Abstract
Genetic factors have been implicated in osteoarthritis (OA), particularly in OA of the hip joint (hip OA). Several instances of familial hip OA that show distinctive modes of inheritance but that differ from chondrodysplasia have been reported. Here, we report the characterization of a large Japanese family with an inherited disease of the hip that is indistinguishable from common hip OA, as evidenced by clinical symptoms and radiographs of the joint. This family contained eight patients in 4 generations. Affected individuals develop pain in the hip joint during adolescence, and the disease progresses to severe crippling before age 60 years. Patients generally are in good health, height is not reduced, and there is no extraskeletal involvement suggestive of chondrodysplasia. The skeletal change is bilateral acetabular dysplasia followed by OA, which occurs after age ∼40 years and is indistinguishable from idiopathic nonfamilial dysplastic hip OA. This trait shows autosomal dominant inheritance, with a considerably consistent phenotype. Genomewide screening revealed linkage at chromosome 13q22, and haplotype analysis narrowed the locus to a 6.0-cM interval between markers D13S1296 and D13S162, with a maximal multipoint LOD score of 3.57. The family described here represents a novel genetic entity as a monogenic form of hip OA. Its further characterization can aid in elucidating the etiology and pathogenesis of a common idiopathic form of OA.
Osteoarthritis (OA [MIM 165720]), particularly that of the hip joint (hip OA), is a growing health issue and causes pain and disability in members of an aging society. Hip OA conventionally falls into two categories, termed “primary” and “secondary” hip OA. Primary hip OA lacks underlying factors and structural abnormalities. Accounting for just 8%–35% of all hip OA, primary hip OA is less common than secondary hip OA.1,2 The more common secondary hip OA results primarily from anatomical deformities in the joint, including acetabular dysplasia, slipped capital femoral epiphysis, and Legg-Calvé-Perthes disease.3
Of these, acetabular dysplasia is the most prevalent causative factor of secondary hip OA3; in Japan, it is the most common cause of hip OA.4 Acetabular dysplasia is an idiopathic, localized developmental dysplasia of the hip that is characterized by a shallow hip socket and decreased coverage of the femoral head. Its radiological criteria include the center-edge angle of Wiberg,5 the Sharp angle,6 and the acetabular roof obliquity.7,8 Most patients with acetabular dysplasia develop OA after midlife,5 and even mild acetabular dysplasia can cause hip OA.9
Hip OA is known to be influenced by genetic factors. Familial clustering has been reported in epidemiological studies that examined prevalence among relatives of patients who had undergone total hip arthroplasty (THA) as a surrogate phenotype of hip OA.10–12 One twin study suggested that the genetic contribution to radiographic hip OA in women may account for up to 60% of the disease risk.13 Genomewide screens have identified suggestive linkage on a number of chromosomes.14–16
Several instances of familial OA that show distinctive modes of inheritance have been reported. In one family, generalized OA associated with mild chondrodysplasia was found to segregate as an autosomal dominant trait17 and to associate with a mutation in the type II collagen gene (COL2A1 [MIM 120140]). Because COL2A1 mutations have been identified in other families with mild-to-severe spondyloepiphyseal dysplasia (SED) congenita (SEDC [MIM 183900]), the OA phenotypes in this family probably represent a mild form of chondrodysplasia. In contrast, Beukes familial hip dysplasia (MIM 142669) shows more localized involvement of joints but still segregates with autosomal dominant inheritance.18 This disorder, identified in an Afrikaner family, is characterized by severely flattened and irregular femoral capital epiphyses of the hip joint. Finally, a large 4-generation family was identified in Iceland.12 As in the other families, the trait segregated as autosomal dominant; affected individuals showed no obvious indication of chondrodysplasias, as characterized by marked short stature; and anomalies contributing to the inherited OA phenotypes were limited to the hip joint. Therefore, each family’s phenotype is indistinguishable from common, idiopathic, nonfamilial OA. Linkage studies have mapped the responsible loci in the Afrikaner and Icelandic pedigrees to 4q35 and 16p, respectively.12,19
In the present study, we describe a Japanese family that is affected by dysplastic hip OA. In this family, acetabular dysplasia is evident beginning during adolescence, and OA develops in all affected individuals after age ∼40 years. This phenotype is transmitted as an autosomal dominant trait and shows linkage to a novel locus on 13q22.
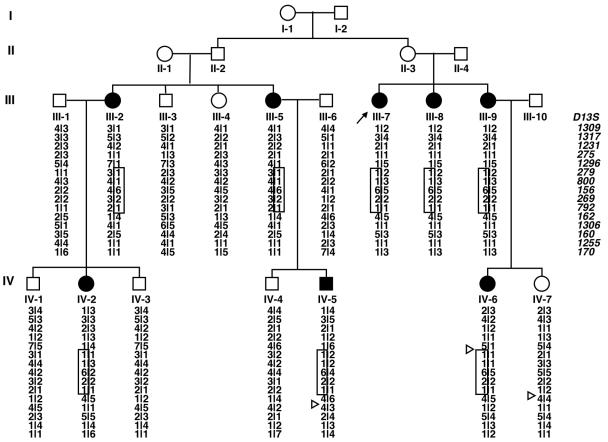
The proband, III-7 (fig. 1), was a 65-year-old woman who first experienced bilateral coxalgia during adolescence. Her coxalgia progressed with age, and she underwent bilateral THA during her 50s. A 4-generation kinship with familial hip OA was identified through her family history, and clinical and radiographic features of family members were examined. Relevant information was obtained from living members of the family, including clinical indicators of OA, such as pain and stiffness in the groin, leg deformity, limitation of hip motion, and limp. The phenotype was ascertained on the basis of medical history and clinical and radiographic presentation of the disorder. Radiographs of hips, hands, spine, and knees were obtained from affected and unaffected family members. From a total of 24 members, which included 5 spouses, 8 individuals were confirmed as affected (fig. 1). There was no history of consanguinity, and the disorder was transmitted to subsequent generations without skipping. Transmission of the disorder was consistent with autosomal dominant inheritance.
Figure 1. .
Family pedigree and the results of haplotype analysis around 13q22. Blackened circles and squares represent affected females and males, respectively. Triangles indicate obligate recombination. The disease haplotype is boxed.
The hip OA phenotype was indistinguishable from that of idiopathic, nonfamilial hip OA associated with acetabular dysplasia. All family members fell within average height and weight ranges for the Japanese population. No history of mental retardation, CNS or peripheral nervous system disease, ocular disease, internal organ abnormality, spontaneous fracture, joint contracture, or hypermobility was observed in this family. Also, there was no apparent evidence of chondrodysplasias, such as SED and multiple epiphyseal dysplasia (MED [MIM 132400]), associating with hip OA. Affected family members first experienced hip pain during adolescence. Early symptoms usually appeared as occasional hip pain or dullness after a long walk. Most patients experienced stiffness and pain in the groin for several years before seeking medical attention. In all patients, hip pain worsened gradually with age, and all five patients aged >50 years presented with terminal-stage OA and underwent surgical intervention with THA. Aside from hip problems, affected individuals were generally in good health.
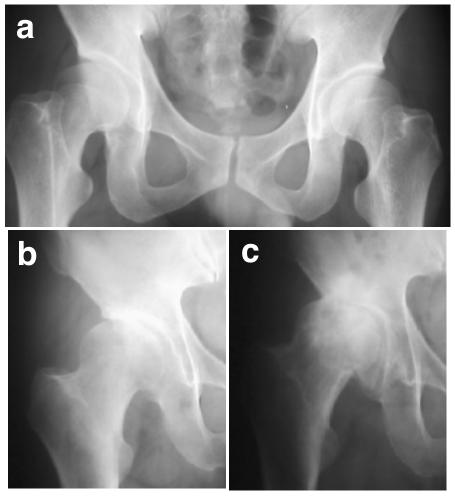
Acetabular dysplasia of the hip was assessed by examining standard anterior-posterior projection of plain radiographs taken in the supine position, and diagnosis was based on modification of criteria proposed by Nakamura et al.8: a center-edge angle of Wiberg ⩽20°, a acetabular roof obliquity ⩾15°, and a Sharp angle ⩾45°. Radiographic hip OA was diagnosed with minimum joint space ⩽2.5 mm, along with the presence of osteophytes and/or bone cysts. Acetabular dysplasia was evident in affected family members beginning during adolescence, and OA appeared at age ∼40 years. Early radiological changes other than acetabular dysplasia were minimal (fig. 2a); neither joint-space narrowing nor osteophyte was observed in the youngest generation. The shape of the femoral head was normal before the progression of OA. Inevitably, OA progressed (fig. 2b and 2c) to the terminal stage, necessitating THA. Vertebral bodies and joints other than the hip were not involved.
Figure 2. .
Hip changes in the study family. a, Radiograph of subject IV-5, at age 38 years, pre-OA. Radiological changes other than acetabular dysplasia are minimal; neither joint-space narrowing nor osteophyte is observed, and the shape of the femoral head is normal. b, Radiograph of subject III-8, at age 62 years, showing early-stage OA and acetabular dysplasia. c, Radiograph of subject III-8 at age 68 years, with advanced-stage OA.
Blood samples were obtained for DNA analysis, with informed consent, from family members aged ⩾18 years. DNA was extracted, using a standard procedure, from 17 members of the kindred, including 8 affected individuals, 7 unaffected related individuals, and 2 unrelated spouses. The affected status of these 17 individuals had been established on the basis of clinical and radiological data. Preliminary analysis, with use of flanking and intragenic microsatellite markers, excluded the following genes from linkage—COL2A1, COL9A1-A3 (MIMs 120210, 120260, and 120270), MATN3 (MIM 602109), and COMP (MIM 600310)—that are involved in chondrodysplasias associated with OA (data not shown). We then conducted a genomewide screen that was based on autosomal dominant transmission, using the ABI PRISM linkage mapping set v2.5 (Applied Biosystems). PCR products were multiplexed and electrophoresed using an ABI PRISM 3700 DNA Analyzer (Applied Biosystems). Genotyping was performed using GeneScan Analysis version 3.5 and Genotyper version 3.6.
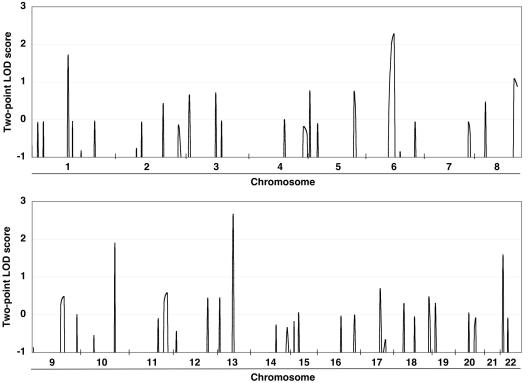
Allelic data were ascertained for Mendelian consistency with Checkfam.20 Parametric two-point linkage analyses were performed using the FASTLINK package version 5.2,21–23 under the assumption of an autosomal dominant trait with 99% penetrance, 0.1% disease frequency, and equal male and female recombination rates. Allelic frequencies for each locus were estimated using genotyped founders and unrelated individuals. Genetic distances between loci were estimated according to sex-averaged genetic maps obtained from the Marshfield Medical Research Foundation. Five regions, represented by six markers, showed LOD scores >1.5: 1p21, 6q15-16, 10q24, 13q22, and 22q11. The highest score was observed at D13S156 (fig. 3).
Figure 3. .
Graphic summary of two-point LOD scores of initial genomewide screen. Five regions showed LOD scores >1.5.
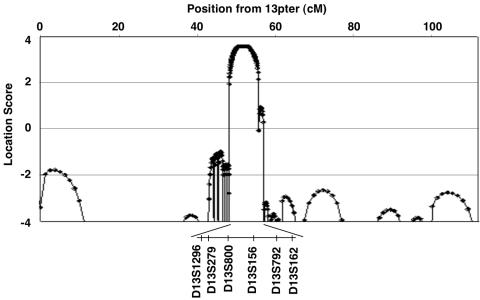
For fine mapping, we designed primer pairs that were based on information from National Center for Biotechnology Information and CEPH databases, and we examined 19 additional markers surrounding these five regions. Two-point LOD scores were calculated for these additional markers, but no region other than 13q22 exceeded the maximum score generated by D13S156 (table 1). Location scores, which are directly comparable to LOD scores,24 were calculated for all markers with use of SimWalk2 version 2.83,24 and the maximum score of 3.57 was observed at D13S279-D13S800-D13S156 (fig. 4). Segregation and the most probable disease haplotype were estimated, using SimWalk2, to span 10 markers. The critical region was localized to a 6.0-cM span between D13S1296 and D13S162. The proximal border of this region was defined by recombination in subject IV-6, between D13S1296 and D13S279; the distal border was found in subject IV-7, between D13S792 and D13S162 (fig. 1).
Table 1. .
Two-Point LOD Scores by the Fine Mapping of 1p21, 6q15-16, 10q24, and 22q11
| LOD at θ = |
||||||||
| Marker | Position (cM) |
0 | .01 | .05 | .1 | .2 | .3 | .4 |
| D1S2793 | 137.3 | .10 | .10 | .08 | .07 | .04 | .02 | .00 |
| D1S206a | 142.3 | 1.72 | 1.68 | 1.51 | 1.29 | .85 | .43 | .12 |
| D1S495 | 145.1 | −∞ | −1.24 | −.41 | −.08 | .10 | .09 | .03 |
| D6S1609 | 94.4 | −.94 | −.80 | −.48 | −.28 | −.10 | −.03 | .00 |
| D6S462a | 101.6 | 2.03 | 1.99 | 1.82 | 1.60 | 1.15 | .68 | .25 |
| D6S300 | 106.2 | −.96 | −.66 | −.19 | .02 | .16 | .15 | .07 |
| D6S434a | 112.3 | 2.26 | 2.21 | 2.03 | 1.79 | 1.29 | .77 | .29 |
| D6S1698 | 122.0 | −∞ | −4.71 | −2.49 | −1.53 | −.65 | −.24 | −.05 |
| D10S1709 | 129.5 | −∞ | −2.94 | −1.37 | −.72 | −.20 | −.03 | .01 |
| D10S192a | 134.3 | 1.90 | 1.87 | 1.73 | 1.54 | 1.15 | .74 | .34 |
| D22S420a | 3.1 | 1.59 | 1.56 | 1.41 | 1.23 | .85 | .48 | .16 |
| D22S427 | 7.5 | −.69 | −.55 | −.21 | −.02 | .11 | .09 | .03 |
Index marker with LOD score >1.5 in the first screen.
Figure 4. .
Location scores resulting from multipoint analysis of chromosome 13. The X-axis shows genetic distance from the telomere (in cM), and the Y-axis shows the location score, calculated using SimWalk2 software. The maximal location score of 3.57 is observed at markers D13S279-D13S800-D13S156.
The disease pattern, as determined by radiographic analysis, in this family is quite similar to common hip OA associated with acetabular dysplasia. There are ethnic and sex differences in the prevalence of acetabular dysplasia,4 and evidence of a genetic contribution has been reported on the basis of a family study.25 In addition, one family with primary acetabular dysplasia has been reported,26 although that study did not evaluate OA.
The familial disorder described here is distinct from other heritable chondrodysplasias in which premature degenerative OA of the hip joint is a major complication. Our family's phenotype resembles Beukes familial hip dysplasia,18 in that both exhibit shallow acetabulum and eventual OA. However, the later onset of symptoms, lack of deformity in the femoral head and/or greater trochanter, and absence of broadening of the femoral neck helps to differentiate our family's phenotype from Beukes hip dysplasia. Our family also differs in two ways from the Icelandic family described elsewhere.12 First, it lacks subchondral cyst of the femoral head, which is characteristic of the Icelandic family. Second, the Icelandic pedigree shows no primary radiographic change in the acetabulum, as is observed here. Genetic analysis also helped to define the distinction; our study revealed no positive linkage to loci associated with OA of the hip joint in the Afrikaner (4p35) and Icelandic (16p) pedigrees.12,19 Together, these phenotypic and genetic differences clearly indicate that our family has a novel entity of familial hip OA.
The small region of chromosome 13q22 that links to our pedigree contains several genes implicated in developmental or pathological events in the skeleton. PCDH9 (MIM 603581), located in the proximal end of this region, belongs to a subfamily of calcium-dependent adhesive proteins within the cadherin superfamily. During limb development and chondrogenesis, N-cadherin is up-regulated, initiates mesenchymal condensation, and is modulated through Wnt signals.27,28 E-cadherin is involved in the morphogenesis of several organs and acts as an important transducer of extracellular signaling for a WNT protein and a bone morphogenetic protein inhibitor. Elsewhere, we highlighted the significance of the calcium-dependent signaling protein calmodulin, which regulates chondrogenesis and associates with hip OA in the Japanese population.29 DACH (MIM 603803), an orthologue of the Drosophila dachshund (dac) gene, also is situated in this region. Flies with a null mutation in dac lack eyes and have truncated limbs.30 Finally, two genes encoding Krüppel-like factors reside in the more telomeric region. Krüppel-like factors are transcription factors, and mutations in the Drosophila orthologue, kr, produce dwarfism. Each of these genes merits consideration for future analysis.
Rapid accumulation of genomic data facilitates the identification of disease gene(s) through positional cloning, and inherited disorders are an excellent starting point for identification of such genes. Pinpointing genes responsible for monogenic traits through use of parametric linkage analysis will inform the search for susceptibility gene(s) in common, polygenic forms of disease. Further study of the family described here should aid in elucidating the etiology and pathogenesis of a common idiopathic form of OA.
Acknowledgments
We thank the family for their cooperation. This work was supported in part by grants-in-aid 15C-1 and 17C-1 for Child Health and Development from the Ministry of Health, Labor and Welfare of Japan.
Web Resource
The URL for data presented herein is as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for OA, COL2A1, SEDC, Beukes familial hip dysplasia, MED, COL9A1-A3, MATN3, COMP, PCDH9, and DACH)
References
- 1.Murray RO (1965) The aetiology of primary osteoarthritis of the hip. Br J Radiol 38:810–824 [DOI] [PubMed] [Google Scholar]
- 2.Solomon L (1976) Patterns of osteoarthritis of the hip. J Bone Joint Surg Br 58:176–183 [DOI] [PubMed] [Google Scholar]
- 3.Harris WH (1986) Etiology of osteoarthritis of the hip. Clin Orthop 213:20–33 [PubMed] [Google Scholar]
- 4.Inoue K, Wicart P, Kawasaki T, Huang J, Ushiyama T, Hukuda S, Courpied J (2000) Prevalence of hip osteoarthritis and acetabular dysplasia in French and Japanese adults. Rheumatology (Oxford) 39:745–748 [DOI] [PubMed] [Google Scholar]
- 5.Wiberg G (1939) Studies on dysplastic acetabulum and congenital subluxation of the hip joint with special reference to the complications of osteoarthritis. Acta Chir Scand 83 (Suppl 58) [Google Scholar]
- 6.Sharp IK (1961) Acetabular dysplasia: the acetabular angle. J Bone Joint Surg Br 43B:268–272 [Google Scholar]
- 7.Massie WK, Howorth MB (1950) Congenital dislocation of the hip. Part I. Method of grading results. J Bone Joint Surg Am 32A:519–531 [PubMed] [Google Scholar]
- 8.Nakamura S, Ninomiya S, Nakamura T (1989) Primary osteoarthritis of the hip joint in Japan. Clin Orthop 241:190–196 [PubMed] [Google Scholar]
- 9.Lane NE, Lin P, Christiansen L, Gore LR, Williams EN, Hochberg MC, Nevitt MC (2000) Association of mild acetabular dysplasia with an increased risk of incident hip osteoarthritis in elderly white women: the study of osteoporotic fractures. Arthritis Rheum 43:400–404 [DOI] [PubMed] [Google Scholar]
- 10.Lindberg H (1986) Prevalence of primary coxarthrosis in siblings of patients with primary coxarthrosis. Clin Orthop Relat Res 203:273–275 [PubMed] [Google Scholar]
- 11.Chitnavis J, Sinsheimer JS, Clipsham K, Loughlin J, Sykes B, Burge PD, Carr AJ (1997) Genetic influences in end-stage osteoarthritis: sibling risks of hip and knee replacement for idiopathic osteoarthritis. J Bone Joint Surg Br 79:660–664 10.1302/0301-620X.79B4.7437 [DOI] [PubMed] [Google Scholar]
- 12.Ingvarsson T, Stefansson SE, Gulcher JR, Jonsson HH, Jonsson H, Frigge ML, Palsdottir E, Olafsdottir G, Jonsdottir T, Walters GB, Lohmander LS, Stefansson K (2001) A large Icelandic family with early osteoarthritis of the hip associated with a susceptibility locus on chromosome 16p. Arthritis Rheum 44:2548–2555 [DOI] [PubMed] [Google Scholar]
- 13.MacGregor AJ, Antoniades L, Matson M, Andrew T, Spector TD (2000) The genetic contribution to radiographic hip osteoarthritis in women: results of a classic twin study. Arthritis Rheum 43:2410–2416 [DOI] [PubMed] [Google Scholar]
- 14.Mustafa Z, Chapman K, Irven C, Carr AJ, Clipsham K, Chitnavis J, Sinsheimer JS, Bloomfield VA, McCartney M, Cox O, Sykes B, Loughlin J (2000) Linkage analysis of candidate genes as susceptibility loci for osteoarthritis-suggestive linkage of COL9A1 to female hip osteoarthritis. Rheumatology (Oxford) 39:299–306 [DOI] [PubMed] [Google Scholar]
- 15.Chapman K, Mustafa Z, Irven C, Carr AJ, Clipsham K, Smith A, Chitnavis J, Sinsheimer JS, Bloomfield VA, McCartney M, Cox O, Cardon LR, Sykes B, Loughlin J (1999) Osteoarthritis-susceptibility locus on chromosome 11q, detected by linkage. Am J Hum Genet 65:167–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loughlin J, Mustafa Z, Smith A, Irven C, Carr AJ, Clipsham K, Chitnavis J, Bloomfield VA, McCartney M, Cox O, Sinsheimer JS, Sykes B, Chapman KE (2000) Linkage analysis of chromosome 2q in osteoarthritis. Rheumatology (Oxford) 39:377–381 [DOI] [PubMed] [Google Scholar]
- 17.Ala-Kokko L, Baldwin CT, Moskowitz RW, Prockop DJ (1990) Single base mutation in the type II procollagen gene (COL2A1) as a cause of primary osteoarthritis associated with a mild chondrodysplasia. Proc Natl Acad Sci USA 87:6565–6568 10.1073/pnas.87.17.6565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cilliers HJ, Beighton P (1990) Beukes familial hip dysplasia: an autosomal dominant entity. Am J Med Genet 36:386–390 10.1002/ajmg.1320360403 [DOI] [PubMed] [Google Scholar]
- 19.Roby P, Eyre S, Worthington J, Ramesar R, Cilliers H, Beighton P, Grant M, Wallis G (1999) Autosomal dominant (Beukes) premature degenerative osteoarthropathy of the hip joint maps to an 11-cM region on chromosome 4q35. Am J Hum Genet 64:904–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saito M, Saito A, Kamatani N (2002) Web-based detection of genotype errors in pedigree data. J Hum Genet 47:377–379 10.1007/s100380200054 [DOI] [PubMed] [Google Scholar]
- 21.Lathrop GM, Lalouel JM, Julier C, Ott J (1984) Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci USA 81:3443–3446 10.1073/pnas.81.11.3443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cottingham RW Jr, Idury RM, Schaffer AA (1993) Faster sequential genetic linkage computations. Am J Hum Genet 53:252–263 [PMC free article] [PubMed] [Google Scholar]
- 23.Schaffer AA, Gupta SK, Shriram K, Cottingham RW Jr (1994) Avoiding recomputation in linkage analysis. Hum Hered 44:225–237 [DOI] [PubMed] [Google Scholar]
- 24.Sobel E, Lange K (1996) Descent graphs in pedigree analysis: applications to haplotyping, location scores, and marker-sharing statistics. Am J Hum Genet 58:1323–1337 [PMC free article] [PubMed] [Google Scholar]
- 25.Wynne-Davies R (1970) Acetabular dysplasia and familial joint laxity: two etiological factors in congenital dislocation of the hip: a review of 589 patients and their families. J Bone Joint Surg Br 52:704–716 [PubMed] [Google Scholar]
- 26.Beals RK (2003) Familial primary acetabular dysplasia and dislocation of the hip. Clin Orthop Relat Res 406:109–115 10.1097/00003086-200301000-00018 [DOI] [PubMed] [Google Scholar]
- 27.Mackie EJ, Thesleff I, Chiquet-Ehrismann R (1987) Tenascin is associated with chondrogenic and osteogenic differentiation in vivo and promotes chondrogenesis in vitro. J Cell Biol 105:2569–2579 10.1083/jcb.105.6.2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tavella S, Raffo P, Tacchetti C, Cancedda R, Castagnola P (1994) N-CAM and N-cadherin expression during in vitro chondrogenesis. Exp Cell Res 215:354–362 10.1006/excr.1994.1352 [DOI] [PubMed] [Google Scholar]
- 29.Mototani H, Mabuchi A, Saito S, Fujioka M, Iida A, Takatori Y, Kotani A, Kubo T, Nakamura K, Sekine A, Murakami Y, Tsunoda T, Notoya K, Nakamura Y, Ikegawa S (2005) A functional single nucleotide polymorphism in the core promoter region of CALM1 is associated with hip osteoarthritis in Japanese. Hum Mol Genet 14:1009–1017 10.1093/hmg/ddi093 [DOI] [PubMed] [Google Scholar]
- 30.Mardon G, Solomon NM, Rubin GM (1994) dachshund Encodes a nuclear protein required for normal eye and leg development in Drosophila. Development 120:3473–3486 [DOI] [PubMed] [Google Scholar]