Abstract
Fetal growth restriction (FGR) affects >200,000 pregnancies in the United States annually and is associated with increased perinatal mortality and morbidity, as well as poorer long-term health for infants with FGR compared with infants without FGR. FGR appears to be a complex trait, but the role of genetic factors in the development of FGR is largely unknown. We conducted a candidate-gene association study of birth weight and FGR in two independent study samples obtained at the Boston Medical Center. We first investigated the association between maternal genotypes of 68 single-nucleotide polymorphisms (SNPs) from 41 candidate genes and fetal growth in a sample of 204 black women selected for a previous study of preeclampsia, 92 of whom had preeclampsia (characterized by high blood pressure and the presence of protein in the urine). We found significant association between SNP rs2297660 in the LRP8 gene and birth weight. Subsequently, we replicated the association in a larger independent sample of 1,094 black women; similar association between LRP8 and FGR was observed in this sample. The “A” allele at rs2297660 was associated with a higher standardized birth weight and a lower risk of FGR. Under the additive genetic model, each additional copy of the “A” allele reduced the risk of FGR by 33% (P<.05). In conclusion, results from the two independent samples of black women provide consistent evidence that SNP rs2297660 in LRP8 is associated with fetal growth.
Fetal growth restriction (FGR) essentially describes the condition of any fetus that does not reach its full growth potential. In clinical practice, the term is usually reserved for those infants whose weights are <10th percentile for the general population at the corresponding gestational week. FGR is one of the most common fetal abnormalities in developed countries1 and affects >200,000 pregnancies in the United States annually. Perinatal mortality in fetuses with FGR is 5–30 times that in normal fetuses.2–6 Perinatal asphyxia involving multiple organs is one of the most significant problems for infants with FGR. Compared with children without FGR, children born with FGR demonstrated twice the rate of attention-deficit/hyperactivity disorder when they were delivered at full term, and an even higher rate when delivered preterm.7 Furthermore, there is evidence that individuals born with FGR are more susceptible to common chronic adult diseases, such as hypertension, ischemic heart disease, and diabetes.8–12
Many strategies have evolved to reduce maternal risk factors associated with FGR, including cessation of smoking, alcohol use, and drug use; bed rest; nutritional supplementation; and anticoagulation. At present, there are no effective treatments for FGR. Antenatal management of FGR is largely empirical, aimed primarily at early identification of at-risk fetuses and selection of the safest time for delivery. FGR, like other complex diseases, is caused by multiple genetic and environmental factors and their interactions. The role of genetic factors in the pathogenesis of FGR remains largely unexplored, except for some rare cases caused by chromosomal abnormalities, single-gene defects, confined placental mosaicism, or inherited thrombophilia.13,14 Previous studies have found that FGR is more common in black women than in women of other ethnicities.15
Hypertensive disorders, including preeclampsia, eclampsia, and gestational hypertension, significantly increase the risk of FGR and account for more FGR cases, on a population basis, than any other medical conditions. Hypertensive disorders of pregnancy affect up to 8% of all pregnancies and remain the major cause of maternal and neonatal mortality and morbidity in the United States and worldwide.16 Chronic hypertension refers to an elevated blood pressure in the mother that predates the pregnancy. Women with chronic hypertension enter pregnancy with a 25%–30% risk of low birth weight due to a combination of preterm delivery and poor fetal growth,17,18 and the risk increases with the degree of hypertension.17,19 When a woman with chronic hypertension develops superimposed preeclampsia, the pregnancy is at extremely high risk.17–19
We had previously conducted a candidate-gene study of chronic hypertension and preeclampsia in subjects enrolled at the Boston Medical Center (BMC) (authors' unpublished data). The purpose of the present study was to examine in samples from the previous study the association between hypertension and/or preeclampsia candidate genes in mothers and the birth weight of babies. In addition, we sought to confirm any significant associations found in the initial analysis by analyzing another independent sample set from the same population.
Material and Methods
Study Population and Data Collection
This study is part of an ongoing molecular epidemiological study of preterm birth and low birth weight that has been conducted at the BMC since 1998. Details on the study site, the population, and data collection procedures were described elsewhere.20 Briefly, this is a case-control study in which cases were defined as either low-birth-weight (<2,500 g) or preterm (at <37 wk) births. Of the low-birth-weight infants, 38% had FGR, and 20% had mothers with preeclampsia. Controls were defined as non–low-birth-weight and non-preterm births. Pregnancies resulting in multiple births and newborns with major birth defects were excluded. The BMC preterm study enrolled a multiethnic population, of whom 51% were black, 12% were white, and 26% were Hispanic. Epidemiological and clinical data on each subject, including maternal age, self-reported ethnicity, parity, prepregnancy height and weight, cigarette smoking, alcohol drinking, past medical history, and pregnancy complications, were collected through a maternal questionnaire-based interview and a review of medical records. Maternal venous blood samples were collected postpartum for nucleic-acid extraction and subsequent laboratory analysis. The institutional review boards of the BMC, the Massachusetts Department of Public Health, the Children’s Memorial Hospital in Chicago, and the Harvard School of Public Health approved the study protocol.
We had previously conducted a candidate-gene study of preeclampsia in 323 mothers (204 black, 61 Hispanic, and 31 white) enrolled at the BMC (authors' unpublished data). In the present study, we first examined the association of the hypertension and/or preeclampsia candidate genes with FGR in the sample of 323 women. To minimize population admixture and to ensure adequate sample size, we limited our analysis to the 204 black women, including 34 cases of FGR. Then, we sought confirmation of the association in another independent sample of black women (n=1,094, including 164 FGR cases) enrolled at the BMC.
Definition of Fetal Growth Phenotypes
The most commonly used definition of FGR is birth weight in the lowest 10th percentile of the reference population at the same gestational week. There are more than a dozen published birth-weight-for-gestational-age standards, some of which are widely used in obstetrics, pediatrics, and research.21–23 However, these published birth-weight cutoff points may differ by several hundred grams at any gestational age, possibly because of differences in data sources (hospital- or population-based), population composition, geographic region, measurement of gestational age, and exclusion criteria.24,25
Since 1995, the Department of Obstetrics and Gynecology at the Boston University Medical Center has maintained a computer database of all pregnant women admitted to the BMC labor and delivery service and the birth outcomes. This database now comprises >15,000 deliveries. In this study, we chose to use this internal population as a reference population, with exclusion of pregancies with multiple fetuses and newborns with major birth defects. We used standardized birth weight (SBWT) as a continuous measure of fetal growth, where SBWT is defined as birth weight standardized by the mean and variance in the stratum of the corresponding ethnic group, sex, and gestational week in the reference population, as used widely elsewhere.26–28 We defined FGR as SBWT <10th percentile of the SBWT in the reference population.
Candidate-Gene and SNP Selection
In a previous study (authors' unpublished data), we identified 129 candidate genes potentially important in preeclampsia and chronic hypertension, the major pathogenic pathways of FGR, on the basis of biological plausibility and supportive literature. A total of 576 SNPs in these genes, most of which are nonsynonymous SNPs, were selected from the public dbSNP database for verification. Among them, 432 were polymorphic in our study population, and 68 were common SNPs with minor-allele frequency ⩾0.05. To maintain adequate power, we focused our analysis on the 68 common SNPs, which spread across 41 genes (table 1).
Table 1.
List of Candidate Genes and SNPs
| Biological Pathway and Gene Symbol |
Gene Name | SNP(s) |
| Infection/inflammation: | ||
| ARTS1 | Adipocyte-derived leucine aminopeptidase | rs27895, rs26618 |
| ICAM1 | Intercellular adhesion molecule 1 | rs5491 |
| IL1A | Interleukin 1-alpha | rs17561 |
| Coagulation: | ||
| F5 | Coagulation factor V | rs6030, rs6019, rs6032 |
| Metabolic: | ||
| APOB | Apolipoprotein B | rs1042031, rs1367117 |
| INSR | Insulin receptor | rs2963 |
| LRP8 | Low-density lipoprotein receptor–related protein 8 (ApoE receptor 2) | rs3737983, rs2297660, rs5174 |
| MTHFR | 5,10-Methylenetetrahydrofolate reductase | rs1801131 |
| SLC2A2 | Solute carrier family 2 | rs1800572 |
| Vascular: | ||
| ABCC8 | ATP-binding cassette, subfamily C, member 8 | rs757110 |
| ADRA1A | Alpha-1A-adrenergic receptor | rs1048101 |
| ADRA1D | Alpha-1D-adrenergic receptor | rs835879, rs1556832 |
| ADRB2 | Beta-2-adrenergic receptor, surface | rs1042714 |
| AGTR2 | Angiotensin II receptor, type 2 | rs5191 |
| APOA4 | Apolipoprotein A-IV | rs675 |
| ATP1A1 | ATPase, Na+/K+ transporting | rs1998449 |
| B3GAT1 | Beta-1,3-glucuronyltransferase 1 | rs1440483, rs1866767 |
| CACNA1C | Calcium channel, voltage dependent, L type, alpha-1C subunit | rs215976 |
| CALCA | Calcitonin/calcitonin-related polypeptide, alpha | rs5241 |
| CALCRL | Calcitonin receptor-like | rs696092, rs858745, rs858750, rs3771083, rs698576, rs698574, rs3771073 |
| DBH | Dopamine beta-hydroxylase | rs1108580, rs5324, rs4531 |
| EDN1 | Endothelin 1 | rs2070698, rs5369 |
| EDNRA | Endothelin receptor, type A | rs702757, rs3942348, rs1517135, rs908581 |
| HCARG | Hypertension-related calcium-regulated gene | rs1209879 |
| HTR2A | 5-hydroxytryptamine (serotonin) receptor 2A | rs6314, rs6308, rs6304 |
| KLK1 | Kallikrein 1, renal/pancreal/salivary | rs3212828 |
| KLKB1 | Kallikrein B, plasma (Fletcher factor) 1 | rs4253301, rs4253325 |
| KNG | Kininogen 1 | rs1656922 |
| LPL | Lipoprotein lipase | rs328 |
| NOS2A | Nitric oxide synthase 2A (inducible, hepatocyte) | rs2297518, rs3730017 |
| NPPA | Natriuretic peptide precursor A | rs5063 |
| NPR1 | Natriuretic peptide receptor A/guanylate cyclase A | rs3891075 |
| NPY2R | Neuropeptide Y receptor Y2 | rs2880415 |
| NR3C2 | Nuclear receptor subfamily 3, group C, member 2 | rs5522 |
| PPARGC1 | Peroxisome proliferator-activated receptor-gamma, coactivator 1 | rs3736265 |
| PRCP | Prolylcarboxypeptidase (angiotensinase C) | rs2298668 |
| RENBP | Renin-binding protein | rs2269371 |
| Other: | ||
| GNA11 | Guanine nucleotide–binding protein, alpha-11 | rs2238625 |
| GNAS | GNAS (guanine nucleotide–binding protein, alphastimulating) complex locus | rs7121 |
| PRKCQ | Protein kinase C, theta form | rs2236379 |
| SLC14A2 | Solute carrier family 14, member 2 | rs3745009 |
Extraction of Nucleic Acids and Genotyping
DNA was extracted from samples of venous whole blood in accordance with standard protocol.29 Verification and initial genotyping of SNPs were performed by Illumina using the BeadArray technology. For the confirmatory analysis, SNP rs2297660 was genotyped using the TaqMan genotyping assay (Applied Biosystems). PCR was performed in a 5-μl reaction containing 10 ng of genomic DNA, 1× master mix, 900 nM of forward and reverse primers, and two 250-nM TaqMan MGB probes by use of 384-well plates on a PTC-225 Tetrad Thermal Cycler (MJ Research) under the following conditions: 95°C for 10 min and 50 cycles at 92°C for 15 s and 60°C for 1 min. After PCR, an end-point plate read of the fluorescence intensity of each well was performed on an ABI Primer 7900 (Applied Biosystems). Genotypes were called automatically using SDS, version 2.1 (Applied Biosystems), and were inspected visually on the plot.
All SNPs were in Hardy-Weinberg equilibrium. As a measure of quality control, we also determined the genotypes at rs2297660 in 167 subjects obtained from the BeadArray method in the initial study and found 100% consistency between the two genotyping methods used in our study.
Statistical Analysis
In both the initial and the confirmatory analyses, we used a generalized linear model to estimate the effects of genotype on SBWT and of genotype relative risks on FGR (using the most frequent homozygote genotype as the reference group), with adjustment for maternal age, parity, smoking, alcohol use, and prepregnancy BMI. In addition, we tested for association under additive, dominant, and recessive genetic models. To test for genotype association with SBWT independent of preeclampsia, we also performed the regression analyses with further adjustment for preeclampsia status. To explore possible genotype interactions with BMI and preeclampsia, we also estimated the genotype effects on SBWT with stratification on BMI tertiles and preeclampsia status.
To adjust for multiple tests that we performed in the single-marker test that included 68 SNPs, each with four models, we performed a permutation procedure to obtain empirical P values for assessment of global significance. In the permutation procedure, we first established an SBWT predictive model in our study sample, using maternal age, parity, smoking, alcohol use, and BMI as covariates, and we calculated the SBWT residue from the predictive model in each subject. In each cycle of permutation, we randomly assigned study subjects to one of the calculated SBWT residues and performed regression analysis on all the genetic models for each SNP, with SBWT residue as the dependent variable. We recorded the minimal P value (Ppermute) among the analyses for all possible combinations of SNPs and genetic models in the cycle. We performed a total of 1,000 permutation cycles and correspondingly obtained 1,000 minimal P values. The global P values of our tests were determined empirically as Pglobal=P(Pobserved⩾Ppermute). All analyses were performed using the R statistical package (The R Project for Statistical Computing).
We also performed haplotype association analysis on the three SNPs (rs5174, rs3737983, and rs2297660) in the LRP8 gene (MIM 602600). We first checked the haplotype structure of LRP8 in the Africa American population derived from the International HapMap Project,30 using HaploView,31 and we found that the three SNPs were in a single block. Then, we performed haplotype association analysis on SBWT, using the Haplo.Stat package32 and adjusting for all covariates, as in the single-marker tests.
Results
Phenotypic Characteristics
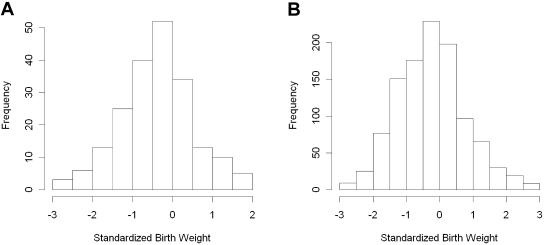
Our initial and confirmatory analyses consisted of 204 and 1,094 black women selected from the BMC preterm cohort, respectively. The initial-analysis samples were originally selected for a case-control study on preeclampsia, whereas the confirmatory-analysis samples were selected from all available subjects in the BMC cohort at the time of the study who were not in the initial analysis. The phenotypic characteristics of the two analysis samples are summarized in table 2. The distributions of age, prepregnancy height, weight, prenatal care during 1st trimester, education level, marital status, and parity were similar in both study samples. Compared with women in the initial sample, women in the confirmatory sample were more likely to drink alcohol during pregnancy; had much lower prevalences of preeclampsia (4.3% vs. 45.1%), preterm deliveries (24.4% vs. 39.7%), and chronic hypertension (2.0% vs. 23.5%); had a longer mean gestation (38.0 wk vs. 36.7 wk); and had babies with a higher mean birth weight (2,959 g vs. 2,625 g). In addition, subjects in the confirmatory sample tended to have a slightly lower BMI and a higher percentage of cigarette smoking, although the differences were not statistically significant. The distribution of SBWT in both study samples was approximately normal, with a slight shift of the mean to the left in the initial sample (fig. 1). The prevalence of FGR in both study samples was also quite similar and was significantly higher than that in the general population.
Table 2.
Characteristics of the Black Women Included in the Initial and Confirmatory Analyses[Note]
|
Genetic Association Analyses |
||
| Characteristic | Initial (n=204) |
Confirmatory (n=1,094) |
| Age (years) | 28.9 ± 7.0 | 27.9 ± 6.6 |
| Age < 20 years | 26 (12.7) | 144 (13.2) |
| Age > 35 years | 45 (22.1) | 179 (16.4) |
| Heighta (cm) | 163.4 ± 6.5 | 163.9 ± 6.9 |
| Weighta (kg) | 71.8 ± 16.5 | 70.3 ± 17.4 |
| BMIa | 26.9 ± 5.9 | 26.1 ± 6.2 |
| BMIa < 20 | 15 (7.4) | 133 (12.2) |
| BMIa > 30 | 48 (23.5) | 209 (19.1) |
| Prenatal care during 1st trimester | 137 (77) | 715 (75.3) |
| Education (high school or beyond) | 138 (68.3) | 785 (72.8) |
| Smoking during pregnancy | 27 (13.2) | 199 (18.2) |
| Alcohol use during pregnancy | 2 (1) | 52 (4.8)b |
| Drug use during pregnancy | 22 (10.8) | 129 (11.8) |
| Married | 66 (32.4) | 364 (33.3) |
| C-section delivery | 72 (35.3) | 325 (30.5) |
| Parity (1+)c | 114 (55.9) | 684 (62.5) |
| Diabetes mellitus | 4 (2) | 16 (1.5) |
| Gestational diabetes | 12 (5.9) | 48 (4.4) |
| Pregnancy-induced hypertensiond | 92 (45.1) | 46 (4.3)b |
| Chronic hypertension | 48 (23.5) | 22 (2.0)b |
| Gestational age at delivery (wk) | 36.7 ± 4.3 | 38.0 ± 3.4b |
| Delivery at <37 wk | 81 (39.7) | 267 (24.4)b |
| Infant’s birth weight (g) | 2,625 ± 957 | 2,959 ± 775b |
| Birth weight < 2,500 g | 83 (40.7) | 252 (23)b |
| Birth weight < 1,500 g | 33 (16.2) | 57 (5.2)b |
| SBWT | −.4 ± .9 | −.3 ± 1.0 |
| FGR | 34 (16.9) | 164 (15.1) |
Note.— Data for quantitative variables are given as mean ± SD. All other data are no. (%) of subjects.
Before pregnancy.
P<.05, obtained by two-tailed t test or χ2 test.
“1+” means at least one previous birth.
Also includes preeclampsia, eclampsia, and HELLP syndrome (a syndrome characterized by hemolysis, elevated liver enzyme levels, and low platelet count).
Figure 1.
A, Distribution of SBWT for the subjects included in the screening study (n=204). B, Distribution of SBWT for the subjects included in the validation study (n=1,094).
The Initial Association Analysis
In the screening analysis, we tested 68 SNPs from 41 candidate genes for association with fetal growth in 204 black women. We found suggestive evidence of association with SBWT for six SNPs in five genes, with an unadjusted P value <.01 in at least one of the tests (table 3). It is noteworthy that all three SNPs in LRP8 were associated with SBWT. In particular, the association between rs2297660 and SBWT (unadjusted P<.0001; adjusted global P<.01) was statistically significant even after adjustment for multiple tests. The birth weights for maternal rs2297660 genotypes AC and AA were 0.48 SD and 0.67 SD higher, respectively, than that for maternal genotype CC. The three LRP8 SNPs were in high linkage disequilibrium in our samples and were found to be in a single haplotype block in both samples (data not shown). Haplotype analysis with the three SNPs produced a blockwide P value of .0004 (table 4). Among all single-marker and haplotype tests on the three SNPs under different genetic models, the strongest association observed was between rs2297660 and SBWT under an additive genetic model. As a result, we sought confirmation of the association between rs2297660 and SBWT in another independent sample.
Table 3.
Selected Results from the Initial Association Analysis of SBWT[Note]
| Gene, SNP, and Genotype |
No. of Women |
β | Praw | Pglobal | Genetic Model |
| LRP8: | |||||
| rs2297660: | .44 | .00004 | .004 | Additive | |
| CC | 130 | ||||
| CA | 63 | ||||
| AA | 11 | ||||
| rs3737983: | .36 | .0005 | .04 | Additive | |
| CC | 114 | ||||
| CT | 76 | ||||
| TT | 14 | ||||
| KLK1: | |||||
| rs3212828: | −.36 | .004 | .30 | Additive | |
| GG | 146 | ||||
| GA | 54 | ||||
| AA | 4 | ||||
| CALCRL: | |||||
| rs698576: | .40 | .007 | .46 | Additive | |
| GG | 162 | ||||
| GA | 41 | ||||
| AA | 1 | ||||
| KNG: | |||||
| rs1656922: | .44 | .007 | .46 | Recessive | |
| TT | 80 | ||||
| TC | 86 | ||||
| CC | 38 | ||||
| F5: | |||||
| rs6019: | −.40 | .013 | .70 | Recessive | |
| GG | 66 | ||||
| GC | 99 | ||||
| CC | 39 |
Note.— Results were computed using a generalized linear model, and only those with P<.01 are listed.
Table 4.
Result of Haplotype Analysis of Association between LRP8 and SBWT
|
Haplotype |
||||
| rs5174 | rs3737983 | rs2297660 |
Haplotype Frequency |
Pa |
| G | C | C | .74 | .003 |
| G | T | C | .05 | .36 |
| G | T | A | .14 | .018 |
| A | T | A | .07 | .009 |
P values were obtained using the haplotype test implemented in Haplo.Stat. The global haplotype P=0.
The Confirmatory and Pooled Analyses
The association between rs2297660 and SBWT was successfully replicated in 1,094 black women selected from the same BMC preterm cohort who were independent from the initial study sample (P<.05) (table 5). However, the differences in the magnitude of the estimated effects of different genotypes on SBWT were smaller in the confirmatory analysis than in the initial analysis. In addition, we estimated the effects of rs2297660 genotype and other covariates on FGR in the pooled sample (table 6). The “A” allele at rs2297660 was associated with a higher SBWT and a lower risk of FGR. Under the additive genetic model, each additional copy of the “A” allele reduced the risk of FGR by 33% (P<.05).
Table 5.
Results of Analyses of Association between rs2297660 and SBWT[Note]
|
CrudeAnalysis |
AdjustedAnalysisa |
|||||||
|
Group and rs2297660 Genotype |
No. of Women |
Mean (±SD) SBWT |
β | SE | P | β | SE | P |
| Screening study: | ||||||||
| CC | 130 | −.58 ± .88 | Ref | … | … | Ref | … | … |
| AC | 63 | −.08 ± .80 | .50 | .13 | .0002 | .48 | .13 | .0004 |
| AA | 11 | .16 ± 1.11 | .74 | .29 | .01 | .67 | .29 | .02 |
| Additiveb | … | −.05 ± .85 | .44 | .11 | .00004 | .41 | .11 | .0001 |
| Validation study: | ||||||||
| CC | 541 | −.26 ± .98 | Ref | … | … | Ref | … | … |
| AC | 245 | −.14 ± 1.02 | .12 | .08 | .12 | .11 | .08 | .14 |
| AA | 46 | .03 ± .99 | .29 | .15 | .06 | .26 | .15 | .08 |
| Additiveb | … | −.11 ± 1.02 | .13 | .06 | .025 | .12 | .06 | .035 |
| Pooled analysis: | ||||||||
| CC | 671 | −.32 ± .97 | Ref | … | … | Ref | … | … |
| AC | 308 | −.13 ± .98 | .19 | .07 | .004 | .19 | .07 | .005 |
| AA | 57 | .05 ± 1.00 | .37 | .14 | .006 | .35 | .13 | .01 |
| Additiveb | … | −.10 ± .99 | .19 | .05 | .0002 | .18 | .05 | .0004 |
Note.— Ref = reference.
Controlled for age, parity, smoking, and BMI.
In additive model, CC = 0, AC = 1, and AA = 2.
Table 6.
Odds Ratios of FGR for rs2297660 Genotype and Other Covariates in the Pooled Sample[Note]
| Covariate | OR (95% CI) | P |
| rs2297660a | .67 (.48–.94) | .02 |
| Age | .97 (.94–1.00) | .03 |
| Parity | 1.01 (.87–1.18) | .8 |
| Smoking | 1.87 (1.18–2.98) | .008 |
| BMI | .99 (.96–1.02) | .6 |
| PIHb | 3.16 (1.96–5.09) | .000002 |
Note.— FGR was defined as SBWT <10th percentile of the SBWT in the reference population.
For rs2297660 genotype, an additive model was used.
PIH = pregnancy-induced hypertension.
Discussion
Preeclampsia is one of the most important risk factors for FGR. In this study, we examined 68 common SNPs in the candidate genes identified on the basis of the major pathogenic pathways from preeclampsia or chronic hypertension to birth weight. We demonstrated a significant association between SNP genotypes in the maternal LRP8 gene and birth weight in two independent samples of black women selected from the BMC preterm cohort. The observed association was independent of preeclampsia status, because there were few changes in estimated genotype effects on SBWT and in levels of significance in the regression analyses with and without adjustment for preeclampsia status. To our knowledge, this is the largest genetic association study of birth weight and FGR in the United States and is the first to implicate maternal LRP8 polymorphisms in the regulation of birth weight among infants of black mothers.
The LRP8 gene, located on chromosome 1p34, consists of 19 exons spanning ∼60 kb and encodes apolipoprotein E (ApoE) receptor 2, which mediates the cellular recognition and internalization of ApoE-containing lipoproteins.33 ApoE is one of the most important regulators of plasma lipids and affects hepatic binding, uptake, and catabolism of several classes of lipoproteins.34 The predicted structure of ApoE receptor 2 contains 963 aa, including a putative 41-aa signal sequence and five functional domains that resemble those of the low-density lipoprotein receptor and the very-low-density lipoprotein receptor. LRP8 is highly expressed in human brain and placenta,35 and its expression is essential for neural development in mice.36 The molecular mechanisms underlying the association between LRP8 and birth weight is unknown, although the gene's high expression level in placenta hints at a possible role in the regulation of the microenvironment of fetal growth.
Of the three LRP8 SNPs that were associated with birth weight, rs2297660 produced the strongest association. The rs2297660 polymorphism elicits a silent nucleotide C→A substitution in the 3rd nucleotide of the 419th codon of LRP8 and does not cause any changes in the structure of the product. We can speculate three mutually exclusive scenarios that might explain the observed association. First, the nucleotide substitution at rs2297660 might affect the stability or translation efficiency of the transcript. Second, the SNP could be in linkage disequilibrium with a true causal variant in close proximity. Third, the association could be spurious. The fact that the same association was replicated in an independent sample in our study suggests that the third scenario is unlikely.
Although we observed a significant association between rs2297660 and SBWT in both study samples, the estimate of the genotype effects from the initial analysis was ∼3 times larger than that from the confirmatory analysis. The major difference between these two study samples is the 10-fold higher percentage of preeclamptic subjects in the initial sample than in the confirmatory one (45.1% vs. 4.3%). In our stratified analysis, the estimated genotype effect was slightly larger in preeclamptic subjects than in non-preeclamptic subjects, although this difference was not statistically significant (P=.48) (table 7). The effects of rs2297660 genotype were 1.35 times larger in preecalmptic subjects than in non-preeclamptic subjects.
Table 7.
Association of LRP8 rs2297660 Genotype and SBWT, Stratified by Pregnancy-Induced Hypertension (PIH) Status[Note]
|
CrudeAnalysis |
Adjusted Analysisa |
||||||
|
Group and rs2297660 Genotype |
No. of Women |
β | SE | P | β | SE | P |
| PIH group: | .30 | .17 | .08 | .23 | .18 | .19 | |
| CC | 73 | ||||||
| AC | 32 | ||||||
| AA | 7 | ||||||
| Non-PIH group: | .18 | .05 | .0008 | .17 | .05 | .001 | |
| CC | 581 | ||||||
| AC | 264 | ||||||
| AA | 49 | ||||||
Note.— An additive inheritance model was used.
Controlled for age, parity, smoking, and BMI.
This study focuses on black women enrolled in the BMC preterm study. The assignment of ethnicity was based on self-reported ethnicity on the questionnaire. The information available does not allow us to further categorize subjects into subpopulations. In the present study, as in most other population-based association studies, population admixture could be an issue that could cause spurious association results.37 In a previous study, we applied a clustering method based on a Bayesian model, to divide subjects enrolled in the BMC preterm study into groups of blacks, Hispanics, and whites on the basis of the genotypes of 31 markers. We found that the self-reported ethnicity groups accounted for, on average, 95% of the underlying admixture.38 It has been suggested that the effects of such admixture can be detected and adjusted for by using a large number of random markers (genomic controls).39 Although this strategy works well in case-control designs, it is not straightforwardly applicable to association tests of quantitative traits like those used in this study.
The frequency of the “A” allele (the protective allele) of rs2297660 was 0.20 in the black sample in our study. In samples of a limited number of white subjects (n=31; 0 cases of FGR) and Hispanic subjects (n=61; 11 cases of FGR) who we genotyped, the frequency of the “A” allele was 0.31 and 0.37, respectively. The low frequency of the protective allele in blacks is consistent with the high prevalence of FGR among blacks. However, the question of whether LRP8 explains racial disparity of FGR requires further data and analyses.
In summary, we have, for the first time, demonstrated a replicable association between maternal genotype of the LRP8 gene and birth weight in black women enrolled at BMC. It will be important to replicate this finding in other study populations and ethnic groups, as well as to examine the effects of LRP8 genotypes in infants and the possible interactions between maternal and infant genotypes. If our findings are confirmed, further study will be warranted to identify functional variants in this gene and to elucidate the underlying biological mechanisms.
Acknowledgments
This study was supported in part by grants 20-FY98-0701 and 20-FY02-56 from the March of Dimes Birth Defects Foundation, grant R01 HD41702 from the National Institute of Child Health and Human Development, and grants R01ES11682, R21 ES11666, and ES-00002 from the National Institute of Environmental Health Sciences. We thank the Boston University Medical Center preterm study advisory group and Drs. Paul Wise, Howard Bauchner, Jerome Klein, Milton Ketochuck, and John Katznica, for their support and guidance throughout the study. We thank Colleen Pearson, Katherine Ortiz, and Emault Louis for their effort in field data collection. We thank the nursing staff of Labor and Delivery at the Boston Medical Center for their assistance with our study. We thank Ann Ramsey, for administrative support, and Lingling Fu, for data entry and management.
Web Resources
The URLs for data presented herein are as follows:
- dbSNP, http://www.ncbi.nlm.nih.gov/SNP/index.html
- International HapMap Project, http://www.hapmap.org/
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for LRP8) [PubMed]
- The R Project for Statistical Computing, http://www.r-project.org/
References
- 1.Creasy RK, Resnik R (1994) Intrauterine growth restriction. In: Creasy RK, Resnik R (eds) Maternal-fatal medicine. WB Saunders, Philadelphia, pp 558–574 [Google Scholar]
- 2.Galbraith RS, Karchmar EJ, Piercy WN, Low JA (1979) The clinical prediction of intrauterine growth retardation. Am J Obstet Gynecol 133:281–286 [DOI] [PubMed] [Google Scholar]
- 3.Kurjak A, Kirkinen P, Latin V (1980) Biometric and dynamic ultrasound assessment of small-for-dates infants: report of 260 cases. Obstet Gynecol 56:281–284 [PubMed] [Google Scholar]
- 4.Hughey MJ (1984) Routine ultrasound for detection and management of the small-for-gestational-age fetus. Obstet Gynecol 64:101–107 [PubMed] [Google Scholar]
- 5.Wennergren M, Wennergren G, Vilbergsson G (1988) Obstetric characteristics and neonatal performance in a four-year small for gestational age population. Obstet Gynecol 72:615–620 [PubMed] [Google Scholar]
- 6.Callan NA, Witter FR (1990) Intrauterine growth retardation: characteristics, risk factors and gestational age. Int J Gynaecol Obstet 33:215–220 10.1016/0020-7292(90)90004-5 [DOI] [PubMed] [Google Scholar]
- 7.Allen MC (1984) Developmental outcome and follow-up of the small for gestational age infant. Semin Perinatol 8:123–156 [PubMed] [Google Scholar]
- 8.McCance DR, Pettitt DJ, Hanson RL, Jacobsson LT, Knowler WC, Bennett PH (1994) Birth weight and non-insulin dependent diabetes: thrifty genotype, thrifty phenotype, or surviving small baby genotype? BMJ 308:942–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lucas A, Fewtrell MS, Cole TJ (1999) Fetal origins of adult disease—the hypothesis revisited. BMJ 319:245–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godfrey KM, Barker DJ (2000) Fetal nutrition and adult disease. Am J Clin Nutr 71:1344S–1352S [DOI] [PubMed] [Google Scholar]
- 11.Vickers MH, Reddy S, Ikenasio BA, Breier BH (2001) Dysregulation of the adipoinsular axis—a mechanism for the pathogenesis of hyperleptinemia and adipogenic diabetes induced by fetal programming. J Endocrinol 170:323–332 10.1677/joe.0.1700323 [DOI] [PubMed] [Google Scholar]
- 12.Li H, Stein AD, Barnhart HX, Ramakrishnan U, Martorell R (2003) Associations between prenatal and postnatal growth and adult body size and composition. Am J Clin Nutr 77:1498–1505 [DOI] [PubMed] [Google Scholar]
- 13.Robinson WP, Barrett IJ, Bernard L, Telenius A, Bernasconi F, Wilson RD, Best RG, Howard-Peebles PN, Langlois S, Kalousek DK (1997) Meiotic origin of trisomy in confined placental mosaicism is correlated with presence of fetal uniparental disomy, high levels of trisomy in trophoblast, and increased risk of fetal intrauterine growth restriction. Am J Hum Genet 60:917–927 [PMC free article] [PubMed] [Google Scholar]
- 14.Anteby EY, Musalam B, Milwidsky A, Blumenfeld A, Gilis S, Valsky D, Hamani Y (2004) Fetal inherited thrombophilias influence the severity of preeclampsia, IUGR and placental abruption. Eur J Obstet Gynecol Reprod Biol 113:31–35 10.1016/j.ejogrb.2003.05.002 [DOI] [PubMed] [Google Scholar]
- 15.Bracken MB, Triche EW, Belanger K, Saftlas A, Beckett WS, Leaderer BP (2003) Asthma symptoms, severity, and drug therapy: a prospective study of effects on 2205 pregnancies. Obstet Gynecol 102:739–752 10.1016/S0029-7844(03)00621-5 [DOI] [PubMed] [Google Scholar]
- 16.Roberts JM, Pearson G, Cutler J, Lindheimer M (2003) Summary of the NHLBI Working Group on Research on Hypertension during Pregnancy. Hypertension 41:437–445 10.1161/01.HYP.0000054981.03589.E9 [DOI] [PubMed] [Google Scholar]
- 17.Sibai BM, Anderson GD (1986) Pregnancy outcome of intensive therapy in severe hypertension in first trimester. Obstet Gynecol 67:517–522 [PubMed] [Google Scholar]
- 18.Mabie WC, Pernoll ML, Biswas MK (1986) Chronic hypertension in pregnancy. Obstet Gynecol 67:197–205 [DOI] [PubMed] [Google Scholar]
- 19.Sibai BM, Abdella TN, Anderson GD (1983) Pregnancy outcome in 211 patients with mild chronic hypertension. Obstet Gynecol 61:571–576 [PubMed] [Google Scholar]
- 20.Wang X, Zuckerman B, Pearson C, Kaufman G, Chen C, Wang G, Niu T, Wise PH, Bauchner H, Xu X (2002) Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. JAMA 287:195–202 10.1001/jama.287.2.195 [DOI] [PubMed] [Google Scholar]
- 21.Brenner WE, Edelman DA, Hendricks CH (1976) A standard of fetal growth for the United States of America. Am J Obstet Gynecol 126:555–564 [DOI] [PubMed] [Google Scholar]
- 22.Lubchenco I, Hansman C, Dressley M, Boyd E (1963) Intrauterine growth as estimated from liveborn birth-weight data at 24 to 42 weeks of gestation. Pediatrics 32:793–800 [PubMed] [Google Scholar]
- 23.Alexander GR, Tompkins ME, Allen MC, Hulsey TC (1999) Trends and racial differences in birth weight and related survival. Matern Child Health J 3:71–79 10.1023/A:1021849209722 [DOI] [PubMed] [Google Scholar]
- 24.Goldenberg RL, Davis RO, Baker RC (1990) Study of preterm birth draws different interpretation. Am J Obstet Gynecol 162:1626–1627 [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Bowes WA Jr (1995) Birth-weight-for-gestational-age patterns by race, sex, and parity in the United States population. Obstet Gynecol 86:200–208 10.1016/0029-7844(95)00142-E [DOI] [PubMed] [Google Scholar]
- 26.Clausson B, Granath F, Ekbom A, Lundgren S, Nordmark A, Signorello LB, Cnattingius S (2002) Effect of caffeine exposure during pregnancy on birth weight and gestational age. Am J Epidemiol 155:429–436 10.1093/aje/155.5.429 [DOI] [PubMed] [Google Scholar]
- 27.Loos RJ, Beunen G, Fagard R, Derom C, Vlietinck R (2002) Birth weight and body composition in young women: a prospective twin study. Am J Clin Nutr 75:676–682 [DOI] [PubMed] [Google Scholar]
- 28.Cnattingius S, Axelsson O, Eklund G, Lindmark G (1985) Smoking, maternal age, and fetal growth. Obstet Gynecol 66:449–452 [PubMed] [Google Scholar]
- 29.Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 30.The International HapMap Consortium (2003) The International HapMap Project. Nature 426:789–796 10.1038/nature02168 [DOI] [PubMed] [Google Scholar]
- 31.Barrett JC, Fry B, Maller J, Daly MJ (2005) Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21:263–265 10.1093/bioinformatics/bth457 [DOI] [PubMed] [Google Scholar]
- 32.Schaid DJ, Rowland CM, Tines DE, Jacobson RM, Poland GA (2002) Score tests for association between traits and haplotypes when linkage phase is ambiguous. Am J Hum Genet 70:425–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim DH, Magoori K, Inoue TR, Mao CC, Kim HJ, Suzuki H, Fujita T, Endo Y, Saeki S, Yamamoto TT (1997) Exon/intron organization, chromosome localization, alternative splicing, and transcription units of the human apolipoprotein E receptor 2 gene. J Biol Chem 272:8498–8504 10.1074/jbc.272.13.8498 [DOI] [PubMed] [Google Scholar]
- 34.Mahley RW, Huang Y (1999) Apolipoprotein E: from atherosclerosis to Alzheimer’s disease and beyond. Curr Opin Lipidol 10:207–217 10.1097/00041433-199906000-00003 [DOI] [PubMed] [Google Scholar]
- 35.Kim DH, Iijima H, Goto K, Sakai J, Ishii H, Kim HJ, Suzuki H, Kondo H, Saeki S, Yamamoto T (1996) Human apolipoprotein E receptor 2: a novel lipoprotein receptor of the low density lipoprotein receptor family predominantly expressed in brain. J Biol Chem 271:8373–8380 10.1074/jbc.271.14.8373 [DOI] [PubMed] [Google Scholar]
- 36.Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, Hammer RE, Richardson JA, Herz J (1999) Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell 97:689–701 10.1016/S0092-8674(00)80782-5 [DOI] [PubMed] [Google Scholar]
- 37.Cardon LR, Palmer LJ (2003) Population stratification and spurious allelic association. Lancet 361:598–604 10.1016/S0140-6736(03)12520-2 [DOI] [PubMed] [Google Scholar]
- 38.Hao K, Wang X, Niu T, Xu X, Li A, Chang W, Wang L, Li G, Laird N (2004) A candidate gene association study on preterm delivery: application of high-throughput genotyping technology and advanced statistical methods. Hum Mol Genet 13:683–691 10.1093/hmg/ddh091 [DOI] [PubMed] [Google Scholar]
- 39.Freedman ML, Reich D, Penney KL, McDonald GJ, Mignault AA, Patterson N, Gabriel SB, Topol EJ, Smoller JW, Pato CN, Pato MT, Petryshen TL, Kolonel LN, Lander ES, Sklar P, Henderson B, Hirschhorn JN, Altshuler D (2004) Assessing the impact of population stratification on genetic association studies. Nat Genet 36:388–393 10.1038/ng1333 [DOI] [PubMed] [Google Scholar]