Abstract
We propose a theoretical mechanism that enables the elaboration of veins to supply distant cells during leaf development. In contrast to the more standard view that a signal (e.g., auxin) is produced at isolated sites to stimulate growth, we determine the consequences of the hypothesis that auxin is produced at a constant rate in every cell. High concentration sites for auxin emerge naturally in a reaction–diffusion model, together with global information about leaf shape and existing venation. Because the global information is encoded as auxin concentration and its gradient, those signals provide individual cells with sufficient information to determine their own fate. Unlike other models, a single substance suffices for the reaction–diffusion at early, but not initial, stages of development. Neither complex interactions nor predetermination are necessary. We predict angiosperm areolation patterns in simulation, and our model further implies the Sachs Canalization Hypothesis and resolves a dilemma regarding the role of auxin in cell growth.
Keywords: canalization hypothesis, emergent behavior, reaction–diffusion
Isolated sites of high auxin concentration have been observed in developing leaves (1), and it has been assumed that auxin production is dominant there. However, evidence (1, 2) is emerging that production may be present at other sites. We demonstrate that discrete regulated sites of synthesis need not exist to develop a spatial pattern of discrete responses. Formally, our “constant production hypothesis” holds that auxin is produced in all cells at the same constant rate. Mathematical analysis of a schematic reaction diffusion model indicates that high concentration sites emerge, which agrees with observations (1) but also shows that distributions carry rich information about the geometry of the leaf and its venation. This information can be interpreted locally as a signal at the cell level, thereby providing global cues. The concentration together with the gradient of concentration have substantial predictive power about vein formation (Fig. 1). Signals for initiating differentiation are readily available locally, removing the need for complex intercellular communication.
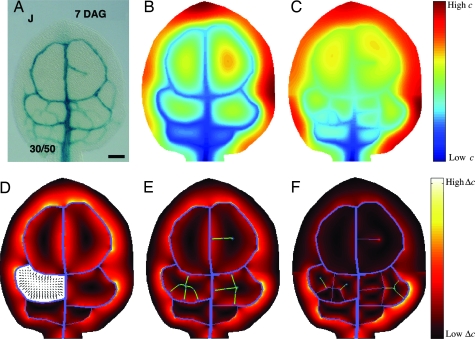
Fig. 1.
Illustration that global signaling information for vein formation can be obtained from constant hormonal production in every cell. As developed in the text, the bottom of the petiole (base of figure) acts as sink, and the hormone diffuses with coefficient Dv through vascular cells and D everywhere else (Dv > D). Developmental time is quantized into two discrete stages for this illustration. (A) Image of stained leaf of Arabidopsis from ref. 3. [Reproduced with permission from ref. 3 (Copyright 2004, Company of Biologists).] (Scale bar: 100 μm.) (B and C) Color-coded concentration levels at stages 1 (B) and 2 (C). Observe how local peaks “move” after creation of new strands. (D) Gradient vectors of concentration in an areole developed further in the work. Color-coded magnitudes of vectors elsewhere in the leaf are shown. (E and F) Color-coded magnitude of greatest possible Δc with immediate neighbors. Veins are in blue, and predictions are in green. Predictions start at a maximum of Δc, follow vectors (e.g., see D), and stop at minimum Δc (see Supporting Text, which is published as supporting information on the PNAS web site). Stage 1 (B and E) was initialized by tracing veins from A (shown in blue in E). Stage 2 (C and F) was initialized using predictions (in blue in F) from stage 1.
Our model differs from others that focus on local phenomena (4–6). We seek to articulate those global pattern features that emerge from purely local phenomena. Such structural questions on a larger scale have, to our knowledge, only been considered in the work of Meinhardt and Gierer (7, 8) and, more recently, in the empirical models by Mündermann et al. (9) and Feugier et al. (10). We show, contrary to Meinhardt (8) and certain current opinion (11, 12), that a single substance traveling purely by diffusion [or its equivalent (13)] is theoretically sufficient as a basis for a mechanistic model of venation patterning when it is uniformly and constantly produced (cf. ref. 10).
Motivation and Assumptions
We demonstrate how simple local behaviors, occurring simultaneously and independently at the cell level, can give rise to complex global patterning. The local behaviors constitute four basic functions that a plant cell will be allowed to perform in this model. Our main result is to show how new venation forms within an areole as a consequence.
Our choices are motivated by the transport of nutrients and a basic tradeoff: although each cell should be provided with sufficient resources, veins should be laid down sparingly because of the energy required. Because this tradeoff must be in effect at all times during leaf growth, especially in plant species with continuously expanding leaves, new venation should be created only when some cells become potentially short of resources. Identifying when this shortage occurs, where these cells are, and how to correct it, are the fundamental questions that we address. New veins should connect the most deprived region to the existing network because nutrients are delivered to the leaf through the petiole, and short connections are more efficient than meandering ones.
How, then, might cells achieve such global behavior? Can they achieve it by performing purely local functions? If so, how does a cell detect that it is too far from the existing vascular network? By what signal does a ground cell initiate the cascade of differentiation processes to become vascular? How are connecting cells identified? These are the operational versions of our fundamental questions.
Turing (14) first showed how chemical substances, by diffusing and reacting together, could give rise to stable patterns. These so-called reaction–diffusion systems inspired Gierer and Meinhardt (7) to propose a technique for generating theories of biological pattern formation. In particular, cells are assumed to produce and assimilate substances that carry information through their peaks of concentration. They postulated that at least two substances are required for venation patterning (8) and showed how such a system answers the above questions.
Sachs (15), building on experiments, proposed that new vascular strands form as the draining paths of auxin. His empirically derived Canalization Hypothesis holds that improved transport along a path enlarges a path’s capacity. This autocatalytic behavior was translated by Mitchison (4) into the idea that the diffusivity of a membrane depends on the flux of the substance through it. Mitchison (4) showed that the canalization hypothesis is sound, but complete vein patterns were not explained (see also ref. 16).
In both cases, production of the controlling substances has eluded experimental verification. The Meinhardt and Gierer proposal awaits identification of the two substances, and the Sachs–Mitchison model implicitly assumes isolated auxin production without addressing where those sites should be. A completely different approach, one where no substances are directly involved, was suggested by Couder et al. (17). It is based on an analogy to crack formation in materials, but it does not guarantee a unique drainage point as required by the petiole in leaves.
We propose a different answer to our operational questions by first considering where auxin might be produced and consumed. Because growth requires nutrients, suppose a leaf may request those resources that it needs. One conceptual request mechanism could be for the leaf to produce a hormone s at a rate proportional to its size and, by using the vascular network, transport it to sites in the plant capable of satisfying the request (e.g., roots). If s is assimilated (or broken down) proportionally to the available resources, then as soon as the capacity of the production sites or of the transport system (the petiole, for example) is exceeded, the hormone concentration will rise. Leaf growth ceases when hormone levels become too high.
This mechanism can be implemented with simple local rules: Each cell should produce s at a constant rate K, allow it to diffuse through its membranes and walls, and measure concentration of s to determine when to stop growing. This way, the collection of n cells in the leaf produces s at a rate of n·K, which is proportional to the overall size. Theoretically, this strategy extends far beyond controlling leaf size, and these rules define our model almost completely.
Model
A leaf is a collection of cells. We distinguish between “ground cells,” those that give rise to all others, and “vascular cells,” those that comprise the venation pattern. We focus on early leaf development and concentrate on establishing signals to initiate the cascade of events that change ground cells into vascular cells within an expanding areole. To keep matters tractable, a cell will be referred to as “c-vascular” (cascade vascular) immediately after this cascade is initiated. The subcollection of c-vascular cells may be thought of as an early prepattern from which veins derive. Ground cells have (essentially) homogeneous characteristics, and areoles are delimited by more developed c-vascular (or mature vascular) cells. Instead of assuming the prepattern is predefined, our model establishes how it emerges from local operations. We refer to both membranes and cell walls together as “cell interfaces” and assume that they act as a single membrane. Each cell performs the following basic cell functions (CFs) independently and simultaneously:
CF1. Produce substance s at the constant rate K.
CF2. Measure c, the concentration of s, inside it and Δc, the difference across cell interfaces.
CF3. Diffuse s through interfaces. C-vascular cells transport s better. Active (polar) transport is abstracted as improved diffusion (13).
CF4. Improve transport of s as triggered by c and Δc.
Notice that the only means of communication among cells is through CF2 because of CF1 and CF3; no other information-exchange mechanism is assumed. The only distinction between ground and c-vascular cells is in how well they transport substance s. In effect, it is the interface between cells that changes permeability. Cells are allowed to have several interfaces, one to each neighboring cell, each with a different permeability to s. A cell will be called c-vascular if at least one of its interfaces has high permeability.
The first three rules are as required for stopping leaf growth. CF4 may be thought of as signaling when a ground cell should become c-vascular. It is, as we show next, a consequence of the other rules.
Analysis
Consider the leaf blade as a collection of cells. If each of them follows the cell functions listed above, veins emerge as required. The key to understanding why and how this venation develops is in the dynamics of s on a larger scale. In particular, the concentration c of the substance viewed as a function over all cells contains all relevant information. The structure of c is the direct consequence of applying CF1 and CF3 everywhere independently, and it is very sensitive to the way in which cells are organized in the collection, that is, to their global pattern. We now develop this structure.
It is useful to think of c as a continuous function. Of course, there are only finitely many cells in a leaf, and, because of CF2, we should only define c for each cell: c(x, y), only where (x, y) is the position of a cell. If, however, there were a large number of cells, then c defined everywhere inside the leaf approximates the continuous c; in fact, the larger the number of cells, the better the approximation. It is in this sense that we will use c in a differential equation with positional derivatives.
Suppose the leaf is endowed with a venation pattern, and focus on an areole. As this aerole expands, new c-vascular strands need to be created. Both the expansion and the formation of new venation are constrained by our model. We now derive the more specific behavior implied by CF4 as imposed by the first three functions.
Cell functions CF1 and CF3 determine the equation governing the distribution of s in the areole. C-vascular cells evacuate the hormone much faster than ground cells. For analysis, assume that s is drained fast enough so that the boundary of the areole may be thought of as a sink for s. Therefore, the temporal change of the concentration inside a region depends on how much is diffused out plus how much is created; in symbols
where D is the diffusion constant of ground cells, ∇2c = cxx + cyy is the Laplacian of concentration over cell position, and K is as in CF1. Eq. 1 is a reaction–diffusion equation that has a steady state: after sufficiently long time, the dynamical system is well approximated by the steady-state ct = 0 (see Fig. 2) Observe that those cells that are further from the boundary have higher concentrations. In fact, the concentration profile is qualitatively similar to that of the function assigning to each cell the shortest distance to a (c-)vascular cell: the so-called “distance transform” (18).
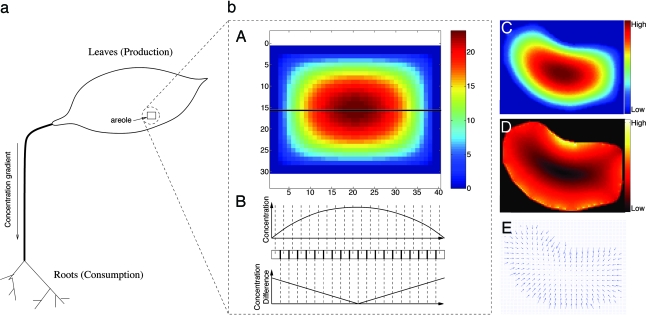
Fig. 2.
Hormone concentration inside an areole. (a) An artificial areole is illustrated with a rectangular boundary of c-vascular cells. Assuming c-vascular cells are much more efficient at transporting s, the boundary may be taken as a sink, and c is governed by Eq. 1. (bA) c at near steady state, ct ≈ 0. (bB) The values of c and Δc along a path (in black) across the areole in bA. Notice how the concentration peaks for cells furthest from the veins, whereas Δc peaks near the vein. (b C–E) Concentration geometry for the areole from Fig. 1D. (bC) Concentration. (bD) Magnitude of gradient. (bE) Gradient vector field. Observe how the gradient vectors point toward largest concentration increase.
When ct = 0, Eq. 1 becomes a Poisson equation. Given our boundary conditions (c = 0 at veins), there is a unique c satisfying it (19). From this equation, we calculate the following:
Result 1.
Consider an areole and suppose that P is a ground cell that is furthest from the c-vascular boundary. Let Q be a c-vascular cell that is closest to P, and denote by L the distance between P and Q. Then
c(P) is proportional to (K/D)L2;
The change in c at the interface of Q nearest to P is proportional to (K/D)L; and
Δc is largest at an interface of the c-vascular boundary, larger than for any ground cell, and is proportional to (K/D)L.
Therefore, by using Result 1a and CF2, a cell may determine whether it has become further than L units from the closest c-vascular (supply) cell by measuring its concentration. This measurement, however, is not sufficient for determining whether the cell should become c-vascular. The example in Fig. 2 shows that if cells differentiated only when c exceeds a threshold, then isolated islands of (pre-)vascular cells would form and never connect. Because the veins must be connected for normal leaves [there are exceptions (20)], a different strategy must be used.
CF2 also allows cells to measure the difference in c across interfaces. In Fig. 2, we plot c and Δc for a cross section of the artificial areole. Observe that Δc decreases as c increases and Δc is highest near existing venation where c is lowest (see Proposition 1 in Supporting Text for an explanation). In fact, Result 1b asserts that Δc at the venation is proportional to L/D and does not depend on the value of c. It also gives the direction toward the furthest cell. This result is sufficient to show that mechanisms for new strand creation should adhere to the following schema.
Schema 1.
Let DI be the diffusion constant across an interface I and Δc be the concentration difference through I. Then increase DI to a higher value when Δc > α(K/DI)L0. (α is a constant of proportionality.) Alternatively, the flux φ = DIΔc = αKL0 may be used.
A comment on language is useful here. We refer to schema rather than mechanism because the increase in DI may be due to a cellular phenomenon such as the synthesis or reorientation of substance carriers (e.g., PIN and AUX1 in the case of auxin) or a (pro)vascular differentiation event that affects many such cellular properties (wall composition, orientation of expansion, etc.). Whatever the cellular mechanism, we require that the transport properties of an interface be improved when Δc is large. In Schema 1 we summarize this condition with a simple threshold, but cellular mechanisms are likely more elaborate. Nevertheless, calculations with this threshold show that the critical size may be regulated. Fig. 3 shows the sequence of events. As a cell becomes c-vascular, it starts evacuating s more rapidly, and Δc increases. Shortly, one interface exceeds the threshold and initiates conversion in the next cell. Because the chain begins at a c-vascular cell, after each change the hormone is readily drained. Also, because the process starts toward a furthest ground cell P, it continues in that direction. Finally, the draining of substance s induces a form of lateral inhibition.
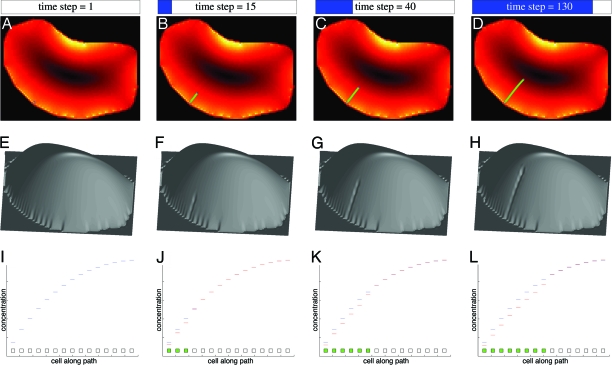
Fig. 3.
Creation of a new c-vascular strand. (A–D) Illustration of our model’s schema for creating a new strand. Four steps in time are shown for the left areole taken from Fig. 1. Color codes are as in Fig. 1F. In general, new strands are initiated at a peak of gradient magnitude (i.e., minima of concentration) and proceed toward the minima of magnitude but may stop before reaching them (see Fig. 6 and Movies 1–5, which are published as supporting information on the PNAS web site). (E–H) Concentration of s inside the areole shown as height. Notice the developing groove as the incipient strand is elaborated: as transport improves, c decreases locally (the groove). (I–L) Illustration of the developing strand in the direction of creation. Squares represent cells along green curve in D: ground cells are in white, and c-vascular cells are in green. Blue curve above cells is initial concentration, and red curve tracks changes with time. Initially, the first cell has lowest concentration, but its interface to the second cell achieves the largest Δc of all interfaces. This value exceeds the threshold τ and causes the interface to increase diffusivity (see Schema 1). The hormone is drained, and the next interface (cell 2 to cell 3) has Δc > τ and the process repeats. Consequently, the interfaces change their D in sequence. This mechanism shows how, contrary to Sachs’s conjecture (ref. 21, p. 205), the concentration gradient may be as relevant as the flux. Note how the strand is created in a rapid burst (three cells in 15 steps), which slows down rapidly (115 steps for the remaining six).
This behavior is not specific to the constraints of our example. In fact, Result 1 holds provided there is at least one point of hormone assimilation. Typically, along c-vascular strands there will be isolated maxima (i.e., the Q) each corresponding to an isolated furthest ground cell (i.e., a P). Fig. 4 compares predictions with observations. It is the collective behavior due to CF1 and CF3 that allows us to keep to a purely local criterion as required by CF4. We now have an answer to both of our questions, selection and connection of a target cell, and it involves a single substance s.
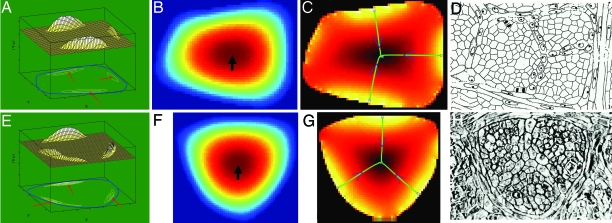
Fig. 4.
Illustration of model predictions of c-vascular formation in Liriodendron areoles. (A and E) Magnitude of gradient of c above a threshold (the plane). The reference plane shows the boundary in blue. Red arrows point to peaks where new strands are initiated. Note that peaks are as expected from Result 1b. (B and F) Magnitude of concentration. Color codes are as in Fig. 1C. Arrow points at peak where the strands end. (C and G) Magnitude of gradient of c (compare with A and E). Color codes are as in Fig. 1F. Green curves are predicted new strands. (D and H) Actual areoles from Pray [Reproduced with permission from ref. 22 (Copyright 1955, The Botanical Society of America).]
Substance s provides a local cue for growth: the concentration gradient. From our model it is necessary and sufficient for growth to be promoted by the magnitude of the gradient and inhibited by the absolute concentration. Thus, where the gradient is low, the concentration of s is high and expansion is slow; where the gradient is high, c is low and expansion is fast. When the draining capacity is reached, the concentration of s rises faster than cells expand. The value of Δc only depends on size, so it changes more slowly than c itself, and, eventually, c becomes large enough to arrest expansion even where Δc is high.
Consequences and Predictions
Dynamics of Vein Creation.
New strands emanate from existing ones and extend toward the ground cells furthest from them. Fig. 1 shows how sites of high concentration (i.e., low magnitude of gradient) appear to “pull” new strands toward them. Incipient strands deplete the region of substance s and eliminate concentration hot spots. Leaf growth then creates new hot spots, and the process continues until maturity. This behavior was reported in ref. 1 for Arabidopsis leaves where s is auxin. The authors propose that moving sites of high auxin production are responsible for the observed phenomena but do not explain why those sites move. We showed that the sites of high concentration are due to substance accumulation (not production), and apparent movement of such sites is accounted for by the dynamics of our model.
Observe that new strands are created in bursts (Fig. 3), giving rise to (essentially) straight segments, which locally approximate the requirement that efficient veins be short and that connections form incrementally. Other comments are in Supporting Text.
Whole-Leaf Interactions.
The simulations in Fig. 4 involved isolated areoles in which both K and D were uniformly constant and a single threshold on Δc was sufficient. At the scale of a leaf, however, there are gradients of cell division frequency (23), which suggests that production, diffusion, and differentiation may also vary. We examine this variation in terms of our model, assuming uniformity at the areole level but allowing parameters to differ from areole to areole.
The three parameters of our model: K, D, and the threshold τ, could theoretically vary in any combination from areole to areole. Result 1 and Schema 1 relate them functionally, so we may fix one and study the other two. Suppose, then, that τ is uniform throughout the leaf. Fig. 5A and B then shows that keeping K and D uniform will not produce the same strands as observed in Fig. 1A. If, however, K and D differ from areole to areole (Fig. 5 C and D, and E and F), then we do obtain good agreement. The logic behind this parameter variation is as follows.
Fig. 5.
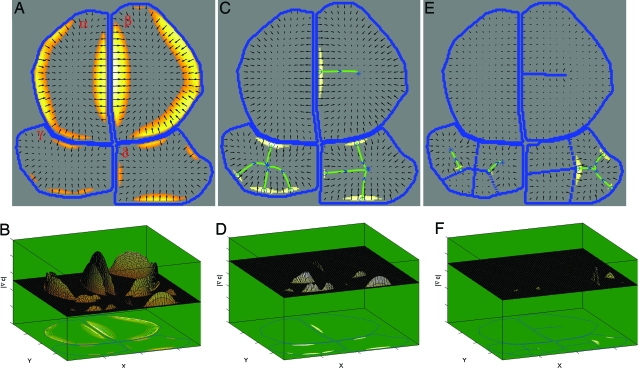
Experiments with the model suggest a variation of parameters across different areoles. We work with the central four areoles from Fig. 1A. Begin by assuming, for a moment, that all parameters (K, D, τ) are uniformly constant. Notice how in A and B, peaks arise to trigger c-vascular differentiation in the upper areoles (α and β) more vigorously than in the lower ones (γ and δ), unlike data in Fig. 1A. More generally, it is impossible to reproduce the observed patterns. However, when the parameters differ in (α, β) relative to (γ, δ) very realistic patterns are obtained (C and E) with a single threshold τ. Comparing areole δ between A and C illustrates how vascular development in β varies the gradient peaks in δ. This illustrates areole–areole timing interactions as well. Such parameter variation across areoles is consistent with gradients of cell division frequency (23). (A and B) All parameters uniformly the same. (C and D) Nonuniform parameters. Kα = Kβ = (1/ ) Kγ, Dα = Dβ = Dγ and Kδ = (1/ )Kγ, Dδ = Dγ. (E and F) Nonuniform parameters using new strands from C. Kα = Kβ = (1/ )Kγ, Dα = Dβ = Dγ and Kδ = Kγ, Dδ = Dγ. (A, C, and E) Four areoles (labeled α, β, γ, δ) from Fig. 1A with predicted new strands. Color codes are as in Fig. 1F. (B, D, and F) Magnitude of gradient of c above threshold. Other parameter variations are available in Fig. 7, which is published as supporting information on the PNAS web site.
Suppose cell division occurs only when a cell reaches a reference size, type A, to result in two daughter cells, type B, (roughly) of equal size. Thus, the space occupied by n type A cells will be occupied by at most 2n type B cells. If each type B cell produces substance s at the same rate as a type A cell, then the concentration change in time due to production within a type B cell is KB = 2KA because the same amount of material is produced by a cell of half the size. Similarly, the same space will contain at most twice the number of interfaces. A region will mostly have type B cells if cell proliferation is high and type A cells if it is low. In our simulations, type A cells occupy two units, and type B cells occupy one unit of space. Thus, if a region of only A cells has substance production of KA and diffusion coefficients of DA per unit, then a region of type B cells should roughly be bounded KB ≤ 2KA and DB ≥ ½DA for each unit of space. More generally, if A is a region of slow or no division and B of fast division, then (KA/DA) ≤ (KB/DB) ≤ 4(KA/DA). Our model thus predicts a longitudinal gradient for the values of K and D in Arabidopsis thaliana leaves consistent with empirical evidence (23, 24) (see Supporting Text).
Reconnections After Vein Damage.
Sachs (14) argues that there is no predetermined pattern in which vascular tissue appears and provides experimental evidence (21) that new vascular strands are induced by wounds incurred on the existing venation. Our model is consistent with these empirical observations when carried to the leaf. In Fig. 8, which is published as supporting information on the PNAS web site, we simulate the removal of three vascular cells. After wounding, the structure of c changes to become the state shown. Notice how the flow of substance s is rearranged so that reconnecting strands form around the wound as in Sachs’s experiments.
Growth and the Role of Auxin.
There is a dilemma in the literature regarding the role of auxin in leaf expansion. On one side, application of the hormone appears to accelerate growth, e.g., leaf expansion (25) or branching and phyllotaxis (26). On the other, increased concentration of the substance on the leaf due to transport inhibition limits leaf blade expansion (27, 28). Our model predicts both types of experimental results on leaves, thereby removing the dilemma.
Within our framework, the above experiments should be analyzed by considering the difference of concentration from cell to cell as well as its value in a cell; clearly, applying auxin changes both. In the transport inhibition experiments, only the diffusion coefficients changed, and not the endogenous production of substance s (here auxin). The value of Δc is proportional to the distance from the neediest cell, but it is inversely proportional to the diffusion coefficient D (Result 1b). Hence, a lower D will, in effect, increase Δc for shorter distances, and c-vascular strands will be shorter (see Schema 1). Also, the transport capacity of the veins will be diminished and reached sooner, so the final organ will be smaller.
In experiments where auxin is externally applied the profile of c is affected. The exogenous auxin concentration exceeds that produced endogenously. Thus, application on a region of the developing blade creates large concentration differences around the boundary of that region, and cells expand more rapidly there even though c is higher.
In effect, the external intervention inverts the relationship between c and Δc around the region: It makes them increase together instead of with opposite signs. The application of a single drop of auxin illustrates this behavior (see Fig. 9, which is published as supporting information on the PNAS web site). Diffusion causes the drop to change c, and at the instant of application the greatest Δc is observed around the point of application. Slowly, the spread increases, and the greatest Δc moves away from the point of application radially with the value of this maximum decreasing until it becomes insignificant.
Discussion
Adopting an axiomatic approach, we developed a theoretical model for leaf venation patterns in which all functions are performed by cells acting on information available locally. The constant production and (diffusive) transport of a single substance was sufficient to coordinate local behavior and provide global information. As a result, the simple rules of our model gave rise to emergent phenomena of leaf vein patterning and provide important cues for shape formation and, possibly, participate in communication between the leaf and the rest of the plant.
Our analysis showed that, within the constraints of the model, there is a schema (Schema 1), which biological mechanisms should follow to create the venation pattern. Although this schema is a form of the Sachs Canalization Hypothesis (21), we derived it from totally different assumptions. We demonstrated that Δc or the flux DΔc can be used to create new c-vascular strands.
New veins appear efficiently as a consequence of local rules and the constraints that they impose, new strands emanate from existing venation toward the furthest region from the network. Thus, the newest c-vascular element is always as short as possible. More generally, our model suggests that the interplay between vein formation and cell expansion is responsible for leaf morphology, because the structure of c may be used as a cue for growth.
Our proposal is schematic in that we have abstracted the known transport apparatus (cell wall, membrane, and auxin carriers) and demonstrated effects that a mechanistic model should exhibit. Because we are concerned with the earliest stages of pattern formation, the cell wall and membrane were assumed to have uniform influence in all directions and transport to be purely diffusive. Carriers such as PIN and AUX1 have not been shown to have well defined orientation at those stages of development, so we have neglected their effects. Our analysis suggests that this orientation is given by the scheme we have developed and that the carriers play a more important role reinforcing the pattern instead of defining it.
Even though the model was very abstract, it was sufficient to demonstrate that a type of reaction–diffusion system, with a single substance and constant kinetics, could account for a number of developmental processes in a leaf. The fact that constant production with diffusion is mathematically sufficient may have implications for the early evolution of plants as well as the growth control of the whole plant (Fig. 2a).
Supplementary Material
Acknowledgments
We thank L. Hickey, N. Kerk, T. Nelson (and his group), and I. Sussex. This work was supported by the Natural Sciences and Engineering Research Council (Canada) and Yale University.
Abbreviation
- c-vascular
cascade vascular.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Aloni R., Schwalm K., Langhans M., Ullrich C. I. Planta. 2003;216:841–853. doi: 10.1007/s00425-002-0937-8. [DOI] [PubMed] [Google Scholar]
- 2.Ljung K., Bhalerao R. P., Sandberg G. Plant J. 2001;28:465–474. doi: 10.1046/j.1365-313x.2001.01173.x. [DOI] [PubMed] [Google Scholar]
- 3.Scarpella E., Francis P., Berleth T. Development (Cambridge, U.K.) 2004;131:3445–3455. doi: 10.1242/dev.01182. [DOI] [PubMed] [Google Scholar]
- 4.Mitchison G. J. Proc. R. Soc. London Ser. B; 1980. pp. 79–109. [Google Scholar]
- 5.Goldsmith M. H. M., Goldsmith T. H., Martin M. H. Proc. Natl. Acad. Sci. USA. 1981;78:976–980. doi: 10.1073/pnas.78.2.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kramer E. M. Trends Plant Sci. 2004;9:578–582. doi: 10.1016/j.tplants.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Gierer A., Meinhardt H. Kybernetik. 1972;12:30–39. doi: 10.1007/BF00289234. [DOI] [PubMed] [Google Scholar]
- 8.Meinhardt H. Differentiation. 1976;6:117–123. doi: 10.1111/j.1432-0436.1976.tb01478.x. [DOI] [PubMed] [Google Scholar]
- 9.Mündermann L., Erasmus Y., Lane B., Coen E., Prusinkiewicz P. Plant Physiol. 2005;139:960–968. doi: 10.1104/pp.105.060483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feugier F. G., Mochizuki A., Iwasa Y. J. Theor. Biol. 2005;236:366–375. doi: 10.1016/j.jtbi.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Leyser O. Cell. 2005;121:819–822. doi: 10.1016/j.cell.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Harrison L. G. Kinetic Theory of Living Pattern. Cambridge, U.K.: Cambridge Univ. Press; 1993. [Google Scholar]
- 13.Mitchison G. J. Proc. R. Soc. London Ser. B; 1980. pp. 489–511. [Google Scholar]
- 14.Turing A. M. Philos. Trans. R. Soc. London B. 1952;237:37–72. [Google Scholar]
- 15.Sachs T. Pattern Formation in Plant Tissues. Cambridge, U.K.: Cambridge Univ. Press; 1991. [Google Scholar]
- 16.Rolland-Lagan A.-G., Prusinkiewicz P. Plant J. 2005;44:854–865. doi: 10.1111/j.1365-313X.2005.02581.x. [DOI] [PubMed] [Google Scholar]
- 17.Couder Y., Pauchard L., Allain C., Adda-Bedia M., Douady S. Eur. Phys. J. B. 2002;28:135–138. [Google Scholar]
- 18.Blum H. J. Theor. Biol. 1973;38:205–287. doi: 10.1016/0022-5193(73)90175-6. [DOI] [PubMed] [Google Scholar]
- 19.Dimitrov P., Zucker S. W. On a Differential Equation Arising in Plant Vascular Biology. New Haven, CT: Yale Univ; 2006. Yale Computer Sci. Tech. Rep. 1345. [Google Scholar]
- 20.Lersten N. Am. J. Bot. 1965;52:767–774. [Google Scholar]
- 21.Sachs T. Adv. Bot. Res. 1981;9:151–262. [Google Scholar]
- 22.Pray T. R. Am. J. Bot. 1955;42:18–27. [Google Scholar]
- 23.Kang J., Dengler N. Planta. 2002;216:212–219. doi: 10.1007/s00425-002-0847-9. [DOI] [PubMed] [Google Scholar]
- 24.Donnelly P. M., Bonetta D., Tsukaya H., Dengler R. E., Dengler N. G. Dev. Biol. 1999;215:407–419. doi: 10.1006/dbio.1999.9443. [DOI] [PubMed] [Google Scholar]
- 25.Lippincott B. B., Lippincott J. A. Am. J. Bot. 1971;58:817–826. [Google Scholar]
- 26.Reinhardt D., Pesce E.-R., Stieger P., Mandel T., Baltensperger K., Bennett M., Traas J., Friml J., Kuhlemeier C. Nature. 2003;426:255–260. doi: 10.1038/nature02081. [DOI] [PubMed] [Google Scholar]
- 27.Sieburth L. E. Plant Physiol. 1999;121:1179–1190. doi: 10.1104/pp.121.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keller C. P., Stahlberg R., Barkawi L. S., Cohen J. D. Plant Physiol. 2004;134:1217–1226. doi: 10.1104/pp.103.032300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.