapy extends renal allograft survival in rhesus macaques. Transplantation. 2002;74(7):933–940. doi: 10.1097/00007890-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 40.Perico N, Amuchastegui S, Bontempelli M, Remuzzi G. CTLA4Ig alone or in combination with low-dose cyclosporine fails to reverse acute rejection of renal allograft in the rat. Transplantation. 1996;61(9):1320–1322. doi: 10.1097/00007890-199605150-00006. [DOI] [PubMed] [Google Scholar]
- 41.Reddy B, Gupta S, Chuzhin Y, Kalergis AM, Budhai L, Zhang M, Droguett G, Horwitz MS, Chowdhury JR, Nathenson SG, et al. The effect of CD28/B7 blockade on alloreactive T and B cells after liver cell transplantation. Transplantation. 2001;71(6):801–811. doi: 10.1097/00007890-200103270-00020. [DOI] [PubMed] [Google Scholar]
- 42.Rehman A, Tu Y, Arima T, Linsley PS, Flye MW. Long-term survival of rat to mouse cardiac xenografts with prolonged blockade of CD28-B7 interaction combined with peritransplant T-cell depletion. Surgery. 1996;120(2):205–212. doi: 10.1016/s0039-6060(96)80289-3. [DOI] [PubMed] [Google Scholar]
- 43.Safley SA, Kapp LM, Tucker-Burden C, Hering B, Kapp JA, Weber CJ. Inhibition of cellular immune responses to encapsulated porcine islet xenografts by simultaneous blockade of two different costimulatory pathways. Transplantation. 2005;79(4):409–418. doi: 10.1097/01.TP.0000150021.06027.DC. [DOI] [PubMed] [Google Scholar]
- 44.Saito K, Sakurai J, Ohata J, Kohsaka T, Hashimoto H, Okumura K, Abe R, Azuma M. Involvement of CD40 ligand-CD40 and CTLA4-B7 pathways in murine acute graft-versus-host disease induced by allogeneic T cells lacking CD28. J Immunol. 1998;160(9):4225–4231. [PubMed] [Google Scholar]
- 45.Sakata N, Kawasome H, Terada N, Johnson GL, Gelfand EW. CD40 and adenosine A2 receptor agonist-cyclic adenosine monophosphate rescue B-cell antigen receptor-induced apoptosis through independent pathways and converge to prevent caspase activation. J Allergy Clin Immunol. 2000;105(3):522–531. doi: 10.1067/mai.2000.104251. [DOI] [PubMed] [Google Scholar]
- 46.Sun W, Wang Q, Zhang L, Liu Y, Zhang M, Wang C, Wang J, Cao X. Blockade of CD40 pathway enhances the induction of immune tolerance by immature dendritic cells genetically modified to express cytotoxic T lymphocyte antigen 4 immunoglobulin. Transplantation. 2003;76(9):1351–1359. doi: 10.1097/01.TP.0000083557.25887.EE. [DOI] [PubMed] [Google Scholar]
- 47.Thiel MA, Steiger JU, O′Connell PJ, Lehnert AM, Coste DJ, Williams KA. Local or short-term systemic costimulatory molecule blockade prolongs rat corneal allograft survival. Clin Experimental Ophthalmol. 2005;33(2):176–180. doi: 10.1111/j.1442-9071.2005.00974.x. [DOI] [PubMed] [Google Scholar]
- 48.Tillman BW, de Gruijl TD, Luykx-de Bakker SA, Scheper RJ, Pinedo HM, Curiel TJ, Gerritsen WR, Curiel DT. Maturation of dendritic cells accompanies high-efficiency gene transfer by a CD40-targeted adenoviral vector. J Immunol. 1999;162(11):6378–6383. [PubMed] [Google Scholar]
- 49.Tu Y, Rehman A, Flye MW. Prolongation of rat to mouse skin and heart xenograft survival by combined CTLA4Ig and anti-CD4/CD8 antibody. Transplant Proc. 1996;28(4):2061–2062. [PubMed] [Google Scholar]
- 50.Walters RW, Grunst T, Bergelson JM, Finberg RW, Welsh MJ, Zabner J. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J Biol Chem. 1999;274(15):10219–10226. doi: 10.1074/jbc.274.15.10219. [DOI] [PubMed] [Google Scholar]
- 51.Walunas TL, Bakker CY, Bluestone JA. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med. 1996;183(6):2541–2550. doi: 10.1084/jem.183.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whitmire JK, Murali-Krishna K, Altman J, Ahmed R. Antiviral CD4 and CD8 T-cell memory: differences in the size of the response and activation requirements. Philos Trans R Soc Lond B Biol Sci. 2000;355(1395):373–379. doi: 10.1098/rstb.2000.0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilson CB, Embree LJ, Schowalter D, Albert R, Aruffo A, Hollenbaugh D, Linsley P, Kay MA. Transient inhibition of CD28 and CD40 ligand interactions prolongs adenovirus-mediated transgene expression in the lung and facilitates expression after secondary vector administration. J Virol. 1998;72(9):7542–7550. doi: 10.1128/jvi.72.9.7542-7550.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Worgall S, Wolff G, Falck-Pedersen E, Crystal RG. Innate immune mechanisms dominate elimination of adenoviral vectors following in vivo administration. Hum Gene Ther. 1997;8(1):37–44. doi: 10.1089/hum.1997.8.1-37. [DOI] [PubMed] [Google Scholar]
- 55.Yamashita K, Masunaga T, Yanagida N, Takehara M, Hashimoto T, Kobayashi T, Echizenya H, Hua N, Fujita M, Murakami M, et al. Long-term acceptance of rat cardiac allografts on the basis of adenovirus mediated CD40Ig plus CTLA4Ig gene therapies. Transplantation. 2003;76(7):1089–1096. doi: 10.1097/01.TP.0000085651.20586.30. [DOI] [PubMed] [Google Scholar]
- 56.Yang Y, Su Q, Grewal IS, Schilz R, Flavell RA, Wilson JM. Transient subversion of CD40 ligand function diminishes immune responses to adenovirus vectors in mouse liver and lung tissues. J Virol. 1996;70(9):6370–6377. doi: 10.1128/jvi.70.9.6370-6377.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu X, Zhan X, D′Costa J, Tanavde VM, Ye Z, Peng T, Malehorn MT, Yang X, Civin CI, Cheng L. Lentiviral vectors with two independent internal promoters transfer high-level expression of multiple transgenes to human hematopoietic stem-progenitor cells. Mol Ther. 2003;7(6):827–838. doi: 10.1016/S1525-0016(03)00104-7. [DOI] [PubMed] [Google Scholar]