Abstract
Role of Late Na Current in Arrhythmogenesis. Ventricular myocardium in larger mammals is composed of three distinct cell types: epicardial, M, and endocardial cells. Epicardial and M cell, but not endocardial cell, action potentials have a prominent Ito-mediated notch. M cells are distinguished from the other cell types in that they display a smaller IKs, but a larger late INa and INa-Ca-. These ionic differences may account for the longer action potential duration (APD) and steeper APD-rate relationship of the M cell. The difference in the time course of repolarization of phase 1 and phase 3 contributes to the inscription of the electrocardiographic J wave and T wave, respectively. These repolarization gradients are modulated by electrotonic interactions, [K+]o, and agents or mutations that alter net repolarizing current. An increase in late INa, as occurring under a variety of pathophysiological states or in response to certain toxins, leads to a preferential prolongation of the M cell action potential, thus prolonging the QT interval and increasing transmural dispersion of repolarization (TDR), which underlies the development of torsade de pointes (TdP) arrhythmias. Agents that reduce late INa are effective in reducing TDR and suppressing TdP. A reduction in peak INa or an increase in net repolarizing current in the early phases of the action potential can lead to a preferential abbreviation of the action potential of epicardium in the right ventricle, and thus the development of a large TDR, phase 2 reentry, and polymorphic ventricular tachycardia associated with the Brugada syndrome.
Keywords: cardiac heterogeneity, M cell, electrocardiogram, long QT syndrome, Brugada syndrome
Electrical Heterogeneity
Heterogeneity of repolarization within the ventricular myocardium was recognized by Einthoven at the turn of the 20th century, and by the great men of science who followed him. Transmural differences in repolarization were largely delineated over the past decade and a half. Today, three distinct myocardial cell types are recognized: epicardial, M, and endocardial cells. Differences in the electrophysiologic characteristics and pharmacologic profiles of these three myocardial cell types have been described in the dog, guinea pig, rabbit, and human ventricles.1
The three predominant cell types differ with respect to the currents that contribute to the early repolarization phase or phase 1. The action potentials of epicardial and M cells have a prominent transient outward current (Ito)-mediated phase 1 that is absent in endocardial cells (see Antzelevitch and Dumaine1 for references). The early repolarization phase gives the epicardial action potential a notched appearance. In the canine heart, Ito and the action potential notch are much larger in the right than in the left ventricular (LV) epicardium2 and M cells.3
The hallmark of the M cell is the ability of its action potential to prolong more than that of epicardium or endocardium with slowing of rate. In the early 1990s, the M cells became the focus of intense investigation after their identification and characterization in the deep structures of the canine ventricle.4-6 M cell distribution in the ventricular wall has been investigated in great detail in the canine LV. M cells with the longest action potential duration (APD) are typically found in the deep subepicardium to midmyocardium in the lateral wall, deep subendocardium to midmyocardium in the anterior wall, and throughout the wall in the region of the outflow tracts. M cells are also present in the deep layers of papillary muscles, trabeculae, and interventricular septum.7 In some species, like the rabbit, cells with M cell characteristics occupy the entire endocardium of the adult ventricle, although cells with the longer APD can be identified in the deep subendocardium at seven weeks of age.
Tissue slices isolated from the M region display an APD at 90% repolarization (APD90) that is more than 100 ms longer than tissues isolated from the epicardium or endocardium at basic cycle lengths of 2,000 ms or greater. In the intact ventricular wall, this disparity in APD90 is less pronounced due to electrotonic coupling of cells. The transmural increase in APD is relatively gradual, except between the epicardium and subepicardium where there is often a sharp increase in APD. This has been shown to be due to an increase in tissue resistivity in this region,8 which may be related to the sharp transition in cell orientation in this region as well as to reduced expression of connexin 43,9,10 which is principally responsible for intracellular communication in the ventricular myocardium. In addition, fibrofatty infiltration tends to localize in the deep subepicardial region, accentuating the electrical insulating properties of this region of the wall. Thus, both the degree of electrotonic coupling and intrinsic APDs play a key role in the expression of electrical heterogeneity in the ventricular myocardium.
The longer APD of the M cell as compared to that of epicardial and endocardial cells has been shown to be due to a number of ionic differences, such as a smaller IKs and a larger late INa11,12 and sodium-calcium exchange current (INa-Ca).13 The net result is a decrease in repolarizing current (i.e., decreased net repolarizing current or “reserve”) during phases 2 and 3 of the M cell action potential. These ionic differences sensitize the M cells to a variety of pharmacological agents. Agents that block IKr, IKs, or increase ICa or late INa generally produce much greater APD prolongation of M than of epicardial or endocardial cells.
Electrical Heterogeneity Contributes to Inscription of J Wave and T Wave of ECG
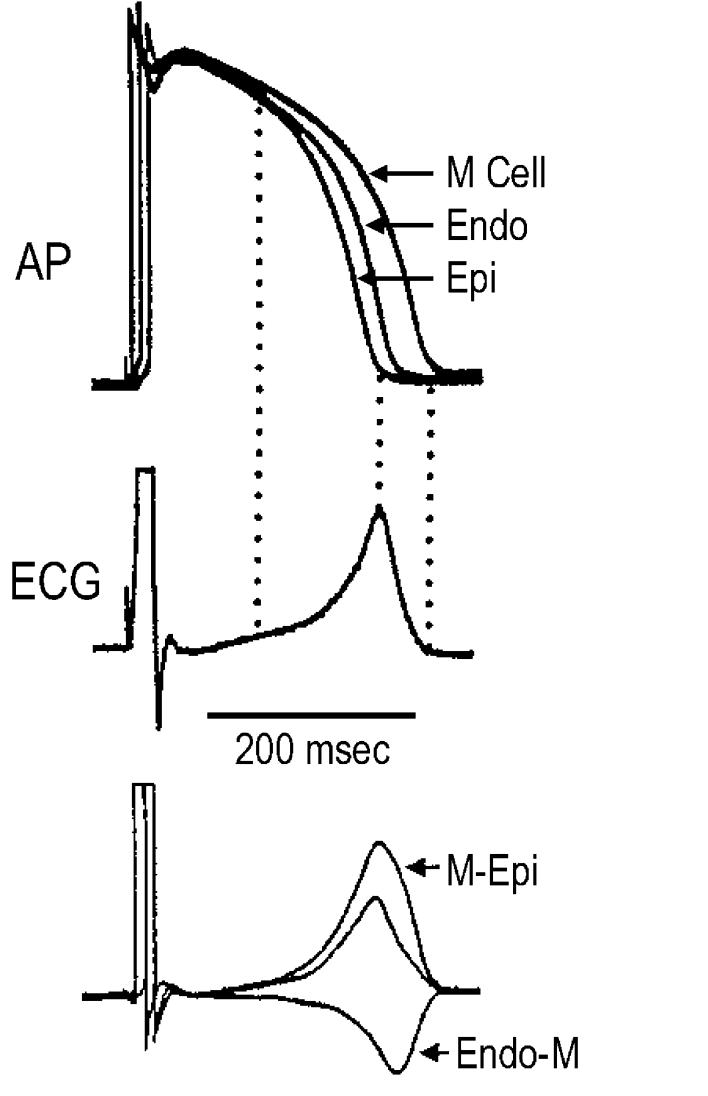
Transmural differences among the three predominant myocardial cell types in early and later repolarization generate voltage gradients that contribute to the inscription of the J wave and T wave of the ECG. The transmural gradient resulting from the presence of an Ito-mediated notch in epicardium but not endocardium gives rise to the J wave, or Osborne wave.14 In the canine heart, voltage gradients developing as a result of the different time course of repolarization of phases 2 and 3 in the three cell types have been shown to give rise to opposing voltage gradients on either side of the M region, which inscribe the T wave (Fig. 1).15 In the case of an upright T wave, the epicardial response is the earliest to repolarize and the M cell action potential is the latest. When studied in wedge preparations isolated from the LV of the canine heart, full repolarization of the epicardial action potential coincides with the peak of the T wave, and repolarization of the M cells is coincident with the end of the T wave. The duration of the M cell action potential determines the QT interval and the duration of the epicardial action potential determines the QTpeak interval. These observations in the perfused wedge preparation suggest that the Tpeak-Tend interval may provide an index of the magnitude of the transmural dispersion of repolarization (TDR) in the in vivo heart, and thus, be useful as an estimate of how TDR may be changing.5,15
Figure 1.
Voltage gradients on either side of the M region are responsible for inscription of the electrocardiographic T wave. Top: Action potentials simultaneously recorded from endocardial, epicardial, and M region sites of an arterially perfused canine left ventricular wedge preparation. Middle: ECG recorded across the wedge. Bottom: Computed voltage differences between the epicardium and M region action potentials (Δ VM-Epi) and between the M region and endocardium responses ( ΔVEndo-M). The center trace, the average of the two opposing voltage gradients, closely resembles the ECG. (Modified from Yan and Antzelevitch,15 with permission.)
Enhanced TDR and Arrhythmogenesis
Long QT Syndrome
The long QT syndrome (LQTS) is characterized by the appearance of long QT intervals in the ECG, an atypical polymorphic ventricular tachycardia (VT) known as torsade de pointes (TdP), and an increased risk for sudden cardiac death.16-18 Congenital LQTS is classified into eight genotypes distinguished by mutations in at least seven different ion channel genes and a structural anchoring protein located on chromosomes 3, 4, 6, 7, 11, 17, and 21.19-25 Acquired LQTS refers to a syndrome similar to the congenital form, but caused by exposure to drugs that prolong the duration of the ventricular action potential,26 or QT prolongation secondary to bradycardia or an electrolyte imbalance. In recent years, this syndrome has been extended to encompass the reduced repolarization reserve (i.e., decrease in net repolarizing current) attending remodeling of the ventricular myocardium that accompanies dilated and hypertrophic cardiomyopathies.27-31
Accentuation of spatial dispersion, secondary to an increase of transmural and trans-septal dispersion of repolarization, and the development of early afterdepolarization (EAD)-induced triggered activity underlie the substrate and trigger for the development of TdP arrhythmias observed under LQTS conditions.1,32 Models of the LQT1, LQT2, and LQT3 forms of the LQTS have been developed using the canine arterially perfused LV wedge preparation.33-36
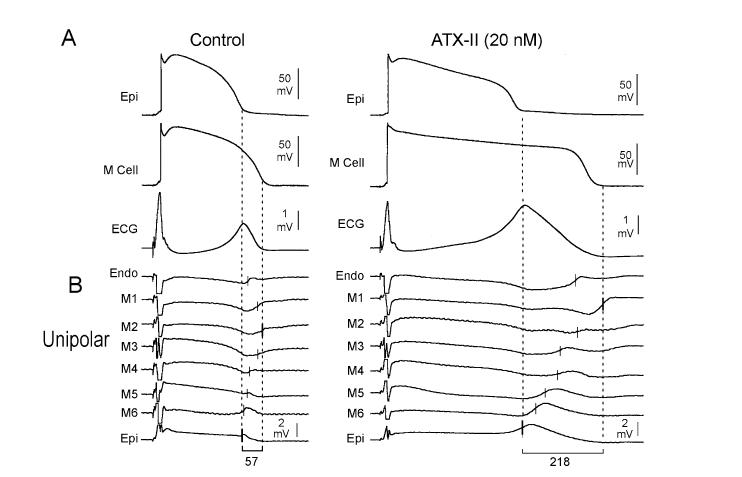
Increased late INa gives rise to the LQT3 form of the congenital LQTS. Experimentally this can be mimicked using ATX-II or anthopleurin-A.37-43 These toxins produce a preferential prolongation of the action potential of the M cell, and have the greatest potential to prolong TDR in association with a prolongation of the QT interval, leading to the development of TdP (Fig. 2).
Figure 2.
ATX-II-induced augmentation of late INa amplifies transmural dispersion of repolarization in the coronary-perfused wedge preparation. Each panel shows: A: Transmembrane action potentials recorded from M (M2) and epicardial sites of a canine left ventricular wedge preparation together with a transmural ECG recorded across the bath (BCL of 2,000 ms) in the absence (left) and the presence (right) of ATX-II (20 nmol/L); B: Eight intramural unipolar electrograms recorded approximately 1.2 mm apart from endocardial (Endo), M (6 sites; M1-M6), and epicardial (Epi) regions (120-μm silver electrodes insulated except at the tip) inserted midway into the wedge preparation. Dashed vertical lines in the unipolar electrograms denote the maximum time of the first derivative (Vmax) of the T wave (local repolarization time). (Modified from Antzelevitch et al.,68 with permission.)
An increase in late INa due to slowing or incomplete inactivation of INa is associated with congenital diseases (e.g., LQT3 syndrome) owing to mutations of the cardiac sodium channel gene SCN5A. Enhancement of late INa is also observed, and is associated with arrhythmogenesis and LV dysfunction in a variety of pathophysiological states, including heart failure,30,44 following hypoxia45 after exposure to free oxygen radicals,46 amphiphiles such as lysophophatidylcholine43 and palmitoyl-L-canitine,47 and in post-ischemia remodeled myocytes.48
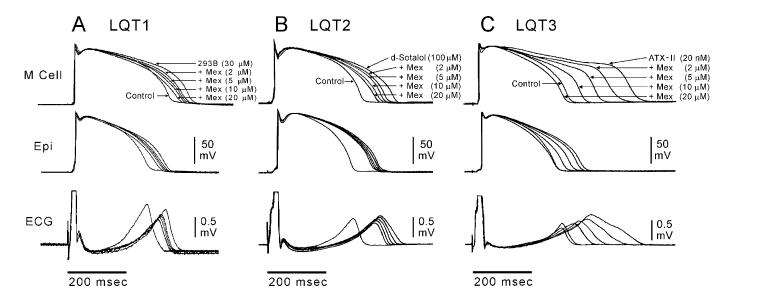
Whereas an increase in late INa is highly arrhythmogenic, its reduction is associated with potent antiarrhythmic activity.34,35,42,49->51 Because of the greater magnitude of late INa in M cells, sodium channel blockers such as mexiletine have a greater effect to abbreviate APD in M cells than in other ventricular cell types (Fig. 3).34,35 The result is a reduction in TDR. These actions of mexiletine have proven to be effective in suppressing TdP in experimental models of LQT1, LQT2, and LQT3.34,35
Figure 3.
Block of late INa with mexiletine reduces TDR in experimental models of the long QT syndrome. Each panel shows transmembrane action potentials recorded from M and epicardial (Epi) sites in canine left ventricular wedge preparations together with a transmural ECG recorded across the bath (BCL of 2,000 ms). Traces are recorded in the presence of the IKs blocker, chromanol 293B (LQT1), IKr blocker D-sotalol (LQT2), and late INa agonist, ATX-II (LQT3), plus increasing concentrations of mexiletine. Mexiletine produced a greater abbreviation of the M cell vs epicardial action potential at every concentration, resulting in a reduction in transmural dispersion of repolarization in all three LQTS models. (Modified from Shimizu and Antzelevitch,34,35 with permission.)
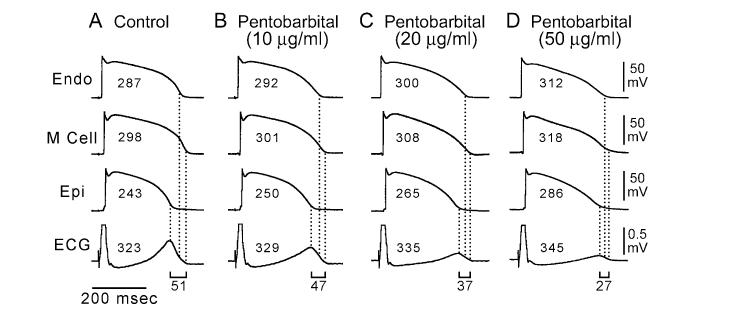
While relatively pure late INa blockers, such as TTX and lidocaine, abbreviate the ventricular action potential and the QT interval, agents that block potassium channels in addition to late INa are capable of prolonging QT while abbreviating TDR. Pentobarbital is an example of such an agent.52 Pentobarbital inhibits IKr, IKs, and late INa.53 This multichannel inhibition leads to a greater prolongation of APD in epicardial and endocardial cells than in M cells, causing a significant reduction in TDR (Fig. 4). Despite its actions to prolong QT, pentobarbital does not induce TdP52 and is effective in suppressing D-sotalol-induced TdP.54
Figure 4.
Pentobarbital-induced QT prolongation but reduction in transmural dispersion of repolarization. Each panel shows transmembrane action potentials recorded from endocardial (Endo), M, and epicardial (Epi) cells in a canine left ventricular wedge preparation together with a transmural ECG recorded across the bath (BCL of 2,000 ms). Numbers in the action potentials denote APD90 values. Numbers above the ECGs denote the QT intervals. (Modified from Shimizu et al.,52 with permission.)
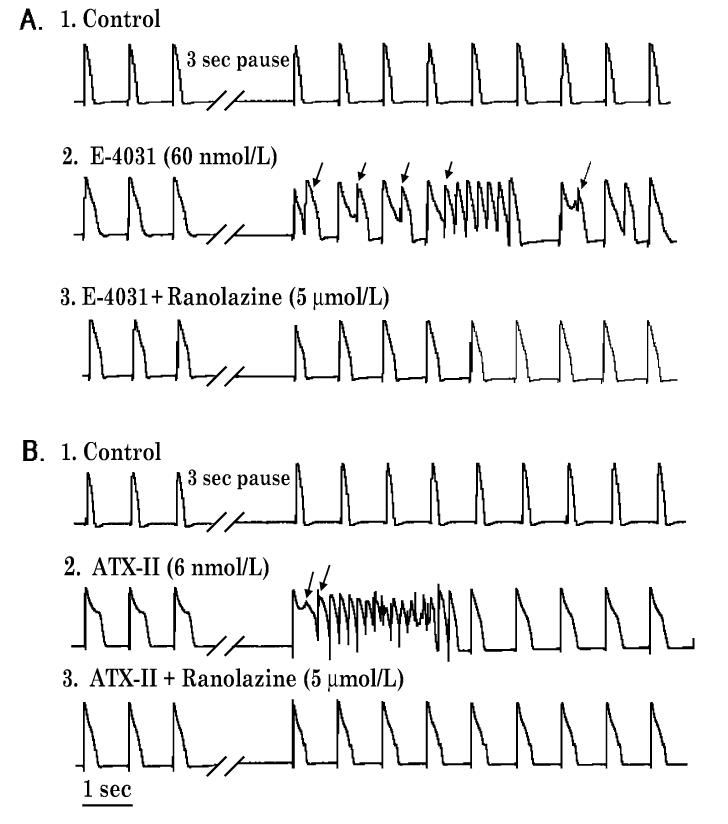
Ranolazine, a novel anti-anginal agent, is a potent late INa blocking action that is capable of producing a modest prolongation of the QT interval51 blocks IKr (IC50 12 μM), late INa, late ICa, peak ICa, INa-Ca (IC50 5.9, = 50, 296, and 91 μM, respectively), and IKs (17%=at 30 μM). In LV tissue and wedge preparations, ranolazine produces a concentration-dependent prolongation of APD in epicardium, but abbreviation of APD of M cells, leading to either no change or a reduction in TDR.51,55 The result is a modest prolongation of the QT interval. Prolongation of APD and QT by ranolazine is fundamentally different from that of other drugs that block IKr and induce TdP in that APD prolongation is rate-independent (i.e., does not display reverse rate-dependent prolongation of APD), and is not associated with EADs, triggered activity, increased spatial dispersion of repolarization, or polymorphic VT. TdP arrhythmias are not observed in response to the drug. Indeed, ranolazine has been found to possess significant antiarrhythmic activity, acting to suppress the arrhythmogenic effects of other QT-prolonging drugs that mimic LQT3 and LQT2 (Fig. 5). In three similar experiments, ranolazine (10-30 μM) completely suppressed PVBs and VTs induced by ATX-II (6 nM; n = 3, LQT3). Ranolazine (10 μM) decreased spontaneous and pause-triggered activity caused by E-4031 (60 nM; LQT2) from a control of 42±6 beats/min to 12±8 beats/min (n = 6, P < 0.05).
Figure 5.
Inhibition by ranolazine of pause-triggered early afterdepolarizations (EADs) and ventricular tachycardias (VTs) in the presence of either E-4031 (panel A) or ATX-9II (panel B) in a female rabbit isolated perfused heart paced at 1 Hz. Records 1-3 in each panel were obtained serially from the same heart; panels A and B represent different hearts. Representative monophasic action potentials (MAPs) were recorded before and after a three-second pause during (A1 and B1) control conditions (no drug) and during perfusion with (A2) E-4031 (60 nmol/L) alone, (A3) E-4031 (60 nmol/L) plus ranolazine (5 μmol/L), (B2) ATX-II (6 nmol/L) alone, or (B3) ATX-II (6 nmol/L) plus ranolazine (30 μmol/L). Arrows indicate EADs, EAD-triggered premature ventricular beats, and VTs ranolazine suppressed EADs and VTs induced in the presence of E-4031 (panel A3), and ATX-II (panel B3). Similar results were obtained from five and two additional hearts treated with either E-4031 or ATX-II, respectively (see text).
These results highlight the fact that IKr block, even if relatively potent, does not in and of itself predict the arrhythmogenic potential of pharmacological agents. Ranolazine’s potent late INa block underlies its action to reduce TDR, eliminate EADs, and suppress TdP.42,43,56,57
Brugada Syndrome
Arrhythmogenesis in the Brugada syndrome is also due to amplification of TDR, but in this case secondary to preferential abbreviation of the right ventricular (RV) epicardial action potential. The arrhythmogenic substrate responsible for the development of extrasystoles and polymorphic VT in the Brugada syndrome is believed to be secondary to amplification of heterogeneities intrinsic to the early phases (phase 1-mediated notch) of the action potential of cells residing in different layers of the RV wall of the heart. Rebalancing of currents active at the end of phase 1 is thought to underlie the accentuation of the action potential notch in RV epicardium, which is responsible for the augmented J wave and ST segment elevation associated with the Brugada syndrome (see Antzelevitch58 for references). The presence of a transient outward current (Ito)-mediated spike and dome morphology, or notch, in ventricular epicardium, but not endocardium, creates a transmural voltage gradient responsible for the inscription of the electrocardiographic J wave.14,59 The ST segment is normally isoelectric due to the absence of transmural voltage gradients at the level of the action potential plateau. Accentuation of the RV action potential notch under pathophysiologic conditions leads to an increase of transmural voltage gradients, and thereby to an accentuation of the J wave or to J point elevation. If the epicardial action potential continues to repolarize before that of endocardium, the T wave remains positive, giving rise to a saddleback configuration of the ST segment elevation. Further accentuation of the notch is accompanied by a prolongation of the epicardial action potential, causing it to repolarize after the endocardium, hence leading to inversion of the T wave. The down-sloping ST segment elevation, or accentuated J wave, observed in experimental wedge models often appears as an R′, suggesting that the appearance of a right bundle branch block morphology in Brugada patients may be due in large part to early repolarization of RV epicardium, rather than major delays in impulse conduction in the right bundle.60 Despite the appearance of a typical Brugada sign, accentuation of the RV epicardial AP notch alone does not give rise to an arrhythmogenic substrate. The arrhythmogenic substrate may develop with a further shift in the balance of current, leading to loss of the action potential dome at some epicardial sites but not others. A marked TDR develops as a consequence, creating a vulnerable window, which when captured by a premature extrasystole can trigger a reentrant arrhythmia. Because loss of the action potential dome in epicardium is generally heterogeneous, epicardial dispersion of repolarization develops as well. Conduction of the action potential dome from sites at which it is maintained to sites at which it is lost causes local re-excitation via phase 2 reentry, leading to the development of a closely coupled extrasystole capable of capturing the vulnerable window across the ventricular wall, thus triggering a circus movement reentry in the form of VT/VF.61,62 Support for these hypotheses derives from experiments involving the arterially perfused RV wedge preparation61 and from recent studies in which MAP electrodes were positioned on the epicardial and endocardial surfaces of the RVOT in patients with the Brugada syndrome.63,64
A shift in the balance of current at the end of phase 1, leading to loss of the epicardial action potential dome and amplification of TDR, can occur as a result of a reduction in rapidly activating INa or as a result of a speeding up of inactivation of INa,65,66 both leading to loss of function. It stands to reason that an agent that slows inactivation of INa may exert an antiarrhythmic effect in the setting of Brugada syndrome. Such a therapeutic potential has been demonstrated for dimethyl lithospermate B, a derivative of Danshen, in the arterially perfused RV wedge model of the Brugada syndrome.67
Conclusion
Amplification of transmural heterogeneities of repolarization in ventricular myocardium can predispose to the development of potentially lethal reentrant arrhythmias (alternative: enhanced late INa). Augmentation of late INa can amplify TDR by causing a preferential prolongation of the M cell action potential, whereas a reduction in peak (early) INa can contribute to a preferential abbreviation of the epicardial action potential. These alterations contribute to the electrophysiological phenotype of the long QT and Brugada syndromes, respectively. Agents that either reduce late INa or augment INa during the action potential notch can exert antiarrhythmic actions in these syndromes.
References
- 1.Antzelevitch C, Dumaine R. Electrical heterogeneity in the heart: Physiological, pharmacological and clinical implications. In: Page E, Fozzard HA, Solaro RJ, editors. Handbook of Physiology. Section 2 The Cardiovascular System. Oxford University Press; New York: 2001. pp. 654–692. [Google Scholar]
- 2.Di Diego JM,, Sun ZQ. Antzelevitch C: Ito and action potential notch are smaller in left vs. right canine ventricular epicardium. Am J Physiol. 1996;271:H548–H561. doi: 10.1152/ajpheart.1996.271.2.H548. [DOI] [PubMed] [Google Scholar]
- 3.Volders PG, Sipido KR, Carmeliet E, Spatjens RL, Wellens HJ, Vos MA. Repolarizing K+ currents ITO1 and IKs are larger in right than left canine ventricular midmyocardium. Circulation. 1999;99(2):206–210. doi: 10.1161/01.cir.99.2.206. [DOI] [PubMed] [Google Scholar]
- 4.Sicouri S, Antzelevitch C. A subpopulation of cells with unique electrophysiological properties in the deep subepicardium of the canine ventricle. The M cell. Circ Res. 1991;68:1729–1741. doi: 10.1161/01.res.68.6.1729. [DOI] [PubMed] [Google Scholar]
- 5.Antzelevitch C, Shimizu W, Yan GX, Sicouri S, Weissenburger J, Nesterenko VV, Burashnikov A, Di Diego JM,, Saffitz JE, Thomas GP. The M cell: Its contribution to the ECG and to normal and abnormal electrical function of the heart. J Cardiovasc Electrophysiol. 1999;10(8):1124–1152. doi: 10.1111/j.1540-8167.1999.tb00287.x. [DOI] [PubMed] [Google Scholar]
- 6.Anyukhovsky EP, Sosunov EA, Gainullin RZ, Rosen MR. The controversial M cell. J Cardiovasc Electrophysiol. 1999;10:244–260. doi: 10.1111/j.1540-8167.1999.tb00667.x. [DOI] [PubMed] [Google Scholar]
- 7.Sicouri S, Fish J, Antzelevitch C. Distribution of M cells in the canine ventricle. J Cardiovasc Electrophysiol. 1994;5:824–837. doi: 10.1111/j.1540-8167.1994.tb01121.x. [DOI] [PubMed] [Google Scholar]
- 8.Yan GX, Shimizu W, Antzelevitch C. Characteristics and distribution of M cells in arterially-perfused canine left ventricular wedge preparations. Circulation. 1998;98:1921–1927. doi: 10.1161/01.cir.98.18.1921. [DOI] [PubMed] [Google Scholar]
- 9.Poelzing S, Akar FG, Baron E, Rosenbaum DS. Heterogeneous connexin43 expression produces electrophysiological heterogeneities across ventricular wall. Am J Physiol Heart Circ Physiol. 2004;286(5):H2001–H2009. doi: 10.1152/ajpheart.00987.2003. [DOI] [PubMed] [Google Scholar]
- 10.Yamada KA, Kanter EM, Green KG, Saffitz JE. Transmural distribution of connexins in rodent hearts. J Cardiovasc Electrophysiol. 2004;15(6):710–715. doi: 10.1046/j.1540-8167.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- 11.Zygmunt AC, Eddlestone GT, Thomas GP, Nesterenko VV, Antzelevitch C. Larger late sodium conductance in M cells contributes to electrical heterogeneity in canine ventricle. Am J Physiol. 2001;281:H689–H697. doi: 10.1152/ajpheart.2001.281.2.H689. [DOI] [PubMed] [Google Scholar]
- 12.Liu DW, Antzelevitch C. Characteristics of the delayed rectifier current (IKr and IKs) in canine ventricular epicardial, midmyocardial, and endocardial myocytes. Circ Res. 1995;76:351–365. doi: 10.1161/01.res.76.3.351. [DOI] [PubMed] [Google Scholar]
- 13.Zygmunt AC, Goodrow RJ, Antzelevitch C. INa-Ca contributes to electrical heterogeneity within the canine ventricle. Am J Physiol. 2000;278:H1671–H1678. doi: 10.1152/ajpheart.2000.278.5.H1671. [DOI] [PubMed] [Google Scholar]
- 14.Yan GX, Antzelevitch C. Cellular basis for the electrocardiographic J wave. Circulation. 1996;93:372–379. doi: 10.1161/01.cir.93.2.372. [DOI] [PubMed] [Google Scholar]
- 15.Yan GX, Antzelevitch C. Cellular basis for the normal T wave and the electrocardiographic manifestations of the long QT syndrome. Circulation. 1998;98:1928–1936. doi: 10.1161/01.cir.98.18.1928. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz PJ. The idiopathic long QT syndrome: Progress and questions. Am Heart J. 1985;109:399–411. doi: 10.1016/0002-8703(85)90626-x. [DOI] [PubMed] [Google Scholar]
- 17.Moss AJ, Schwartz PJ, Crampton RS, Tzivoni D, Locati EH, MacCluer JW, Hall WJ, Weitkamp LR, Vincent GM, Garson A, Robinson JL, Benhorin J, Choi S. The long QT syndrome: Prospective longitudinal study of 328 families. Circulation. 1991;84:1136–1144. doi: 10.1161/01.cir.84.3.1136. [DOI] [PubMed] [Google Scholar]
- 18.Zipes DP. The long QT interval syndrome: A Rosetta stone for sympathetic related ventricular tachyarrhythmias. Circulation. 1991;84:1414–1419. doi: 10.1161/01.cir.84.3.1414. [DOI] [PubMed] [Google Scholar]
- 19.Wang Q, Shen J, Splawski I, Atkinson DL, Li ZZ, Robinson JL, Moss AJ, Towbin JA, Keating MT. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell. 1995;80:805–811. doi: 10.1016/0092-8674(95)90359-3. [DOI] [PubMed] [Google Scholar]
- 20.Mohler PJ, Schott JJ, Gramolini AO, Dilly KW, Guatimosim S, duBell WH, Song LS, Haurogne K, Kyndt F, Ali ME, Rogers TB, Lederer WJ, Escande D, Le Marec H,, Bennett V. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature. 2003;421(6923):634–639. doi: 10.1038/nature01335. [DOI] [PubMed] [Google Scholar]
- 21.Plaster NM, Tawil R, Tristani-Firouzi M,, Canun S, Bendahhou S, Tsunoda A, Donaldson MR, Iannaccone ST, Brunt E, Barohn R, Clark J, Deymeer F, George AL, Fish FA, Hahn A, Nitu A, Ozdemir C, Serdaroglu P, Subramony SH, Wolfe G, Fu YH, Ptacek LJ. Mutations in Kir2.1 cause the developmental and episodic electrical phenotypes of Andersen’s syndrome. Cell. 2001;105:511–519. doi: 10.1016/s0092-8674(01)00342-7. [DOI] [PubMed] [Google Scholar]
- 22.Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- 23.Wang Q, Curran ME, Splawski I, Burn TC, Millholland JM, Van Raay TJ,, Shen J, Timothy KW, Vincent GM, De Jager T,, Schwartz PJ, Towbin JA, Moss AJ, Atkinson DL, Landes GM, Connors TD, Keating MT. Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nat Genet. 1996;12:17–23. doi: 10.1038/ng0196-17. [DOI] [PubMed] [Google Scholar]
- 24.Splawski I, Tristani-Firouzi M,, Lehmann MH, Sanguinetti MC, Keating MT. Mutations in the hminK gene cause long QT syndrome and suppress IKs function. Nat Genet. 1997;17:338–340. doi: 10.1038/ng1197-338. [DOI] [PubMed] [Google Scholar]
- 25.Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, Napolitano C, Schwartz PJ, Joseph RM, Condouris K, Tager-Flusberg H,, Priori SG, Sanguinetti MC, Keating MT. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:1–19. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 26.Bednar MM, Harrigan EP, Anziano RJ, Camm AJ, Ruskin JN. The QT interval. Prog Cardiovasc Dis. 2001;43(5 Pt 2):1–45. doi: 10.1053/pcad.2001.21469. [DOI] [PubMed] [Google Scholar]
- 27.Tomaselli GF, Marban E. Electrophysiological remodeling in hypertrophy and heart failure. Cardiovasc Res. 1999;42:270–283. doi: 10.1016/s0008-6363(99)00017-6. [DOI] [PubMed] [Google Scholar]
- 28.Sipido KR, Volders PG, De Groot SH,, Verdonck F, Van de WF,, Wellens HJ, Vos MA. Enhanced Ca(2+) release and Na/Ca exchange activity in hypertrophied canine ventricular myocytes: Potential link between contractile adaptation and arrhythmogenesis. Circulation. 2000;102(17):2137–2144. doi: 10.1161/01.cir.102.17.2137. [DOI] [PubMed] [Google Scholar]
- 29.Volders PG, Sipido KR, Vos MA, Spatjens RL, Leunissen JD, Carmeliet E, Wellens HJ. Downregulation of delayed rectifier K() currents in dogs with chronic complete atrioventricular block and acquired + torsades de pointes. Circulation. 1999;100(24):2455–2461. doi: 10.1161/01.cir.100.24.2455. [DOI] [PubMed] [Google Scholar]
- 30.Undrovinas AI, Maltsev VA, Sabbah HN. Repolarization abnormalities in cardiomyocytes of dogs with chronic heart failure: Role of sustained inward current. Cell Mol Life Sci. 1999;55(3):494–505. doi: 10.1007/s000180050306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maltsev VA, Sabbah HN, Higgins RS, Silverman N, Lesch M, Undrovinas AI. Novel, ultraslow inactivating sodium current in human ventricular cardiomyocytes. Circulation. 1998;98(23):2545–2552. doi: 10.1161/01.cir.98.23.2545. [DOI] [PubMed] [Google Scholar]
- 32.Antzelevitch C, Shimizu W. Cellular mechanisms underlying the long QT syndrome. Curr Opin Cardiol. 2002;17(1):43–51. doi: 10.1097/00001573-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Shimizu W, Antzelevitch C. Effects of a K(+) channel opener to reduce transmural dispersion of repolarization and prevent torsade de pointes in LQT1, LQT2, and LQT3 models of the long-QT syndrome. Circulation. 2000;102(6):706–712. doi: 10.1161/01.cir.102.6.706. [DOI] [PubMed] [Google Scholar]
- 34.Shimizu W, Antzelevitch C. Cellular basis for the ECG features of the LQT1 form of the long QT syndrome: Effects of β-adrenergic agonists and antagonists and sodium channel blockers on transmural dispersion of repolarization and torsade de pointes. Circulation. 1998;98:2314–2322. doi: 10.1161/01.cir.98.21.2314. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu W, Antzelevitch C. Sodium channel block with mexiletine is effective in reducing dispersion of repolarization and preventing torsade de pointes in LQT2 and LQT3 models of the long-QT syndrome. Circulation. 1997;96:2038–2047. doi: 10.1161/01.cir.96.6.2038. [DOI] [PubMed] [Google Scholar]
- 36.Shimizu W, Antzelevitch C. Differential effects of beta-adrenergic agonists and antagonists in LQT1, LQT2 and LQT3 models of the long QT syndrome. J Am Coll Cardiol. 2000;35:778–786. doi: 10.1016/s0735-1097(99)00582-3. [DOI] [PubMed] [Google Scholar]
- 37.Shimizu W, Kamakura S. Catecholamines in children with congenital long QT syndrome and Brugada syndrome. J Electrocardiol. 2001;34(Suppl):173–175. doi: 10.1054/jelc.2001.28864. [DOI] [PubMed] [Google Scholar]
- 38.Shimizu W, Antzelevitch C. Characteristics of spontaneous as well as stimulation-induced torsade de pointes in LQT2 and LQT3 models of the long QT syndrome. Circulation. 1997;96:I–554. doi: 10.1161/01.cir.96.6.2038. (Abstract) [DOI] [PubMed] [Google Scholar]
- 39.El-Sherif N,, Caref EB, Chinushi M, Restivo M. Mechanism of arrhythmogenicity of the short-long cardiac sequence that precedes ventricular tachyarrhythmias in the long QT syndrome. J Am Coll Cardiol. 1999;33(5):1415–1423. doi: 10.1016/s0735-1097(98)00700-1. [DOI] [PubMed] [Google Scholar]
- 40.Restivo M, Caref EB, Kozhevnikov DO, El-Sherif N: Spatial dispersion of repolarization is a key factor in the arrhythmogenicity of long QT syndrome. J Cardiovasc Electrophysiol. 2004;15(3):323–331. doi: 10.1046/j.1540-8167.2004.03493.x. [DOI] [PubMed] [Google Scholar]
- 41.Milberg P, Reinsch N, Wasmer K, Monnig G, Stypmann J, Osada N, Breithardt G, Haverkamp W, Eckardt L. Transmural dispersion of repolarization as a key factor of arrhythmogenicity in a novel intact heart model of LQT3. Cardiovasc Res. 2005;65(2):397–404. doi: 10.1016/j.cardiores.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 42.Wu L, Shryock JC, Song Y, Li Y, Antzelevitch C, Belardinelli L. Antiarrhythmic effects of ranolazine in a guinea pig in vitro model of long-QT syndrome. J Pharmacol Exp Ther. 2004;310(2):599–605. doi: 10.1124/jpet.104.066100. [DOI] [PubMed] [Google Scholar]
- 43.Song Y, Shryock JC, Wu L, Belardinelli L. Antagonism by ranolazine of the pro-arrhythmic effects of increasing late INa in guinea pig ventricular myocytes. J Cardiovasc Pharmacol. 2004;44(2):192–199. doi: 10.1097/00005344-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Valdivia CR, Chu WW, Pu J, Foell JD, Haworth RA, Wolff MR, Kamp TJ, Makielski JC. Increased late sodium current in myocytes from a canine heart failure model and from failing human heart. J Mol Cell Cardiol. 2005;38(3):475–483. doi: 10.1016/j.yjmcc.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 45.Ju YK, Saint DA, Gage PW. Hypoxia increases persistent sodium current in rat ventricular myocytes. J Physiol. 1996;497(Pt 2):337–347. doi: 10.1113/jphysiol.1996.sp021772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ward CA, Giles WR. Ionic mechanism of the effects of hydrogen peroxide in rat ventricular myocytes. J Physiol. 1997;500(Pt 3):631–642. doi: 10.1113/jphysiol.1997.sp022048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu J, Corr PB. Palmitoyl carnitine modifies sodium currents and induces transient inward current in ventricular myocytes. Am J Physiol. 1994;266(3 Pt 2):H1034–H1046. doi: 10.1152/ajpheart.1994.266.3.H1034. [DOI] [PubMed] [Google Scholar]
- 48.Huang B, El Sherif T,, Gidh-Jain M,, Qin D, El-Sherif N: Alterations of sodium channel kinetics and gene expression in the postinfarction remodeled myocardium. J Cardiovasc Electrophysiol. 2001;12(2):218–225. doi: 10.1046/j.1540-8167.2001.00218.x. [DOI] [PubMed] [Google Scholar]
- 49.Antzelevitch C. Electrical heterogeneity, cardiac arrhythmias, and the sodium channel [In Process Citation] Circ Res. 2000;87(11):964–965. doi: 10.1161/01.res.87.11.964. [DOI] [PubMed] [Google Scholar]
- 50.Antzelevitch C, Belardinelli L, Wu L, Fraser H, Zygmunt AC, Burashnikov A, Diego JM, Fish JM, Cordeiro JM, Goodrow RJ, Scornik F, Perez G. Electrophysiologic properties and antiarrhythmic actions of a novel antianginal agent 2. J Cardiovasc Pharmacol Ther. 2004;9(Suppl 1):S65–S83. doi: 10.1177/107424840400900106. [DOI] [PubMed] [Google Scholar]
- 51.Antzelevitch C, Belardinelli L, Zygmunt AC, Burashnikov A, Di Diego JM,, Fish JM, Cordeiro JM, Thomas GP. Electrophysiologic effects of ranolazine: A novel anti-anginal agent with antiarrhythmic properties. Circulation. 2004;110:904–910. doi: 10.1161/01.CIR.0000139333.83620.5D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shimizu W, McMahon B, Antzelevitch C. Sodium pentobarbital reduces transmural dispersion of repolarization and prevents torsade de pointes in models of acquired and congenital long QT syndrome. J Cardiovasc Electrophysiol. 1999;10:156–164. doi: 10.1111/j.1540-8167.1999.tb00656.x. [DOI] [PubMed] [Google Scholar]
- 53.Sun ZQ, Eddlestone GT, Antzelevitch C. Ionic mechanisms underlying the effects of sodium pentobarbital to diminish transmural dispersion of repolarization. Pacing Clin Electrophysiol. 1997;20:11–1116. (Abstract) [Google Scholar]
- 54.Weissenburger J, Nesterenko VV, Antzelevitch C. Transmural heterogeneity of ventricular repolarization under baseline and long QT conditions in the canine heart in vivo: Torsades de pointes develops with halothane but not pentobarbital anesthesia. J Cardiovasc Electrophysiol. 2000;11:290–304. doi: 10.1111/j.1540-8167.2000.tb01798.x. [DOI] [PubMed] [Google Scholar]
- 55.Antzelevitch C, Belardinelli L, Wu L, Fraser H, Zygmunt AC, Burashnikov A, Di Diego JM,, Fish JM, Cordeiro JM, Goodrow RJ, Scornik FS, Perez GJ. Electrophysiologic properties and antiarrhythmic actions of a novel anti-anginal agent. J Cardiovasc Pharmacol Ther. 2004;9(Suppl 1):S65–S83. doi: 10.1177/107424840400900106. [DOI] [PubMed] [Google Scholar]
- 56.Antzelevitch C, Belardinelli L, Wu L, Fraser H, Zygmunt AC, Burashnikov A, Di Diego JM,, Fish JM, Cordeiro JM, Goodrow RJ, Scornik FS, Perez GJ. Electrophysiologic properties of ranolazine: A novel anti-anginal agent. J Cardiovasc Pharmacol Ther. 2004;9(Suppl 1):S65–S83. doi: 10.1177/107424840400900106. [DOI] [PubMed] [Google Scholar]
- 57.Shryock JC, Song Y, Wu L, Fraser H, Belardinelli L. A mechanistic approach to assess the proarrhythmic risk of QT-prolonging drugs in preclinical pharmacologic studies. J Electrocardiol. 2004;37(Suppl):34–39. doi: 10.1016/j.jelectrocard.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 58.Antzelevitch C. The Brugada syndrome: Ionic basis and arrhythmia mechanisms. J Cardiovasc Electrophysiol. 2001;12(2):268–272. doi: 10.1046/j.1540-8167.2001.00268.x. [DOI] [PubMed] [Google Scholar]
- 59.Fish JM, Antzelevitch C. Role of sodium and calcium channel block in unmasking the Brugada syndrome. Heart Rhythm. 2004;1(2):210–217. doi: 10.1016/j.hrthm.2004.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gussak I, Antzelevitch C, Bjerregaard P, Towbin JA, Chaitman BR. The Brugada syndrome: Clinical, electrophysiologic and genetic aspects. J Am Coll Cardiol. 1999;33:5–15. doi: 10.1016/s0735-1097(98)00528-2. [DOI] [PubMed] [Google Scholar]
- 61.Yan GX, Antzelevitch C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST segment elevation. Circulation. 1999;100:1660–1666. doi: 10.1161/01.cir.100.15.1660. [DOI] [PubMed] [Google Scholar]
- 62.Lukas A, Antzelevitch C. Phase 2 reentry as a mechanism of initiation of circus movement reentry in canine epicardium exposed to simulated ischemia. Cardiovasc Res. 1996;32:593–603. [PubMed] [Google Scholar]
- 63.Antzelevitch C, Brugada P, Brugada J, Brugada R, Shimizu W, Gussak I, Perez Riera AR: Brugada syndrome. A decade of progress. Circ Res. 2002;91(12):1114–1119. doi: 10.1161/01.res.0000046046.53721.90. [DOI] [PubMed] [Google Scholar]
- 64.Kurita T, Shimizu W, Inagaki M, Suyama K, Taguchi A, Satomi K, Aihara N, Kamakura S, Kobayashi J, Kosakai Y. The electrophysiologic mechanism of ST-segment elevation in Brugada syndrome. J Am Coll Cardiol. 2002;40(2):330–334. doi: 10.1016/s0735-1097(02)01964-2. [DOI] [PubMed] [Google Scholar]
- 65.Antzelevitch C, Brugada P, Borggrefe M, Brugada J, Brugada R, Corrado D, Gussak I, LeMarec H, Nademanee K, Riera ARP, Tan H, Shimizu W, Schultze-Bahr E, Wilde A. Brugada syndrome: Overview. In: Antzelevitch C, Brugada P, Brugada J, Brugada R, editors. The Brugada Syndrome: From Bench to Bedside. Blackwell Futura; Oxford: 2004. pp. 1–22. [Google Scholar]
- 66.Dumaine R, Towbin JA, Brugada P, Vatta M, Nesterenko VV, Nesterenko DV, Brugada J, Brugada R, Antzelevitch C. Ionic mechanisms responsible for the electrocardiographic phenotype of the Brugada syndrome are temperature dependent. Circ Res. 1999;85:803–809. doi: 10.1161/01.res.85.9.803. [DOI] [PubMed] [Google Scholar]
- 67.Fish JM, Welchons D, Kim YS, Ho WK, Antzelevitch C. Dimethyl lithospermate B, an extract of Danshen, suppresses arrhythmogenesis associated with the Brugada syndrome. Circulation. 2006 doi: 10.1161/CIRCULATIONAHA.105.601690. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Antzelevitch C, Shimizu W, Yan GX, Sicouri S, Weissenburger J, Nesterenko VV, Burashnikov A, Di Diego JM,, Saffitz JE. Thomas GP: The M cell. Its contribution to the ECG and to normal and abnormal electrical function of the heart. J Cardiovasc Electrophysiol. 1999;10:1124–1152. doi: 10.1111/j.1540-8167.1999.tb00287.x. [DOI] [PubMed] [Google Scholar]