Abstract
Ovarian steroids, estrogen and progesterone, influence the sensitivity of certain neural processes to cannabinoid treatment by modulation of brain dopaminergic activity. We examined the effects of the active ingredient of cannabis, Δ9-tetrahydrocannabinol (THC), on sexual behavior in female rats and its influence on steroid hormone receptors and neurotransmitters in the facilitation of sexual receptivity. Our results revealed that the facilitatory effect of THC was inhibited by antagonists to both progesterone and dopamine D1 receptors. To test further the idea that progesterone receptors (PR) and/or dopamine receptors (D1R) in the hypothalamus are required for THC-facilitated sexual behavior in rodents, antisense and sense oligonucleotides to PR and D1R were administered intracerebroventricularly (ICV) into the third cerebral ventricle of ovariectomized, estradiol benzoate-primed rats. Progesterone- and THC-facilitated sexual behavior was inhibited in animals treated with antisense oligonucleotides to PR or to D1R. Antagonists to cannabinoid receptor-1 subtype (CB1), but not to cannabinoid receptor-2 subtype (CB2) inhibited progesterone- and dopamine-facilitated sexual receptivity in female rats. Our studies indicate that THC acts on the CB1 cannabinoid receptor to initiate a signal transduction response that requires both membrane dopamine and intracellular progesterone receptors for effective induction of sexual behavior.
Keywords: transcription factors, cannabinoids, signal transduction cross talk
The major psychoactive cannabinoid in marijuana, Δ9-tetrahydrocannabinol (THC), alters many reproductive parameters in both male and female laboratory animals and humans (1). In males, THC has been reported to reduce testosterone concentrations in the plasma (2, 3), suppress spermatogenesis, reduce the weight of testes and accessory reproductive organs (4), and decrease components of male sexual behavior (5–7). In the females, THC has been demonstrated to prolong estrous cycle of rats (8), decrease proestrous luteinizing hormone (LH) surge leading to inhibition of ovulation (9, 10), and stimulate sexual behavior (11, 12). THC-mediated effects on central dopaminergic systems lead to increased extracellular dopamine concentration (13). These effects on brain dopaminergic activity vary as a function of the gonadal status of the animal (14). Furthermore, ovarian sex steroid hormones also alter the density and affinity of cannabinoid (CB) receptors in steroid-sensitive brain areas, suggesting that ovarian steroid hormones could alter the sensitivity of certain neuronal processes to THC treatment.
Ovarian steroid hormones, estrogen (E) and progesterone (P), regulate cellular functions in the brain that control sexual behavior in the female rat (15, 16). Estrogen priming causes an increase in progesterone receptor (PR) levels in the hypothalamus and preoptic areas of the rat brain (17). We and others previously have demonstrated a critical role for hypothalamic PRs in the induction of sexual behavior by using PR antagonists and antisense oligonucleotides (18–21) in female rats. We also reported that neurotransmitters such as dopamine, acting by means of D1 subtype receptors, could activate PRs by a ligand-independent mechanism (19), thus providing evidence for the existence of crosstalk between steroid hormone- and neurotransmitter-initiated pathways in sexual behavior in female rats and mice (22, 23). Because E and P modulate THC effects on CB receptors and brain dopaminergic activity in female rats (24), it is possible that steroid hormone receptors mediate certain neurobehavioral effects of THC. The present study was designed to examine whether crosstalk exists between THC and steroid hormone-mediated pathways. To this end, we used the rat reproductive behavior model to examine the interactions between these pathways. In this paper, we first present evidence for the facilitatory effects of THC on sexual receptivity in female rats. The CB receptor-1 subtype (CB1) receptors and not CB2 receptors mediate the facilitatory effects of THC. We then demonstrate the requirement of not only PRs but also of dopamine D1B subtype receptors for THC-facilitated sexual receptivity in female rats. These experiments substantiate the requirement for crosstalk between multiple signal transduction pathways to achieve integration of neural communication in the neuroendocrine system.
Materials and Methods
Ovariectomized Sprague–Dawley rats (160–180 g body weight) were commercially obtained from the supplier (Harlan, Houston). The animals were maintained on a 12:12 h reversed light cycle with lights off at 1200 h and food and water available ad libitum and were in compliance with federal guidelines for animal care. All steroids were purchased from Sigma. Other compounds were obtained from Research Biochemicals (Natick, MA); THC was from National Institute for Drug Abuse (NIDA), and the cannabinoid receptor antagonists, SR 141716A (CB1) and SR 144528 (CB2), were generously provided by NIDA. PR antagonist RU 38486 was obtained from Roussal Uclaf (Paris), and ZK 99299 was obtained from Schering, AG (Berlin). Oligonucleotides were synthesized by Sigma–Genosys (The Woodlands, TX).
Behavioral Testing Procedures.
All behavior tests were conducted during the dark phase of the reversed light–dark cycle. A week after their arrival, the animals were administered hormones and tested for sex behavior. All hormones were dissolved in sesame oil and were injected s.c. unless specified otherwise. Estradiol benzoate (EB; 2 μg) was administered 48 h before 100 μg P, and behavioral testing was done in a 50 × 45 × 24-cm polystyrene arena in the presence of sexually active males 4 h later. Receptive behavior of the female rat was evaluated as the lordosis response of the female rat upon mounting by the male rat. Lordosis response of each female was observed for 10 mounts by the male, scored, and recorded as published (19, 22). A lordosis quotient (LQ), calculated as a percentage of the total number of lordosis responses by the total number of mounts, was used as a measure of sexual receptivity. All animals, which exhibited high levels of lordosis, were used in the experiments. The observer was blind to the treatment conditions.
Stereotaxic Surgery and Central Administration of Compounds.
A stainless steel cannula (23 gauge) was implanted adjacent to the ventro medial nucleus of the hypothalamus, into the third cerebral ventricle of anesthetized female rats by using a Lab Standard stereotaxic instrument (Stoelting, Wood Dale, IL). The coordinates used for the third ventricle were: anteroposterior (AP), bregma, 3.3 mm; lateral, just on the midline (above superior longitudinal sinus) and vertical, 8.5 mm. The procedure for cannulation was similar to that described by Mani et al. (19, 22). Animals were allowed to recover from surgery for 1 wk before use in experiments.
Dose–Response with THC.
Cannulated animals were primed with EB (2 μg; s.c.). Forty-eight hours later, intracerebroventricular (ICV) administration of THC at varying doses (50, 100, 200, and 400 ng) was performed. Lordosis response of female rats in the presence of males was observed and recorded 30 min later and LQs were calculated. Vehicle, EB-primed, and EB + P-treatment groups were included as controls.
Administration of Antagonists to PR, D1 and CB1 and CB2.
Forty-eight hours after EB-priming (2 μg; s.c.), female rats with indwelling cannulae were given ICV injections of PR antagonist RU 38486 (2 μg) or ZK 99299 (2 μg) or D1 antagonist SCH 23390 (100 ng) or cannabinoid receptor antagonists SR 141716A (CB1; 1 ng) and SR 144528 (CB2; 1 ng). These were followed by ICV injections of P (2 μg) or THC (100 ng) 1 h later. The animals were tested and scored for THC-facilitated lordosis response 30 min after the ICV administration. Control animals received vehicle instead of test substances. The doses of the antagonists for PR and D1 and dose of P were based on our earlier published studies (22). The dose of cannabinoid receptor antagonists was based on the dose–response curves generated in the study.
Administration of Sense and Antisense Oligonucleotides.
Antisense (PRAs) and sense (PRS) phosphorothioated oligonucleotides to the PR mRNA sequence 5′-TGTTGTCCCCGCTCATGAGC-3′ were the same as described in our earlier publications (19, 20). The phosphorothioated antisense (D1As) and sense (D1S) oligonucleotides to D1A receptor were designed to the D1A receptor mRNA sequence 5′-GTGACGACAAGATGGCGTTCTTG-3′. The phosphorothioated antisense (D1BAs) and sense (D1BS) oligonucleotides to D1B receptor oligonucleotides were synthesized to the D1B mRNA sequence 5′-TCAGCGCGACATGCTGCCTC-3′.
Cannulated female rats were injected s.c. with EB (2 μg). At the same time, 4 nmol of antisense and sense phosphorothioated oligonucleotides were administered ICV into the third ventricle. The oligonucleotides were administered ICV 24 h later. Forty-eight hours after EB priming, THC (100 ng) was administered ICV and sexual behavior was observed 30 min later. Positive controls included EB-primed (2 μg) rats with indwelling cannulae that received ICV injection of P (2 μg) or THC (100 ng) 48 h later, and observation of sexual behavior at 30 min after P or THC.
Data Analysis.
Statistical analysis was done by the following methods as appropriate: For each significant ANOVA, post hoc comparisons were made by using Dunnett's method, or one-way ANOVA followed by Tukey–Kramer or Dunn's method for comparison. INSTAT software (GraphPad, San Diego) was used for statistical analysis.
Results
THC-Facilitated Lordosis Response in Female Rats: Effects of Dose–Response.
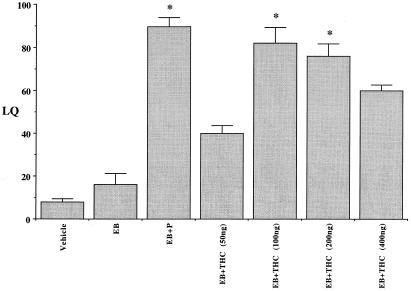
ICV administration of THC into the third cerebral ventricle of EB-primed rats facilitated a dose-dependent lordosis response within 30–60 min. A bell-shaped curve response was observed; with lower and high doses typified by lower LQs, whereas significantly higher levels of lordosis (LQ > 80) were observed at 100- to 200-ng doses (Fig. 1). The general locomotor activity remained unaffected after low doses (50–200 ng) of THC administration in EB-treated rats. However, higher doses (400 ng and above) of THC treatment rendered the animals cataleptic with reduced locomotor activity. Vehicle and EB alone were not capable of significantly inducing lordosis response. The administration of P, 48 h post EB-priming, resulted in the animals exhibiting high levels of lordosis. One-way ANOVA detected a statistically significant overall effect (P < 0.001) in THC-facilitated lordosis. The Tukey–Kramer method of multiple comparisons indicated statistically significant (P < 0.001) differences in the lordosis response between EB + THC-treated groups compared with EB treatment alone.
Figure 1.
Effect of varying doses of THC on lordosis of female rats. Ovariectomized rats with indwelling stainless steel cannulae stereotaxically implanted into the third cerebral ventricle were injected s.c. with 2 μg of EB in 0.1 ml sesame oil. Various doses of THC (50–400 ng) in saline were administered ICV 48 h later. The animals were examined for their receptive behavior in the presence of a male, 30 min after THC administration. The results of the experiments were expressed as LQ, defined as the percentage of the number of complete lordosis responses by the female divided by the number of mounts by the male. Control groups of rats received vehicle or EB or EB + P treatments. Values are represented as mean LQ (lordosis responses/mounts × 100) ± SEM (n = 6 animals for each group).
THC Facilitation of Lordosis Response Is Mediated by CB1 Receptors.
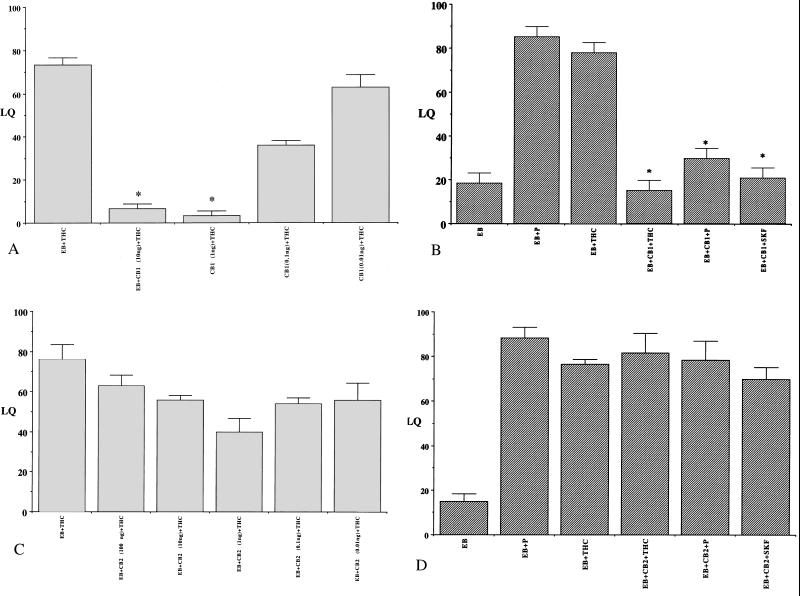
ICV administration of CB1 receptor antagonist SR 141716A significantly inhibited THC-facilitated lordosis response in EB-primed female rats (P < 0.001) in a dose-dependent manner (Fig. 2A). The antagonist also reduced P-facilitated lordosis response in EB-primed animals. ANOVA followed by post hoc Tukey–Kramer test for multiple comparisons indicated a significant reduction in THC, P, and SKF-facilitated lordosis responses of EB-primed animals that received CB1 antagonist compared with those that did not (P < 0.001; Fig. 2B). A dose–response generated by the administration of CB2 antagonist SR 144528 indicated no significant effect of the antagonist on THC-facilitated lordosis response at any of the doses administered (Fig. 2C). Fig. 2D reveals no significant differences in the THC, P, or SKF-facilitated lordosis response of EB-primed rats that received ICV administration of CB2 receptor antagonist SR 144528, compared with the controls (P > 0.05). These results suggest that an operative CB1 receptor pathway might be required for responses to progesterone or dopamine.
Figure 2.
Cannabinoid receptor antagonists on THC-facilitated lordosis response in EB-primed female rats. (A) Dose–response and (B) effects of CB1 receptor antagonist, SR 141716A (CB1); (C) dose–response and (D) effects of CB2 receptor antagonist, SR 144528 (CB2) on THC (100 ng)-facilitated lordosis response in EB-primed rats. Ovariectomized female rats with indwelling cannulae in their third ventricle were administered varying doses (as indicated) of CB1 or CB2 ICV 48 h after EB priming (2 μg, s.c.). The animals were tested for their lordosis response 30 min after THC administration, and the receptive behavior was expressed as LQ (A and C). Ovariectomized, EB-primed rats were administered (B) CB1 (1 ng) or (D) CB2 (1 ng) ICV 48 h after EB priming. P (2 μg) or THC (100 ng) or D1 receptor agonist, SKF 38393 (SKF; 100 ng) were microinjected ICV into the third cerebral ventricle 1 h later. The animals were tested for their lordosis response 30 min after the administration of the compounds, and the receptive behavior was expressed as LQ. Controls included rats that received vehicle, EB, EB + P, or EB + SKF. Values presented are means ± SEM of LQs. (*, P < 0.001); n = 6–8 animals for each group).
THC-Facilitated Lordosis Response Is Inhibited by Antagonists to PR and D1 Receptor.
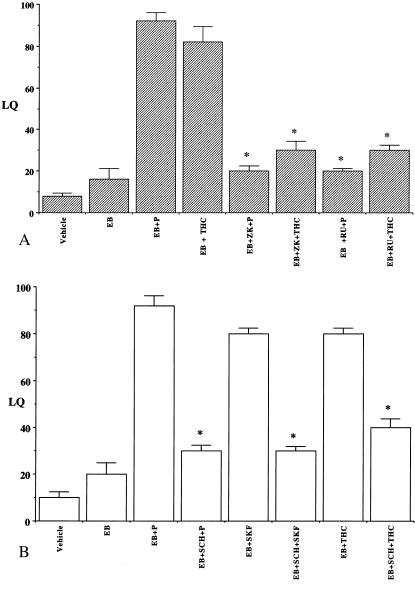
To further explore the interrelationships of the THC, progesterone, and dopamine transduction pathways, ICV injections of PR antagonists RU 486 and ZK98299 were administered 1 h before THC was given; the progestin antagonists suppressed THC-facilitated lordosis response in EB-treated female rats (Fig. 3A). A similar reduction in SKF- and THC-facilitated lordosis response was observed upon ICV administration of the D1 receptor antagonist SCH23390 in EB-primed rats (Fig. 3B). No significant lordosis responses were observed in control EB and vehicle-treated animals. One-way ANOVA followed by Dunn's test for multiple comparisons indicated a significant effect of antagonists on THC-facilitated lordosis compared with the EB + P/SKF/THC-treated controls (P < 0.001). Our results suggest that functional PR and D1 receptor also are required for THC-mediated sexual behavior.
Figure 3.
Inhibition of THC-facilitated sexual behavior in EB-primed rats by (A) progesterone and (B) dopamine receptor antagonists. Ovariectomized, EB-primed (2 μg; s.c.) rats with stainless steel cannulae in the third cerebral ventricle were used in these studies. The rats were given ICV injections of (A) progesterone antagonists, RU 38486 (RU) or ZK 98299 (ZK) or (B) D1 dopamine receptor antagonist, SCH 23390 (SCH) after 48 h. P (2 μg) or THC (100 ng) or D1 receptor agonist, SKF 38393 (SKF; 100 ng) were microinjected ICV into the third cerebral ventricle 1 h later. The animals were tested for their lordosis response 30 min after THC administration and the receptive behavior was expressed as LQ. Controls included rats that received vehicle, EB, EB + P, or EB + SKF. Values presented are means ± SEM of LQs. (*, P < 0.001); n = 6–8 animals for each group).
PR Are Involved in Mediating THC Effects on Female Sexual Behavior.
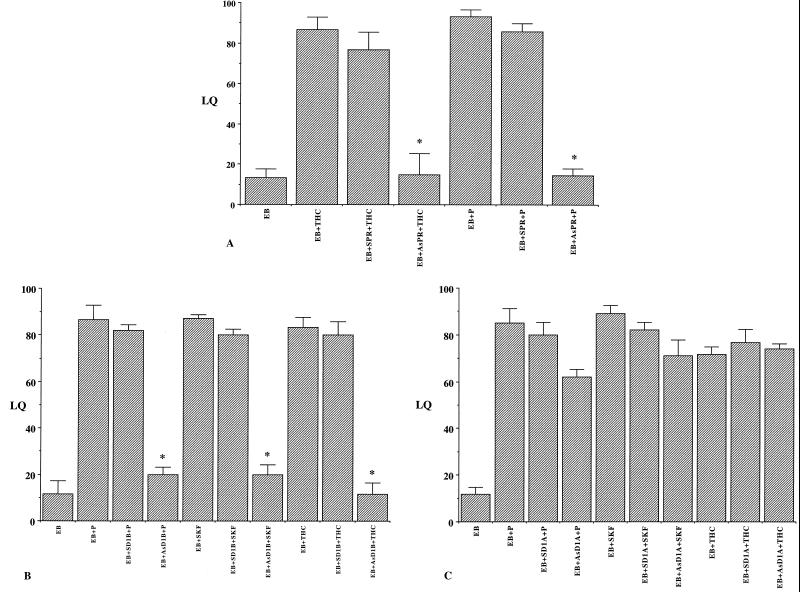
THC-facilitated lordosis was significantly reduced in EB-primed animals treated ICV with the phosphorothioated antisense oligonucleotide to rat PRmRNA (AsPR) at 4 nmol dose (Fig. 4A). In contrast, rats that received the sense phosphorothioated oligonucleotide (SPR) showed high levels of lordosis similar to EB + THC-treated controls. P-facilitated lordosis also was inhibited by PRAs, whereas the sense oligonucleotides (SPR) had no significant effects compared with the EB + P-treated controls. One-way ANOVA followed by Dunnet's multiple comparisons indicated statistically significant (P < 0.01) differences in P or THC-facilitated lordosis in animals that received AsPR compared with EB + P or EB + THC controls, respectively. Sense oligonucleotides had no significant effect (P > 0.05) on P or THC-facilitated lordosis. The results were confirmed with three separate sets of oligonucleotides to PR mRNA and their matched sense oligonucleotide controls, and substantiated a role for PR in THC-mediated lordosis response.
Figure 4.
Effect of antisense (As) and sense (S) oligonucleotides to (A) PR mRNA (PR) and (B) dopamine receptor (D1 B), (C) dopamine receptor (D1 A) on P-, SKF-, and THC-facilitated lordosis response in EB-primed rats (2 μg, s.c.). Ovariectomized rats with indwelling cannulae in the third cerebral ventricle were given ICV injections of 4 nmol of the oligonucleotides at the time of EB priming. Animals were injected the same dose of the oligonucleotides 24 h later and tested for lordosis response 30 min after P or THC administration. The animals were scored for their lordosis response and the results were expressed as means ± SEM of LQs. Control animals received vehicle EB alone or EB followed by P/THC or SKF 48 h after EB priming. (*, P < 0.001; n = 6 animals per group).
THC-Facilitated Lordosis Response Depends on D1B Receptors.
ICV administration of antisense oligonucleotides to D1B receptor (AsD1B) into the third cerebral ventricle significantly suppressed THC-facilitated lordosis of EB-primed rats (P < 0.001), whereas sense oligonucleotides had no significant effects (Fig. 4B). Antisense oligonucleotides to D1A receptor (AsD1A) had no significant effects (P > 0.05) on the THC-facilitated lordosis responses of EB-treated animals compared with those that received sense oligonucleotides (SD1A) or EB + P/THC-treated controls (Fig. 4C). As we have reported previously, P-facilitated lordosis also was inhibited by AsD1B, whereas the sense oligonucleotides (SD1B) had no significant effects (P > 0.05). One-way ANOVA followed by Dunn's multiple comparisons indicated statistically significant (P < 0.01) differences in P or THC-facilitated lordosis in animals that received AsD1B compared with EB + P or EB + THC controls, respectively. Again, the experiment was confirmed with three separate sets of oligonucleotides to D1A and D1B mRNA and their matched sense oligonucleotide controls. The above results using antisense pretreatments substantiate a role for progesterone and dopamine D1B receptors in the THC response.
Discussion
It is well known that ovarian steroid hormones E and P are essential for the display of sexual behavior in female rodents (15–17). In addition to the steroid hormones, several compounds like the neurotransmitter dopamine (DA) facilitate sexual receptivity by means of crosstalk with PRs in the hypothalamus of the female rat (19, 22, 23). In the current study, we now provide evidence for the convergence of yet another signal transduction pathway, in the facilitation of female reproductive behavior. In concordance with the previous reports on the ability of THC to stimulate lordosis response in rats (11) and hamsters (12), the current study demonstrates facilitation by THC on sexual receptivity in female rats. Furthermore, this THC facilitation of sexual receptivity is mediated by the CB1 receptors. Surprisingly, the response to THC also appears to require both PR and dopamine D1R receptors and indicates that crosstalk between the CB1-initiated signaling pathway(s) and the DA and PR pathways are required for THC-facilitated female reproductive behavior.
The cellular effects of THC are mediated through specific cannabinoid receptors, members of the G protein-coupled receptor super family (25). To date, two subtypes of the cannabinoid receptor, CB1 and CB2, have been identified (26, 27). CB1 is predominantly expressed in the brain (26), although mRNA for CB1 also has been identified in testis (28), spleen cells (29), and leucocytes (30). CB2 subtype is expressed principally in immune tissue (31) where it may be involved in cannabinoid-mediated immune responses. CB2 mRNA has been detected in cerebellar granule cells and cerebellum of the mouse (32). Radioligand autoradiography (33), in situ hybridization, and immunohistochemical studies (34) demonstrate CB1 localization in brain regions whose functionality is associated with the pharmacological action of cannabinoids. Autoradiography (33) and receptor binding studies (24) indicate the presence of CB1 receptors in the medial basal hypothalamus of the rat brain, a region known to be associated with female sexual behavior. The current behavioral observations are in agreement with a role for the hypothalamic CB1 receptors in THC-facilitated reproductive behavior.
Our studies revealed that antagonists to CB1 receptors inhibited P- and SKF-facilitated responses, and vice versa, suggesting that P and DA signaling pathways are interacting with the CB1-mediated pathways. In situ hybridization studies indicate that CB1 receptors are present in the lateral region of the ventromedial nucleus of the hypothalamus (34), an area that also contains a high density of PRs (35). It could be hypothesized that THC could directly interact with hypothalamic steroid receptors to stimulate their transcriptional activation. However, this does not appear to be the case because cannabinoid compounds including THC fail to activate steroid receptors in in vitro cell cultures (36). The converse also is true. Steroid ligands including 17-β-estradiol, progesterone, pregnenolone sulfate, androsterone, cortisone, and corticosterone have no direct interactions with the CB1 receptors in the rat brain (37–39). Thus, it is unlikely that THC exerts its effects by direct interactions with steroid receptors in the brain. Rather, it is likely that components of the CB1-initiated signaling cascade converge with the P- and/or DA-initiated pathways, to effect sexual receptivity. We have previously reported a two-way convergence between P- and DA-initiated signaling pathways in the facilitation of sexual receptivity (19, 23). The present results now indicate that a three-way convergence is likely.
These suggested relationships are not without precedent. In this context, it is worthy to note that dopamine traditionally has been thought to be involved in the mediation of the effects of THC (40). Microdialysis studies indicate that THC increases extracellular dopamine concentrations in the mesolimbic pathways in the rats (13). Acute administration of THC increases the activity of dopaminergic neurons, both in the ventral tegmental area and in the stubstantia nigra, the regions known to be involved in positive reinforcement and motor control, respectively (41). An increase in dopaminergic activity also has been reported in the reproductively relevant mediobasal hypothalamus (MBH) in response to THC (42, 43). Dopaminergic neurons have been demonstrated in the arcuate nucleus with projections to the external layer of median eminence (44). CB1 receptors in the brain colocalize with dopaminergic pathways, suggesting a functional interaction with the dopaminergic system in those regions of the brain (45). Similar interactions could occur in the medial basal hypothalamic region of the brain, which contains a high density of CB1 receptors and cell bodies or terminals of dopaminergic neurons (24, 33). The expression of D1B in the PR-rich ventromedial nucleus of the rat brain also has been recently reported (46). The present data clearly indicate the involvement of dopamine D1B receptors in THC-facilitated receptivity, suggesting an interaction between the two pathways. Collectively, the data indicate an involvement of both DA- and P- regulated pathways in the signaling by THC.
Interestingly, in addition to dopaminergic neurons, THC also has been demonstrated to affect noradrenergic (47, 48), serotonergic (49), and peptidergic neurons (50), including those of luteinizing hormone releasing hormone (LHRH), which are involved, directly or indirectly, in the mediation of sexual receptivity in female rodents (51, 52). ICV administration of THC elevated LHRH content in the MBH within 30 min followed by a decrease at 60 min, concurrent with lowered plasma LH levels in male rats (53). In vitro studies indicated that the suppressive effect of THC on LH release was because of the reduction in noradrenergic stimulation of prostaglandin E2 (PGE2) and LHRH release in MBH (54). De Miguel et al. (42) have provided evidence for a role of CB1 receptors in the suppressive effects of THC on LH release. Those authors also reported an enhancement of γ-aminobutyric acid (GABA-ergic) activity in the MBH. Thus, the lordosis response observed in the animals could be because of the increased GABA activity in the medial hypothalamus (55, 56). It also is possible that the elevated LHRH levels in the MBH within 30 min post-THC administration was responsible for the enhanced lordosis response observed in the current study.
The precise mechanisms for signal transduction from cell surface CB1 receptors to nuclear steroid receptors that involve D1B receptors require further investigation. Although it is known that steroid receptors function as general transcription factors for neural communication by the dopaminergic system, the plausibility of the cannabinoid receptor system using similar pathways cannot be ignored. Likely mechanisms could include THC mediating its effects by the CB1 receptor to promote dopamine release followed by dopamine D1B receptor activation, leading to activation of PR or its associated coactivators. The existence of the dopamine-PR pathway has been substantiated previously (19, 22, 23). Thus, THC-initiated increases in dopaminergic activity in the MBH could lead to increased cAMP levels, increased protein kinase A (PKA) activity, leading to enhanced phosphorylation of dopamine and cAMP-regulated phosphoprotein (DARPP-32) as demonstrated by us recently (23). Alternatively, CB1 receptor-mediated signaling by THC could involve downstream convergence, for example at mitogen-activated protein kinases (MAPKs); cannabinoid receptor agonists have been shown to stimulate MAPKs by a G protein involving cAMP-independent mechanisms (30). MAPK also has been reported to be involved in the DA-mediated effects (57). D1 receptor agonists have been demonstrated to activate p38 MAPK and c-Jun amino-terminal kinase (JNK) by a PKA-dependent mechanism in SK-N-MC neuroblastoma cells in vitro (58). Furthermore, a role for MAPK in the regulation of phosphorylation of coactivators of PRs, leading to their transcriptional activation also has been demonstrated (59). Thus, convergence and mutual interdependence of these multiple intracellular signaling pathways by either of the above mechanisms could reinforce the steroid-mediated transduction pathways to achieve the appropriate integration required for this complex neuroendocrine behavior.
Acknowledgments
We acknowledge the word processing help of Mr. John Ellsworth. We thank the National Institute of Drug Addiction for generously providing Δ9-tetrahydrocannabinol and antagonists to CB1 and CB2 receptors used in these studies. This research was supported by National Institutes of Health Grants HD74095 (to B.W.O.) and MH57442 (to S.K.M.).
Abbreviations
- THC
Δ9-tetrahydrocannabinol
- LH
luteinizing hormone
- CB
cannabinoid
- E
estrogen
- P
progesterone
- PR
P receptor
- CB1
CB receptor-1 subtype
- EB
estradiol benzoate
- LQ
lordosis quotient
- ICV
intracerebroventricular
- MAPK
mitogen-activated protein kinase
Footnotes
See commentary on page 793.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.031563998.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.031563998
References
- 1.Murphy L L, Stegar R W, Bartke A. In: Biochemistry and Physiology of Drug Abuse. Watson R R, editor. Boca Raton, FL: CRC; 1990. pp. 73–93. [Google Scholar]
- 2.Kolodny R C, Masters W H, Kolodner R M, Toro G. N Engl J Med. 1974;290:872–874. doi: 10.1056/NEJM197404182901602. [DOI] [PubMed] [Google Scholar]
- 3.Symons A M, Teale J D, Marks V. J Endocrinol. 1976;68:43. [PubMed] [Google Scholar]
- 4.Dixit V P, Sharma V N, Lohiya N K. Eur J Pharmacol. 1974;26:111–114. doi: 10.1016/0014-2999(74)90081-8. [DOI] [PubMed] [Google Scholar]
- 5.Corcoran M E, Amitt Z, Malsbury C W, Daykin S. Res Commun Chem Pathol Pharmacol. 1974;7:779–782. [PubMed] [Google Scholar]
- 6.Merari A A, Barak A, Plaves M. Psychopharmacologia. 1973;28:243–246. doi: 10.1007/BF00429304. [DOI] [PubMed] [Google Scholar]
- 7.Murphy L L, Gher J, Steger R W, Bartke A. Pharmacol Biochem Behav. 1994;48:1011–1017. doi: 10.1016/0091-3057(94)90213-5. [DOI] [PubMed] [Google Scholar]
- 8.Chakravarty I, Ghosh J J. Sci Res Cannabis. 1973;38:1–4. [Google Scholar]
- 9.Chakravarty I, Sheth A R, Ghosh J J. Fertil Steril. 1975;26:947–948. doi: 10.1016/s0015-0282(16)41364-6. [DOI] [PubMed] [Google Scholar]
- 10.Nir I, Ayalon A, Tsafriri T, Corodova T, Lindner H R. Nature (London) 1973;244:470–471. doi: 10.1038/243470a0. [DOI] [PubMed] [Google Scholar]
- 11.Gordon J H, Bromley B L, Gorski R A, Zimmerman E. Pharmacol Biochem Behav. 1978;8:603–608. [PubMed] [Google Scholar]
- 12.Turley W A, Floody O R. Pharmacol Biochem Behav. 1981;14:745–747. doi: 10.1016/0091-3057(81)90142-8. [DOI] [PubMed] [Google Scholar]
- 13.Tanda G, Pontieri F E, di Chiara G. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez-Ruiz J J, Rodriguiz de Fonseca F, Navarro M, Ramos J A. In: Biochemistry and Physiology of Drug Abuse. Watson R R, editor. Boca Raton, FL: CRC; 1992. pp. 119–163. [Google Scholar]
- 15.Blaustein J D, Olster D H. In: Advances in Comparative and Environmental Physiology. Balthazart J, editor. Vol. 3. Berlin: Springer; 1989. pp. 31–104. [Google Scholar]
- 16.Pfaff D W, Schwartz-Giblin S, McCarthy M M, Kow L. In: Physiology of Reproduction. Knobil E, Neill J D, editors. New York: Plenum; 1994. pp. pp.107–220. [Google Scholar]
- 17.Parsons B, Rainbow T C, Pfaff D W, McEwen B S. Nature (London) 1981;292:58–59. doi: 10.1038/292058a0. [DOI] [PubMed] [Google Scholar]
- 18.Mani S K, Blaustein J D, Allen J M C, Law S W, O'Malley B W, Clark J H. Endocrinology. 1994;135:1409–1414. doi: 10.1210/endo.135.4.7925102. [DOI] [PubMed] [Google Scholar]
- 19.Mani S K, Allen J M C, Clark J H, Blaustein J D, O' Malley B W. Science. 1994;265:1246–1249. doi: 10.1126/science.7915049. [DOI] [PubMed] [Google Scholar]
- 20.Ogawa S, Olazabal S, Pfaff D W. J Neurosci. 1994;14:1766–1774. doi: 10.1523/JNEUROSCI.14-03-01766.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pollio G, Xue P, Zanisi A, Nicolin A, Maggi A. Mol Brain Res. 1993;19:135–139. doi: 10.1016/0169-328x(93)90158-l. [DOI] [PubMed] [Google Scholar]
- 22.Mani S K, Allen J M C, Lydon J P, Mulac-Jericevic B, Blaustein J D, DeMayo F J, Conneely O M, O'Malley B W. Mol Endocrinol. 1996;10:1728–1737. doi: 10.1210/mend.10.12.8961281. [DOI] [PubMed] [Google Scholar]
- 23.Mani S K, Fienberg A F, O' Callaghan J P, Snyder G L, Allen P B, Dash P K, Moore A N, Mitchell A J, Bibb J, Greengard P, et al. Science. 2000;287:1053–1056. doi: 10.1126/science.287.5455.1053. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez de Fonseca F, Cebeira M, Ramos J A, Martin M, Fernandez-Ruiz J J. Life Sci. 1994;54:159–170. doi: 10.1016/0024-3205(94)00585-0. [DOI] [PubMed] [Google Scholar]
- 25.Devane W A, Dysarz F A, Johnson S R, Melvin L S, Howlett A C. Mol Pharmacol. 1988;36:605–613. [PubMed] [Google Scholar]
- 26.Matsuda L A, Lolait S J, Brownstein B J, Young A C, Bonner T L. Nature (London) 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 27.Munro S, Thomas K L, Abu-Shaar M. Nature (London) 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 28.Gerard C M, Mollereau V, Vassart G, Parmentier M. Biochem J. 1991;279:129–134. doi: 10.1042/bj2790129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaminski N E, Abood M, Kessler F K, Martin B R, Schatz A R. Mol Pharmacol. 1992;42:736–742. [PMC free article] [PubMed] [Google Scholar]
- 30.Bouaboula M, Bourrie B, Rinaldi-Carmona M, Shire D, Le Fur G, Casellas P. J Biol Chem. 1995;270:13973–13980. doi: 10.1074/jbc.270.23.13973. [DOI] [PubMed] [Google Scholar]
- 31.Galiegue S, Mary S, Marchand J, Duossossoy D, Carriere D, Carayon P, Bouaboula M, Shire D, Le Fur G, Cassellas P. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- 32.Skaper S D, Buriani A, Dal Toso R, Petrelli L, Romanello S, Facci L, Leon A. Proc Natl Acad Sci USA. 1996;93:3984–3989. doi: 10.1073/pnas.93.9.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herkenham M, Lynn A B, Johnson M R, Melvin L S, de Costa B R, Rice K C. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mailleux P, Vanderhaeghen J J. Neuroscience. 1992;48:655–668. doi: 10.1016/0306-4522(92)90409-u. [DOI] [PubMed] [Google Scholar]
- 35.Parsons B, Rainbow T C, Maclusky N J, McEwen B S. J Neuroendocrinol. 1982;2:1446–1452. doi: 10.1523/JNEUROSCI.02-10-01446.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruh M F, Taylor J A, Howlett A C, Welshons W V. Biochem Pharmacol. 1997;53:35–41. doi: 10.1016/s0006-2952(96)00659-4. [DOI] [PubMed] [Google Scholar]
- 37.Bidaut-Russell M, Devane W A, Howlett A C. J Neurochem. 1990;55:21–26. doi: 10.1111/j.1471-4159.1990.tb08815.x. [DOI] [PubMed] [Google Scholar]
- 38.Howlett A C, Evans D M, Houston D B. In: Marijuana/Cannabinoids: Neurobiology and Neurophysiology. Murphy L, Bartke A, editors. Boca Raton, FL: CRC; 1992. pp. 35–72. [Google Scholar]
- 39.Kuster J E, Stevenson J I, Ward S J, D'Amdra T E, Haycock D A. J Pharmacol Exp Ther. 1993;264:1352–1363. [PubMed] [Google Scholar]
- 40.Piomelli D, Giuffrida A, Calignano A, Rodriguez de Fonseca F. Trends Pharmacol Sci. 2000;21:218–224. doi: 10.1016/s0165-6147(00)01482-6. [DOI] [PubMed] [Google Scholar]
- 41.Wu X, French E D. Neuropharmacology. 2000;39:391–398. doi: 10.1016/s0028-3908(99)00140-9. [DOI] [PubMed] [Google Scholar]
- 42.De Miguel R, Romero J, Munoz R M, Garcia-Gil L, Gonzalez S, Villanua M A, Makriyannis A, Ramos J A, Feranadez-Ruiz J J. Biochem Pharmacol. 1998;56:1331–1338. doi: 10.1016/s0006-2952(98)00185-3. [DOI] [PubMed] [Google Scholar]
- 43.Corchero J, Fuentes J A, Manzanares J. Eur J Pharmacol. 1997;323:193–195. doi: 10.1016/s0014-2999(97)00144-1. [DOI] [PubMed] [Google Scholar]
- 44.Fuxe K, Hokfelt T. In: Frontiers in Neuroendocrinology. Martini L L, Ganong W F, editors. New York: Oxford Univ. Press; 1969. pp. 47–98. [Google Scholar]
- 45.Perwee R G. Pharmacol Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- 46.Zhou D, Apostolakis E M, O'Malley B W. Biochem Biophys Res Commun. 1999;266:556–559. doi: 10.1006/bbrc.1999.1851. [DOI] [PubMed] [Google Scholar]
- 47.Murphy L L, Steger R W, Smith M S, Bartke A. Neuroendocrinology. 1990;52:316–321. doi: 10.1159/000125604. [DOI] [PubMed] [Google Scholar]
- 48.Murphy L L, Chandrashekar V, Bartke A. Neuroendocr Lett. 1994;16:1–7. [Google Scholar]
- 49.Kramer J, Ben-David M. Endocrinology. 1978;103:452–458. doi: 10.1210/endo-103-2-452. [DOI] [PubMed] [Google Scholar]
- 50.Steger R W, DePaolo L, Asch R H, Silverman A Y. Neuroendocrinology. 1983;37:361–370. doi: 10.1159/000123576. [DOI] [PubMed] [Google Scholar]
- 51.Moss R L, McCann S M. Science. 1973;181:177–179. doi: 10.1126/science.181.4095.177. [DOI] [PubMed] [Google Scholar]
- 52.Pfaff D W. Science. 1973;182:1148–1149. doi: 10.1126/science.182.4117.1148. [DOI] [PubMed] [Google Scholar]
- 53.Wenger T, Rettori V, Snyder G D, Dalterio S, McCann S M. Neuroendocrinology. 1987;46:488–493. doi: 10.1159/000124870. [DOI] [PubMed] [Google Scholar]
- 54.Rettori V, Aguila M C, Gimeno M F, Franchi A M, McCann S M. Proc Natl Acad Sci USA. 1990;87:10063–10066. doi: 10.1073/pnas.87.24.10063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McCarthy M M, Masters D B, Fiber J M, Lopez-Colome A M, Beyer C, Komisaruk B R, Feder H H. Neuroendocrinology. 1991;53:473–479. doi: 10.1159/000125760. [DOI] [PubMed] [Google Scholar]
- 56.McCarthy M M, Masters D B, Rimvall K, Schwartz-Giblin S, Pfaff D W. Brain Res. 1994;14:209–220. doi: 10.1016/0006-8993(94)91019-7. [DOI] [PubMed] [Google Scholar]
- 57.Yan Z, Feng J, Fienberg A F, Greengard P. Proc Natl Acad Sci USA. 1999;96:11607–11612. doi: 10.1073/pnas.96.20.11607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhen X, Uryu K, Wang H-Y, Friedman E. Mol Pharmacol. 1998;54:453–458. doi: 10.1124/mol.54.3.453. [DOI] [PubMed] [Google Scholar]
- 59.Rowen B G, Weigel N L, O'Malley B W. J Biol Chem. 2000;275:4475–4483. doi: 10.1074/jbc.275.6.4475. [DOI] [PubMed] [Google Scholar]