Abstract
Early (EAD) and delayed (DAD) afterdepolarizations-induced triggered activity is capable of initiating and maintaining cardiac arrhythmias. EAD-induced triggered responses are traditionally thought to be involved in the generation of ventricular arrhythmias under long QT conditions and are precipitated by bradycardia or long pauses. In contrast, DAD-induced triggered activity commonly underlies arrhythmias precipitated by tachycardia. Spontaneous release of calcium from the sarcoplasmic reticulum (SR) secondary to cellular calcium overload induces DADs and some forms of EADs. Recent studies from our laboratory have uncovered a novel mechanism giving rise to triggered activity, termed “late-phase 3 EAD,” which combines properties of both EAD and DAD, but has its own unique character. Late-phase 3 EAD-induced triggered extrasystoles represent a new concept of arrhythmogenesis in which abbreviated repolarization permits “normal SR calcium release#x201D; to induce an EAD-mediated closely coupled triggered response, particularly under conditions permitting intracellular calcium loading. This review briefly describes the mechanisms and properties of late-phase 3 EADs, how they differ from conventional EADs and DADs, as well as their role in the initiation of cardiac arrhythmias, such as atrial fibrillation. (PACE 2006; 29:290-295)
Keywords: triggered activity, atrial fibrillation
Conventional EAD and DAD
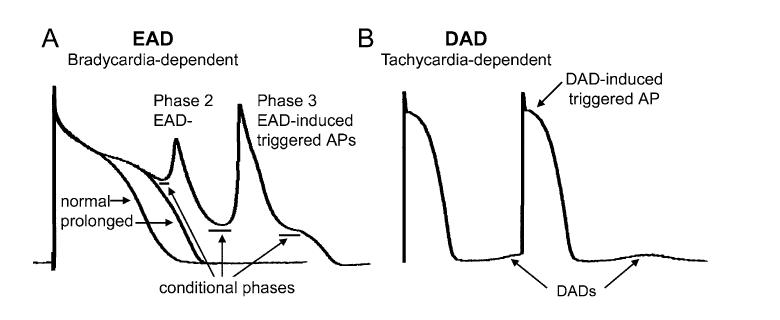
Early (EAD) and delayed (DAD) afterdepolarizations are defined as oscillations that attend or follow the cardiac action potential and depend on preceding activation for their manifestation (Fig. 1). EADs interrupt or retard repolarization during phase 2 and/or phase 3 of the cardiac action potential, whereas DADs arise after full repolarization. EADs appearing at potential positive to -30 mV are typically referred to as phase 2 EAD. EADs occurring at a more negative potential are generally termed as phase 3 EAD. EAD- or DAD-induced triggered action potentials (i.e., triggered activity) appear when EAD or DAD amplitude brings the membrane to its threshold potential (Fig. 1). These triggered events may be responsible for extrasystoles and tachyarrhythmias that develop under conditions predisposing to the development of afterdepolarizations. Another important role of EADs in arrhythmogenesis is an aggravation of dispersion of repolarization, occurring when EAD or EAD-induced action potential causes a local (nonpropagating) response. Dispersion of repolarization predisposes to the development of reentrant arrhythmias.
Figure 1.
Early (EAD) and delayed (DAD) afterdepolarizations and EAD- and DAD-induced triggered action potentials (AP). (A) Phase 2 EAD and phase 3 EAD-induced APs in canine isolated Purkinje fiber preparation treated with d-sotalol (I Kr block). The conditional phase of EAD is defined as the time interval spanning from the moment when membrane potential starts to deviate from normal course to the moment that immediately precedes the EAD upstroke or downstroke. (B) DAD and DAD-induced triggered activity in canine ventricular preparation induced by rapid pacing in the presence of isoproterenol (β-adrenergic agonist, augmenting intracellular calcium activity).
Like action potential duration (APD), EAD and DAD manifestation is a sensitive function of heart rate. EADs are commonly associated with prolongation of repolarization and promoted by bradycardia or pauses.1,2 In contrast, the appearance of DADs is commonly associated with rapid heart rates.1 In the presence of β-adrenergic receptor stimulation, EADs and DADs may appear at the same rate, equivalent to the resting sinus rhythm.2 EADs can be transiently induced or accentuated by an acceleration from a slow to normal rates.3 The conventional EAD, however, does not occur during tachycardia.
The ionic mechanism responsible for the DAD is fairly well established and invariably related to cellular calcium loading, which occurs at rapid activation rates and is commonly facilitated by digitalis, β-adrenoreceptor stimulation, and low extracellular potassium. DADs are initiated by spontaneous sarcoplasmic reticulum (SR) calcium release following the end of the action potential. This spontaneous SR calcium release activates transient inward current (Iti), which underlies the DAD voltage oscillation (Fig. 1). Iti largely comprises inward Na/Ca exchange (INa-Ca) and calcium-activated chloride (ICa(Cl)) currents.
Ionic mechanisms underlying the conventional EAD are quite variable and depend on the mode of EAD induction, tissues/cells used, and voltage at which the EAD occurs. A reduction of the rapidly and slowly activating delayed rectified potassium currents (IKr and IKs) and augmentation of the L-type calcium inward current (ICa(L))or late sodium current (late INa) prolongs to APD and predisposes to the development of EADs. EADs can appear without involvement of intracellular calcium activity (Cai); however, augmentation of Cai under conditions of APD prolongation can greatly facilitate the development of EADs (e.g., by β-adrenergic receptor stimulation or acceleration of pacing rate).2-4 β-adrenergic receptor stimulation (isoproterenol) alone may induce EAD in isolated single cardiac myocytes,2 but not in multi-cellular preparations.5 SR calcium release is more likely to be involved in the generation of EAD in the ventricular muscle, but not or less so in Purkinje fibers,6 presumably due to a poorly developed SR system in the Purkinje cell.
DADs and Cai-dependent EADs have a number of features in common.2 Spontaneous SR calcium release initiates the DAD and, when it occurs during the course of the action potential, determines the initial phase, termed the conditional phase, of Cai-dependent EAD.2,4,7 Na/Ca exchanger current is thought to be largely responsible for the conditional phase of both phase 2 and phase 3 EAD.2,3,7
EAD and DAD in Ventricles and Atria
DAD can readily develop in Purkinje fibers and ventricular and atrial muscle.1,5,8 While numerous investigators have observed EAD in Purkinje and ventricular muscle under appropriate conditions,1,3,9 EAD has been reported only in very few cases in atria. These atrial EADs are associated with augmented intracellular calcium activity and rapid rates.10-12 Atrial muscles seem to be resistant to generating EADs in response to agents that prolong repolarization.13,14 It is consistent with the clinical observation that Class III antiarrhythmic agents are generally associated with ventricular, but not atrial, proarrhythmia. Some congenital long QT patients as well as cesium-treated dogs, however, have been reported to develop “atrial torsade de pointes” and atrial EAD-like deflections on monophasic action potential recordings.15,16 Such atrial arrhythmias are not prevalent in the clinic.17
Atrial cells seem to be capable of producing only phase 3 EAD.10-12 Ventricular cells develop only phase 2 EAD, whereas Purkinje fibers develop both phase 2 and phase 3 EAD.3,9,18,19 In the ventricle, Purkinje fiber and M cell tissues, which have reduced levels of IKs, develop EADs and DADs more readily than epicardial and endocardial tissues, which have a more robust IKs.5,9,20,21 When IKs is reduced pharmacologically, the ventricular epicardium and endocardium readily develop both EADs and DADs under appropriate conditions.5,9
Clinical Arrhythmias Associated with Conventional EAD and DAD Activity
EAD- and DAD-induced triggered activity can initiate and maintain both atrial and ventricular arrhythmias. Triggered activity can provide the extrasystole that precipitates tachyarrhythmias that are maintained by reentrant mechanisms (e.g., ventricular or atrial tachycardia and fibrillation).19 Some forms of ventricular and atrial tachyarrhythmia may be maintained by a focal mechanism, presumably DAD-induced triggered activity.22,23 DAD activity is thought to participate in the generation of the arrhythmias occurring in the setting of cellular calcium loading (e.g., digitalis toxicity, augmented sympathetic tone, exercise, hypokalemia). EAD activity is involved in precipitating polymorphic ventricular tachycardia associated with the congenital or acquired long QT syndromes (i.e., Torsade de pointes (TdP)). TdP is thought to be initiated by EAD-induced triggered activity and is maintained by reentry. While EAD is most likely to initiate TdP in the congenital LQT2/LQT6 (reduction of IKr) and LQT3 (augmentation INa(late)) forms of the long QT syndrome, LQT1/LQT5-related TdP (reduction in IKs) may be initiated by DAD, rather than EAD.5,19
Acquired forms of the long QT syndrome most often develop in response to drugs that block IKr, although many block IKs as well. These pharmacological agents precipitate TdP via an EAD-induced triggered mechanism. Ventricular hyper-trophy and heart failure, which are also associated with life-threatening arrhythmias, are known to promote DAD- and EAD-induced triggered activity. Acute ischemia and particularly reperfusion are also associated with cellular calcium loading that can promote the appearance of DAD.
Late-Phase 3 EAD
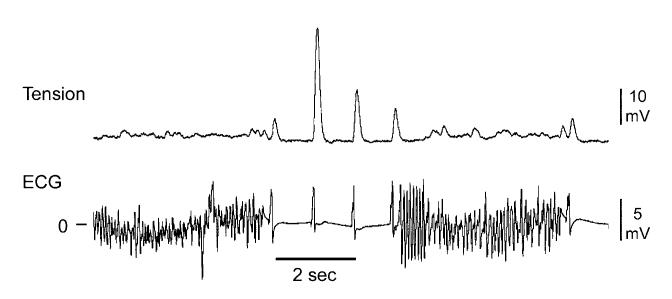
Immediate reinduction of atrial fibrillation (AF) occurs in up to 56% of patients after successful electrical cardioversion of the arrhythmia.24-26 A reentrant mechanism is thought to underlie the maintenance of the arrhythmia, but recent evidence points to a novel mechanism, known as late-phase 3 EAD, as the basis for the premature beat(s) that reinduce AF (Fig. 2).11 We first described this interesting extrasystolic mechanism in isolated coronary-perfused canine right atrial preparation used to create experimental models of AF. The parasympathetic neurotransmitter acetylcholine was used to abbreviate APD, allowing very rapid pacing and AF induction. AF susceptibility is commonly associated with an abbreviation of the atrial APD. Late-phase 3 EAD-induced triggered beat(s) were observed to arise after termination of AF and to be responsible for the immediate reinduction of AF (Fig. 3). Dramatic APD abbreviation, very rapid rates of excitation, and strong SR calcium release were required to elicit EADs in the initial period following termination of AF or rapid pacing. These EADs are distinguished by the fact that they interrupt the final phase of repolarization of the action potential (late-phase 3). These characteristics of the EAD are distinctly different from those generally encountered and represent a hybrid between the EAD and DAD activity normally encountered in ventricular myocardium. However, in striking contrast to all previously described DAD or Cai-depended EAD, it is likely to be normal, not spontaneous SR calcium release that is responsible for the generation of the EAD under these conditions. To distinguish these EADs from those commonly encountered in the presence of agents with Class III antiarrhythmic action and/or β-adrenergic agonist actions, we have termed these as “late-phase 3 EAD.” Note that Purkinje fiber may develop EAD during late-phase 3 repolarizations, but these occur only at slow rates when repolarization is dramatically prolonged, and, if Cai is involved, it is spontaneous release, rather than normal SR calcium release mediated.1,7 Normal SR calcium release is induced by transmembrane entry of calcium via ICa(L) (so-called calcium-induced calcium release).
Figure 2.
An example of spontaneous termination of acetylcholine-mediated AF and its immediate reinitiation in a canine isolated coronary-perfused right atrial preparation. Note the significant transient augmentation of phasic tension (which approximates the amount of sarcoplasmic reticulum calcium release) following the termination of AF and its association with the immediate AF recurrence. (Reproduced with permission from Ref. 11.)
Figure 3.
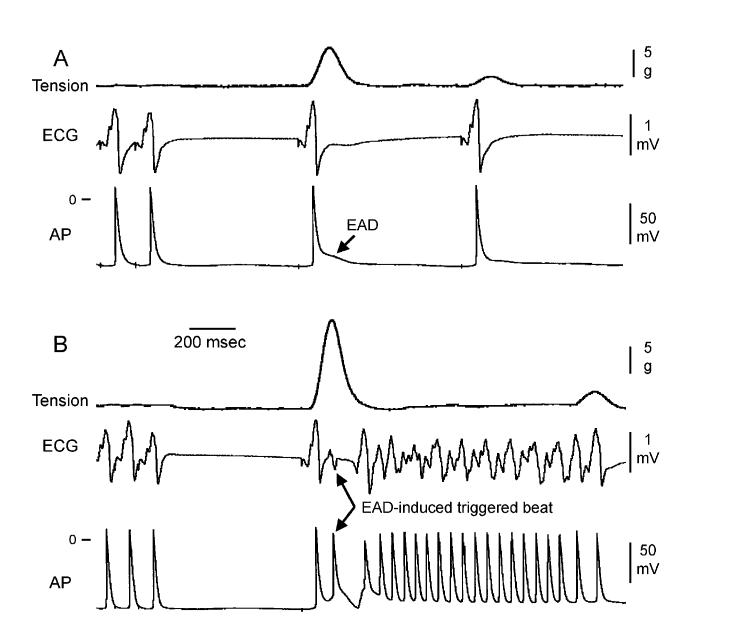
Late-phase 3 EAD-induced extrasystole arising following termination of rapid pacing initiates AF. Tension, ECG, and transmembrane action potential (AP) recordings recorded as the pacing CL was increased from 150 to 700 ms (A) or from 100 to 700 ms (B) are shown. (A) With rapid pacing at a CL of 150 ms, only a modest increase in tension and a prolongation of late repolarization are observed in the first AP recorded after termination of rapid pacing. (B) With rapid pacing at a CL of 100 ms, a more dramatic increase in phasic tension is associated with the development of a late-phase 3 EAD-induced triggered beat, which initiates a run of AF. (Reproduced with permission from Ref. 11.)
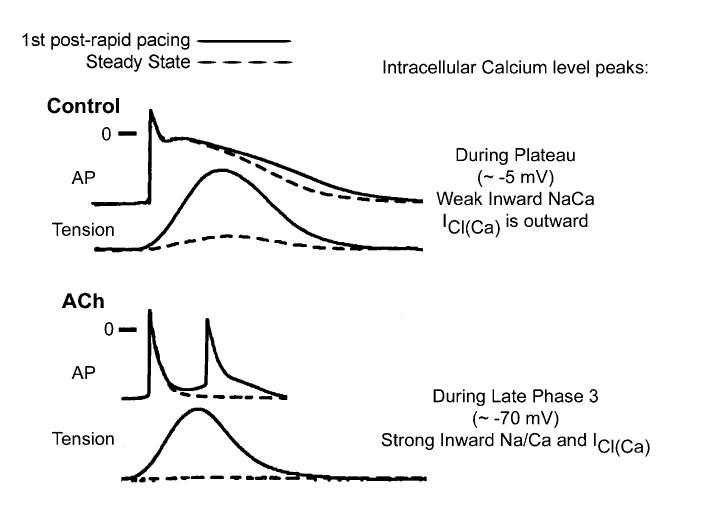
Although potentiated contractions due to augmented SR calcium release are observed upon termination of rapid activation rates in control and in the presence of Ach, late-phase 3 EADs are observed only when APD is markedly abbreviated as with acetylcholine.11 A possible explanation for how and why late-phase 3 EADs and triggered activity develop under conditions of short but not normal APD is summarized in Figure 4. Transient APD prolongation, EADs, and extrasystoles observed immediately after termination of AF or rapid pacing appear to be secondary to accentuated SR calcium release, since SR calcium release blocker ryanodine eliminates the appearance of all.11 Based on the time course of contraction, levels of intracellular calcium (Cai) would be expected to peak during the plateau of the action potential (membrane potential of approximately -5 mV) under control conditions, but during the late phase of repolarization (membrane potential of approximately -70 mV) in the presence of acetylcholine. As a consequence, the two principal calcium-mediated currents, INa-Ca and ICl(Ca), would be expected to be weakly inward or even outward (ICl(Ca)) when APD is normal (control), but strongly inward when APD is very short (acetylcholine). Thus, abbreviation of the atrial APD allows for a much stronger recruitment of both INa-Caand ICl(Ca) in the generation of a late-phase 3 EAD. It is noteworthy that the proposed mechanism is similar to that thought to underlie the development of DADs and conventional Cai-dependent EAD.2,27 The principal difference is that in the case of these DADs/EADs, INa-Ca, and ICl(Ca) are recruited secondary to a spontaneous release of calcium from the SR, whereas in the case of late-phase 3 EADs, these currents are accentuated as a consequence of the normal SR release mechanisms.
Figure 4.
Proposed mechanism for the development of late-phase 3 EADs. Superimposed action potential (AP) and phasic tension recordings obtained under steady-state conditions and during the first regular postrapid pacing beat in control and in the presence of acetylcholine are shown. See text for further discussion. (Re-produced with permission from Ref. 11.)
The appearance of late-phase 3 EAD immediately following termination of AF or rapid pacing has been recently reported by another group of investigators in canine atria in vivo.28 Patterson et al.12 have recently described “tachycardia pause”-induced EAD in isolated superfused canine pulmonary vein muscular sleeve preparations in the presence of both simultaneous parasympathetic (to abbreviate APD) and sympathetic (to augment Cai) nerve stimulation. This EAD also appears during late phase 3 of the action potential.12 Patterson et al.12 suggested the same mechanism (i.e., “Cai outlasting repolarization” and “normal SR calcium release-induced EAD”), as originally suggested for the late-phase 3 EAD,11 to explain EAD in their experiments. A similar mechanism has recently been invoked to explain catecholamine-induced afterdepolarizations and ventricular tachycardia in mice.29
The mechanism responsible for the transient increase in atrial and ventricular contractility (i.e., SR calcium release) immediately following the transition from rapid to normal rates is fairly well established.30 Rapid activation rates result in an increase in intracellular Na+, leading to cellular calcium loading mediated by Na/Ca exchange (generating an outward current). A transient period of hypercontractility occurs immediately following return to normal rates because of augmented SR calcium loading and release. The strong SR calcium release at normal rates then stimulates extrusion of calcium through Na/Ca exchanger, explaining the transient nature of hypercontractility. The electrogenic inward current generated by the exchanger is likely responsible for the transient APD prolongation and EAD development.
Clinical Implications for Late-Phase 3 EAD
Late-phase 3 EAD-induced triggered beats may be responsible for the immediate reinitiation of AF following termination of paroxysmal AF, but perhaps less likely to be involved in AF reinitiation in cases of persistent AF or AF reinitiation occurring days or months after AF termination. Indeed, a transient period of post-AF hypercontractility seems to take place following termination of short, but not long-lasting, AF.31 It is noteworthy that the highest incidence of immediate reinduction of AF occurs following the termination of a short-lasting AF (<1-3 hours).25,26 Early AF reinitiation most often occurs within 1-2 minutes after successful cardioversion of AF of any duration.25,26,32
The conditions that give rise to late-phase 3 EADs may also occur immediately following termination of other tachyarrhythmias (atrial flutter or tachycardia, ventricular tachycardia or fibrillation). All of these are known to abbreviate repolarization and induce a transient potentiation of SR calcium release upon return to normal (sinus) rhythm.30 Extrasystolic activity or bigeminy can also lead to postextrasystolic potentiation of SR calcium release. Bigeminal pacing protocols are known to promote the appearance of premature beats and increase atrial vulnerability.33 It remains to be determined if “normal SR calcium release”-induced triggered activity may be involved in the initiation of arrhythmias in the short QT or Brugada syndromes, as well as the other conditions associated with abbreviated repolarization.
An abbreviated atrial repolarization is a typical feature of AF-susceptible patients. APD abbreviation is traditionally considered to lead to the development of an arrhythmogenic substrate by reducing the wavelength for reentry.19 The re-entrant wavelength is calculated as the product of conduction velocity and refractory period (or APD). The results of our11 and other studies12,28 have demonstrated that abbreviation of repolarization when combined with an augmentation Cai may also contribute to arrhythmogenesis by allowing for the development of late-phase 3-EAD-induced triggered activity. The initiation of some clinical AF is thought to be related to an interaction of both sympathetic and parasympathetic systems.34 It has been shown that simultaneous stimulation of both branches of the autonomic nervous system (causing both APD abbreviation and augmentation of Cai) may lead to the induction of late-phase 3 EAD in canine pulmonary vein muscular sleeves.12 Pulmonary veins are known to be a principal source of electrical activity initiating paroxysmal AF.35
Footnotes
Supported by grant HL47678 from NHLBI (CA) and grants from the American Heart Association (AB and CA), and the Masons of NYS and Florida.
References
- 1.Wit AL, Rosen MR. Afterdepolarizations and triggered activity: Distinction from automaticity as an arrhythmogenic mechanism. In: Fozzard HA, Haber E, Jennings RB, Katz AM, Morgan HE, editors. The Heart and Cardiovascular System. Raven Press; New York: 1992. pp. 2113–2164. [Google Scholar]
- 2.Volders PG, Vos MA, Szabo B, et al. Progress in the understanding of cardiac early afterdepolarizations and torsades de pointes: Time to revise current concepts. Cardiovasc Res. 2000;46:376–392. doi: 10.1016/s0008-6363(00)00022-5. [DOI] [PubMed] [Google Scholar]
- 3.Burashnikov A, Antzelevitch C. Acceleration-induced action potential prolongation and early afterdepolarizations. J Cardiovasc Electrophysiol. 1998;9:934–948. doi: 10.1111/j.1540-8167.1998.tb00134.x. [DOI] [PubMed] [Google Scholar]
- 4.Patterson E, Scherlag BJ, Szabo B, Lazzara R. Facilitation of epinephrine-induced afterdepolarizations by class III antiarrhythmic drugs. J Electrocardiol. 1997;30:217–224. doi: 10.1016/s0022-0736(97)80007-6. [DOI] [PubMed] [Google Scholar]
- 5.Burashnikov A, Antzelevitch C. Block of IKs does not induce early afterdepolarization activity but promotes β-adrenergic agonist-induced delayed afterdepolarization activity. J Cardiovasc Electro-physiol. 2000;11:458–465. doi: 10.1111/j.1540-8167.2000.tb00342.x. [DOI] [PubMed] [Google Scholar]
- 6.Burashnikov A, Antzelevitch C. Mechanisms underlying early afterdepolarization activity are different in canine Purkinje and M cell preparations. Role of intracellular calcium. (abstract) Circulation. 1996;94:I–527. [Google Scholar]
- 7.Szabo B, Sweidan R, Rajagopalan C, Lazzara R. Role of Na+: Ca2+ exchange current in Cs-induced early afterdepolarizations in Purkinje fibers. J Cardiovasc + Electrophysiol. 1994;5:933–944. doi: 10.1111/j.1540-8167.1994.tb01133.x. [DOI] [PubMed] [Google Scholar]
- 8.Wang YG, Huser J, Blatter LA, Lipsius SL. Withdrawal of acetylcholine elicits Ca2+-induced delayed afterdepolarizations in cat atrial myocytes. Circulation. 1997;96:1275–1281. doi: 10.1161/01.cir.96.4.1275. [DOI] [PubMed] [Google Scholar]
- 9.Burashnikov A, Antzelevitch C. Prominent IKs in epicardium and endocardium contributes to development of transmural dispersion of repolarization but protects against development of early afterdepolarizations. J Cardiovasc Electrophysiol. 2002;13:172–177. doi: 10.1046/j.1540-8167.2002.00172.x. [DOI] [PubMed] [Google Scholar]
- 10.Chen YJ, Chen SA, Chen YC, et al. Effects of rapid atrial pacing on the arrhythmogenic activity of single cardiomyocytes from pulmonary veins: Implication in initiation of atrial fibrillation. Circulation. 2001;104:2849–2854. doi: 10.1161/hc4801.099736. [DOI] [PubMed] [Google Scholar]
- 11.Burashnikov A, Antzelevitch C. Reinduction of atrial fibrillation immediately after termination of the arrhythmia is mediated by late phase 3 early afterdepolarization-induced triggered activity. Circulation. 2003;107:2355–2360. doi: 10.1161/01.CIR.0000065578.00869.7C. [DOI] [PubMed] [Google Scholar]
- 12.Patterson E, Po SS, Scherlag BJ, Lazzara R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm. 2005;2:624–631. doi: 10.1016/j.hrthm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Wang ZG, Pelletier LC, Talajic M, Nattel S. Effects of flecainide and quinidine on human atrial action potentials: Role of rate-dependence and comparison with guinea pig, rabbit, and dog tissues. Circulation. 1990;82:274–283. doi: 10.1161/01.cir.82.1.274. [DOI] [PubMed] [Google Scholar]
- 14.Burashnikov A, Mannava S, Antzelevitch C. Transmembrane action potential heterogeneity in the canine isolated arterially-perfused atrium: Effect of IKr and Ito/IKur block. Am J Physiol. 2004;286:H2393–H2400. doi: 10.1152/ajpheart.01242.2003. [DOI] [PubMed] [Google Scholar]
- 15.Kirchhof P, Eckardt L, Franz MR, et al. Prolonged atrial action potential durations and polymorphic atrial tachyarrhythmias in patients with long QT syndrome. J Cardiovasc Electrophysiol. 2003;14:1027–1033. doi: 10.1046/j.1540-8167.2003.03165.x. [DOI] [PubMed] [Google Scholar]
- 16.Satoh T, Zipes DP. Cesium-induced atrial tachycardia degenerating into atrial fibrillation in dogs: Atrial torsades de pointes. J Cardiovasc Electrophysiol. 1998;9:970–975. doi: 10.1111/j.1540-8167.1998.tb00137.x. [DOI] [PubMed] [Google Scholar]
- 17.Vincent GM. Atrial arrhythmias in the inherited long QT syndrome. J Cardiovasc Electrophysiol. 2003;14:1034–1035. doi: 10.1046/j.1540-8167.2003.03365.x. [DOI] [PubMed] [Google Scholar]
- 18.Davidenko JM, Cohen L, Goodrow RJ, Antzelevitch C. Quinidine-induced action potential prolongation, early afterdepolarizations, and triggered activity in canine Purkinje fibers. Effects of stimulation rate, potassium, and magnesium. Circulation. 1989;79:674–686. doi: 10.1161/01.cir.79.3.674. [DOI] [PubMed] [Google Scholar]
- 19.Antzelevitch C, Burashnikov A. Mechanisms of arrhythmogenesis. In: Podrid PJ, Kowey PR, editors. Cardiac Arrhythmia: Mechanisms, Diagnosis and Management. 2nd Ed Lippincott Williams & Wilkins; Philadelphia: 2001. pp. 51–79. [Google Scholar]
- 20.Antzelevitch C, Sicouri S. Clinical relevance of cardiac arrhythmias generated by afterdepolarizations. Role of M cells in the generation of U waves, triggered activity and torsade de pointes. J Am Coll Cardiol. 1994;23:259–277. doi: 10.1016/0735-1097(94)90529-0. [DOI] [PubMed] [Google Scholar]
- 21.Liu DW, Antzelevitch C. Characteristics of the delayed rectifier current (IKr and IKs) in canine ventricular epicardial, midmyocardial, and endocardial myocytes. Circ Res. 1995;76:351–365. doi: 10.1161/01.res.76.3.351. [DOI] [PubMed] [Google Scholar]
- 22.Lerman BB, Stein K, Engelstein ED, et al. Mechanism of repetitive monomorphic ventricular tachycardia. Circulation. 1995;92:421–429. doi: 10.1161/01.cir.92.3.421. [DOI] [PubMed] [Google Scholar]
- 23.Zhou S, Chang CM, Wu TJ, et al. Nonreentrant focal activations in pulmonary veins in canine model of sustained atrial fibrillation. Am J Physiol. 2002;283:H1244–H1252. doi: 10.1152/ajpheart.01109.2001. [DOI] [PubMed] [Google Scholar]
- 24.Wellens HJ, Lau CP, Luderitz B, et al. Atrioverter: An implantable device for the treatment of atrial fibrillation. Circulation. 1998;98:1651–1656. doi: 10.1161/01.cir.98.16.1651. [DOI] [PubMed] [Google Scholar]
- 25.Oral H, Ozaydin M, Sticherling C, et al. Effect of atrial fibrillation duration on probability of immediate recurrence after transthoracic cardioversion. J Cardiovasc Electrophysiol. 2003;14:182–185. [PubMed] [Google Scholar]
- 26.Schwartzman D, Musley SK, Swerdlow C, Hoyt RH, Warman EN. Early recurrence of atrial fibrillation after ambulatory shock conversion. J Am Coll Cardiol. 2002;40:93–99. doi: 10.1016/s0735-1097(02)01912-5. [DOI] [PubMed] [Google Scholar]
- 27.Zygmunt AC, Goodrow RJ, Weigel CM. INaCa and ICl(Ca) contribute to isoproterenol-induced delayed afterdepolarizations in midmyocardial cells. Am J Physiol. 1998;275:H1979–H1992. doi: 10.1152/ajpheart.1998.275.6.H1979. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe I, Okumura Y, Ohkubo K, et al. Steady-state and nonsteady-state action potentials in fibrillating canine atrium: Alternans of action potential and late phase 3 early afterdepolarization as a precursor of atrial fibrillation. (abstract) Heart Rhythm. 2005;2:S259. [Google Scholar]
- 29.Kirchhof P, Klimas J, Fabritz L, et al. Mechanism of catecholamine-induced ventricular tachycardias in mice with heart-directed expression of junctin and triadin: Shortening of action potentials and prolonging calcium transients. (abstract) Heart Rhythm. 2005;2:S69. [Google Scholar]
- 30.Bers DM. 2nd Ed Kluwer Academic Publishers; Amsterdam: 2001. Excitation-Contraction Coupling and Cardiac Contractile Force. [Google Scholar]
- 31.Leistad E, Christensen G, Ilebekk A. Atrial contractile performance after cessation of atrial fibrillation. Am J Physiol. 1993;264:H104–H109. doi: 10.1152/ajpheart.1993.264.1.H104. [DOI] [PubMed] [Google Scholar]
- 32.Timmermans C, Rodriguez LM, Smeets JL, Wellens HJ. Immediate reinitiation of atrial fibrillation following internal atrial defibrillation. J Cardiovasc Electrophysiol. 1998;9:122–128. doi: 10.1111/j.1540-8167.1998.tb00893.x. [DOI] [PubMed] [Google Scholar]
- 33.Attuel P, Leclercq JF, Halimi F, Fiorello P, Stiubei M, Seing S. Bigeminy pacing: A new protocol to unmask atrial vulnerability. J Cardiovasc Electrophysiol. 2003;14:10–15. doi: 10.1046/j.1540-8167.2003.02194.x. [DOI] [PubMed] [Google Scholar]
- 34.Bettoni M, Zimmermann M. Autonomic tone variations before the onset of paroxysmal atrial fibrillation. Circulation. 2002;105:2753–2759. doi: 10.1161/01.cir.0000018443.44005.d8. [DOI] [PubMed] [Google Scholar]
- 35.Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]