Abstract
Premature ovarian failure (POF) is characterized by elevated gonadotropins and amenorrhea in women aged <40 years. In a Lebanese family with five sisters who received the diagnosis of POF, we established linkage to the long arm of the X chromosome (between Xq21.1 and Xq21.3.3), using whole-genome SNP typing and homozygosity-by-descent mapping. By sequencing one candidate gene within that region, POF1B, we identified a point mutation localized in exon 10. This substitution of a nucleotide (G→A), at position 1123, results in an arginine→glutamine mutation of the protein sequence at position 329 (mutation R329Q). All the affected family members were homozygous for the mutation, whereas the unaffected members were heterozygous. Because POF1B shares high homology with the tail portion of the human myosin, we assessed the ability of both wild-type and mutant POF1B proteins to bind nonmuscle actin filaments in vitro. We found that the capacity of the mutant protein to bind nonmuscle actin filaments was diminished fourfold compared with the wild type, suggesting a function of POF1B in germ-cell division. Our study suggests that a homozygous point mutation in POF1B influences the pathogenesis of POF by altering POF1B binding to nonmuscle actin filaments.
A number of physiological changes are associated with menopause: decline in the number of ovarian follicles, menstrual irregularities, ovarian hormonal deficiency, anovulation, decreased fertility, and, finally, a complete and irreversible cessation of menses.1,2 In Western populations, it has been estimated that this phenomenon occurs at a mean age of 50 years. However, in 2%–3% of women, cessation of menses occurs before age 40 years.3,4 This condition, called “premature ovarian failure” (POF),5 is characterized by primary or secondary amenorrhea, hypoestrogenism, and elevated serum-gonadotropin levels.
The etiology of POF is poorly understood because its known causes are highly heterogeneous. POF is most commonly caused by the presence of autoantibodies,6 but the disorder can also have genetic origins.7,8 Although the X chromosome undergoes inactivation in somatic tissues, it is reactivated in oogonia, and both alleles of the X-chromosome genes involved in ovarian morphogenesis are required for normal ovarian development. As a result, X monosomy or X rearrangements disrupting genes of the X chromosomes are sufficient to cause ovarian failure. Autosomal mutations in the follicle-stimulating hormone (FSH) and in both FSH and luteinizing-hormone (LH) receptor genes have already been reported.9
It has often been observed that abnormalities in the X chromosome are present in women affected by POF. A “critical region” for normal ovarian function has been reported on the long arm of the X chromosome that spans Xq13.3-q26/27.10 Within this region, according to breakpoint mapping of X-autosome translocations, two regions called “POF1” and “POF2” have been identified. The number of genes involved in POF is small and localized to either POF1 or POF2. POF1 extends from Xq21-qter and is home to XPNPEP2 on Xq2511 and the FMR1 gene. POF2 extends from Xq13.3 to Xq21.1 and harbors DACH2 in Xq21.3,12 DIAPH2 in proximal Xq22,13 and POF1B, the gene that is the subject of the present study. Individuals with the FMR1 premutation have idiopathic POF.
POF1B is thus located within the critical region for normal ovarian function, is known to escape X inactivation, and was identified as a candidate gene for POF in a patient with a breakpoint in the third intron because of an X;1 translocation.14,15 The function of the POF1B protein remains unknown, and there is no genetic evidence of its role in POF. A mutation analysis of POF1B performed in an Italian population showed that a number of heterozygous sequence variants were present in both patients with POF and healthy controls. It was concluded that the POF1B gene was not associated with POF.16
In the present study, we describe a Lebanese familial case of POF, with linkage to Xq21. We identify a point mutation in the candidate gene POF1B and demonstrate that this mutation disrupts the normal binding of POF1B to nonmuscle actin filaments.
Material and Methods
Pedigree
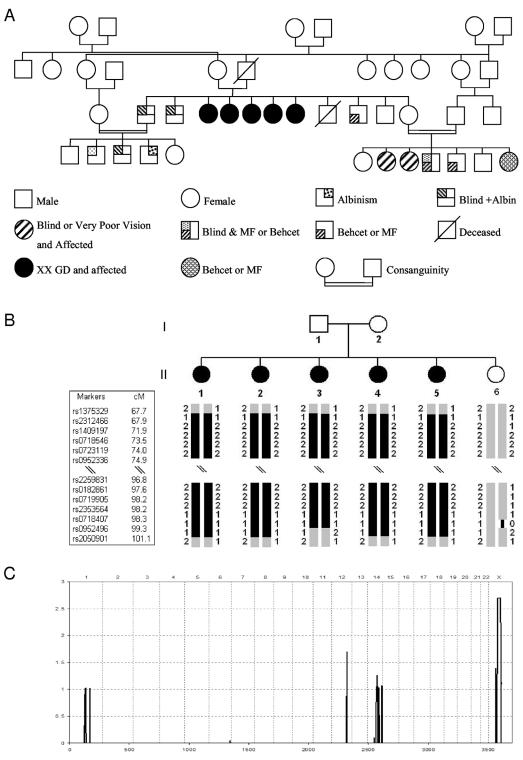
We report a familial Lebanese case of POF wherein the proband's parents, who are consanguineous, had five affected daughters, one healthy son, four other sons (two with albinism and blindness due to retinitis pigmentosa, one with familial Mediterranean fever/Behcet disease [FMF/Behcet], and one who died at age 14 years), and one healthy daughter (fig. 1A). The fertile, unaffected daughter is also in a consanguineous marriage and has several affected children with blindness due to retinitis pigmentosa and/or with FMF/Behcet. We had access to the DNA of the healthy son, all the daughters, and the mother but not the father of all the affected daughters, who was deceased, and the three sons with albinism/blindness or FMF/Behcet, who declined to be interviewed for the study. All three of these individuals were married and had progeny, but we have little clinical information available beyond what was given from participating family members.
Figure 1. .
Pedigree and linkage. A, Pedigree. B, Haplotype structure of the pedigree. Homozygous blocks on the X chromosome that are shared by all five affected daughters are shown as blackened bars. Each marker is labeled with its name, and the genetic distance appears to the right; blackened symbols indicate affected individuals. Fifty SNPs in the middle of each block were omitted because of the space constraint. Note that all affected daughters share identical haplotypes over a 21-Mb homozygous interval that the healthy daughter does not share. C, Genomewide-scan linkage analysis under an assumption of recessive inheritance and first-cousin consanguineous mating. Multipoint LOD scores are plotted along the Y-axis, and chromosomal position is plotted along the X-axis. Chromosomes are listed in numerical order across the top.
POF Diagnosis
The proband (individual 41) was aged 31 years at presentation. She is single, has never been pregnant, and had a history of delayed puberty with primary amenorrhea. She denied any history of galactorrhea, anosmia, or headaches. She continued to increase in height until she began sex-hormone treatment. Relevant aspects of her physical exam showed a height of 181 cm and weight of 81 kg, normal axillary hair, and breasts of Tanner stage 3. She also has documented osteoporosis and “weak teeth.” She had five sisters who were aged 45, 41, 39, 34, and 29 years at the time of her diagnosis. The eldest was married to a first cousin with whom she had children; among her children are two daughters who are both blind due to retinitis pigmentosa and who have normal menses, LH, and FSH. The latter four sisters also have primary amenorrhea; in at least one sister, osteoporosis and dental problems similar to those of the proband are documented. One of the proband’s albino brothers also married his first cousin and has a daughter who is infertile, but no further clinical information on her was available. The second albino brother married an unrelated partner and has two daughters who have normal menses. The proband was treated with Premarin and Provera, which resulted in resumption of her menses and advancement of her breasts and pubic hair to Tanner stage 5 after 2 years of therapy. Her electrolytes were within normal limits. Before treatment with sex hormones, her LH was 11.5 IU/liter, FSH was 56.7 IU/liter, E2 was 8.9 IU/liter, and testosterone was 39 IU/liter. Her karyotype was 46,XX. Adrenocorticotropic hormone (ACTH)-stimulation test showed dehydroepiandrosterone sulfate of 2,009 IU/liter and 17OH-P of 0.35 at baseline, which went to 2,363 and 3.15, respectively, 60 min after ACTH stimulation.
Linkage
After obtaining informed consent from all study individuals, blood samples were collected and genomic DNA was extracted by standard salt-precipitation technique.17 The research protocol was approved by the local institutional review board. Linkage was tested using a homozygosity-by-descent mapping approach, with data derived from the GeneChip Mapping 10K array (Affymetrix). To test for linkage, SNP calls generated by GeneChip DNA Analysis Software were converted to different formats by scripts written in the lab for each program used (available from H.L. on request). Mendelian and non-Mendelian errors were detected with Merlin (Multipoint Engine for Rapid Likelihood Interference) and MINX (Merlin for X chromosome). MAPMAKER/HOMOZ version 0.9 was used to perform homozygosity-mapping analysis of 11,149 SNPs from the 10K mapping array. Multipoint LOD score was calculated with MAPMAKER/HOMOZ for the region flanking the autozygous segment of interest. Because of the program constraints, the genome scan was run using a 50-marker window. Asian allele frequencies provided by Affymetrix were used for the analysis. Haplotypes were constructed with Merlin, and a list of candidate genes was generated using University of California–Santa Cruz (UCSC) resources from the genomic region identified by the linkage result. There are 270 known genes in this region.
Sequencing
From the list of candidate genes, we performed a mutation screening of diaphanous (MIM 300108) and POF1B (MIM 311360), two genes known to be interrupted by a breakpoint in patients affected by POF. Primers were designed for each exon and then were amplified by PCR. PCR product was sequenced by an automated DNA sequence analyzer (ABI Prism Tm 373 [Perkin-Elmer]). Finally, the sequences were analyzed and screened for mutation.
Incidence of the R329Q Variant in the Lebanese Population
Presence of the R329Q variant was investigated in 92 women from the same ethnic background as our family. Individuals were known to be fertile and had a normal obstetric history; therefore, they were appropriate controls for our study. The DNA was collected in an anonymous way, with complete deidentification. Mutation screening in exon 10 of each control was performed using a dideoxy sequencing method (Agencourt Bioscience).
Protein Domain Prediction
To assess POF1B protein function, the protein sequence was submitted to different database sources: National Center for Biotechnology Information (NCBI) protein alignment, NCBI protein domain prediction, and Predict Protein software.
Plasmids
POF1B cDNA was purchased from OriGene. To produce the cDNA mutant in vitro, we used the QuikChange Site-Directed Mutagenesis Kit (Stratagene) and followed the manufacturer’s instructions. The mutated oligonucleotide primers were designed as follows: forward, 5′-GCTAAATGTGGACAGCACTAGTTGGAGTGACTTATCAG-3′; reverse, 5′-CTGATAAGTCACTCCAACTAGTGCTGTCCACATTTAGC-3′. Sequences and presence of the point mutation were checked by automated DNA sequencing (ABI Prism TSm 373 [Perkin-Elmer]).
In Vitro Protein Production
Both wild-type and mutant cDNA were used as a template to produce proteins in vitro, with use of a transcription translation kit (TNT Quick Coupled Transcription/Translation kit [Promega]). Yields of protein were estimated from the incorporation of 35S-methionine in reactions that were performed in parallel with nonradiolabeled syntheses. The 35S-radiolabeled wild-type and mutant POF1B proteins were run on a 12% SDS-PAGE gel. The gel was dried, exposed, and developed using a phosphorimager cassette.
Actin-Myosin Binding Study
The ability of both wild-type and mutant POF1B protein to bind nonmuscle actin filaments in vitro was assessed using a nonmuscle actin kit purchased from Cytoskeleton. A constant and equal quantity of wild-type and mutant stock protein were incubated with nonmuscle actin at room temperature for 30 min. Each experiment was run in duplicate and also with a control in which actin was substituted by an identical volume of actin buffer. Ultracentrifugation was performed at 150,000 g for 1.5 h at 4°C. Both supernatants and pellets were run on a 4%–20% (w/v) SDS-PAGE gel. The gel was dried, exposed, and developed using a phosphorimager cassette.
Results
Linkage
A panel of >10,000 SNPs was typed on all individuals with use of the 10K-SNP mapping assay (Affymetrix). The data from this microarray-based approach resulted in high information content throughout the genome. The mean call rate (±SD) was 94%±0.03% (with a range of 89.6%–96.63%). The distribution of genotype calls was consistent over AA, AB, and BB calls, showing slightly lower AB calls in most of the samples, as is expected for an inbred family. Mendelian error rate and non-Mendelian error rate were 0.08% and 0.16%, respectively. Genotypes for SNPs with at least one error were changed to “No Call” across all the family members before starting the analysis. Since there was no information on the genetic relationship between the parents of the affected women, we counted the possible autozygous segments in the autosomal chromosomes of the descendants, to determine whether it was likely that the affected daughters were from a consanguineous mating, which would permit us to use homozygosity by descent to map the disease interval.18 Identification of the autozygous segments by counting the long homozygous segments was possible because of the high-density SNPs with mean genetic map distance of 0.32 cM and mean heterozygosity of 0.37 cM. Since regions of autozygosity are expected to be large with first- or second-cousin inbreeding,18 we counted the number of segments with a minimum of 10 consecutive homozygous SNPs that spanned at least 10 cM in all autosomes. The estimated number of autozygous blocks >10 cM is 6–10 for first-cousin mating, 3–5 for first-cousin-once-removed mating, and 1–3 for second-cousin mating. The six siblings in our study have an average (±SD) of 7±2.7 homozygous blocks, which strongly suggests that they are descendents of a consanguineous marriage of recent relatives (first-cousin-once-removed marriage or first-cousin marriage).
We searched through the genome and detected a single long segment of homozygosity on the X chromosome that was shared only by the affected daughters (fig. 1B). The unaffected daughter was heterozygous for that region. Haplotypes were constructed with Merlin, and the homozygous segment spanned bases 41926093–92958387 (UCSC May 2004 assembly). To rule out the possibility that there could be shared autozygous segments on the autosomal chromosomes, we scanned the remaining genome with MAPMAKER/HOMOZ for homozygosity-mapping analysis. Assuming the parents are first cousins, we performed analysis under an autosomal recessive inheritance model. Throughout the autosomal chromosomes, there was no homozygous stretch shared by only the affected daughters and not by the unaffected daughter. The locus of linkage was localized to the long arm of the X chromosome (genomic region between Xq21.1 and Xq 21.3.3), corresponding to a 21-Mb region (fig. 1C). Because of the long length, this interval is likely autozygous in all of the affected daughters. On the basis of the physical interval, a list of 270 known genes was generated, and two candidate genes (POF1B and diaphanous) were analyzed.
Family Members with POF Who Carry a Point Mutation in Exon 10 of POF1B
To identify a possible involvement for one of the candidates in POF, we screened each exon by direct sequencing of two candidates that have been demonstrated to be disrupted by a breakpoint (localized in introns) in patients affected by POF: diaphanous and POF1B.
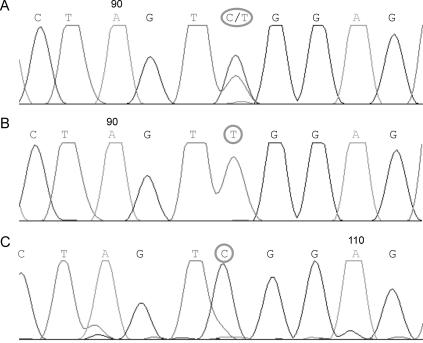
Sequencing of diaphanous exons failed to reveal a mutation for any of the family members (data not shown). However, when screening POF1B, we identified a point mutation in exon 10, a G→A substitution at np 1132 (fig. 2). This variant results in an amino acid change of arginine→glutamine at position 329 (R329Q) in the protein sequence. The unaffected women screened in the family (one daughter and the mother) are both heterozygous at this position, whereas all five affected daughters are homozygous for the mutation. Since the father died before the time of investigation, we were unable to screen his DNA.
Figure 2. .
Representative sequence chromatogram of exon 10 of the POF1B gene after sequencing of the members of the family. A, Sequence analysis of the mother’s and unaffected daughter’s DNA. At np 1132, those members carry either a cytosine (C) or a thymine (T), which shows they are heterozygous. B, Sequence analysis of an affected daughter's DNA. At the same np 1132, all patients carry a T and are homozygous for the mutation. C, Sequence analysis of DNA of an unrelated female control. The control individual is a homozygote and carries a C.
It is conceivable that the variant detected is a common SNP. To measure the incidence of the R329Q variant in the general Lebanese population, we tested whether the nucleotide variant is present in 92 individuals from a similar ethnic background. Although none carried the mutation in a homozygous state, results of sequence screening (data not shown) revealed that the mutation was present, in a heterozygous state, in 4 of the 92 control women. Thus, the estimated allele frequency in Lebanon is 2.2%. The predicted rate of homozygosity in this population would be 0.048% and could account for a fraction of the POF detected in this population.
POF1B Protein Shares a High Degree of Homology with Human Myosin
Although POF1B was identified as a candidate for POF,15 little is known about its expression and potential role. To begin investigation of its function, we performed protein-sequence comparisons against different databases. Results from NCBI protein alignment indicate that the protein shares 42% similarity and 17% identity with the human perinatal myosin, also named “heavy polypeptide 8.”
This identity localizes to the C terminus and is, in fact, a myosin tail. No similarity with other proteins was found for sequence at the N-terminus.
A “predict protein” search indicated that the variant alters the arginine with an amino acid triplet, serine-leucine-arginine, which is part of a protein kinase C (PKC) phosphorylation-recognition site. Thus, this rare variant may have functional consequences.
Mutant POF1B Binds Nonmuscle Actin with Less Affinity Than Wild-Type POF1B In Vitro
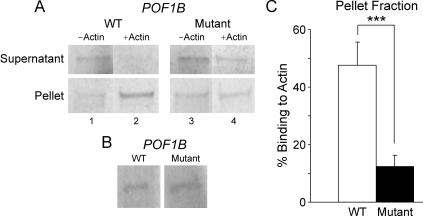
Previous studies have shown that, during ovarian development, the alteration of the final stock of oocytes could lead to POF. Given these findings and the similarity of the C terminus of POF1B to the myosin tail of the human myosin, we next tested the hypothesis that POF1B could interact with actin filaments and therefore have a possible role in the cell-division process. To do so, we assessed the ability of both the wild-type and mutant proteins to assemble with nonmuscle actin in vitro (fig. 3). We show that, in the supernatant fraction, which mainly contains protein not bound to actin, the decrease of intensity of the signal after actin binding in wild-type POF1B is larger than in R329Q mutant (fig. 3A). Conversely, in the pellet, in the presence of actin, the signal was more intense in the wild type than in R329Q mutant (fig. 3A). After quantification of three independent experiments (each in duplicate), we recorded an average 48% binding to actin filament with the wild-type POF1B, whereas it was 12% with the R329Q variant (P=.0024) (fig. 3C). There was no difference in the yield of protein produced after the in vitro translation (fig. 3B).
Figure 3. .
Nonmuscle actin–binding assay of wild-type (WT) and mutant POF1B. A, A constant and equal concentration of 35S-radiolabeled wild-type and mutant POF1B was incubated with 12 μM nonmuscle actin and was spun down at 150,000 g for 1.5 h at 4°C. As a control, the same concentrations of 35S-radiolabeled proteins were incubated under the same conditions, but nonmuscle actin was replaced with the same volume of actin buffer. The top panel represents SDS-PAGE gel picture after 70-h exposure from supernatant of each sample run in duplicate (Supernatant), whereas the bottom panel corresponds to SDS-PAGE gel picture from pellets (Pellet) after the same time of exposure. Lanes correspond to wild-type POF1B (1 and 2) or mutant POF1B (3 and 4), in the presence (2 and 4) or absence (1 and 3) of nonmuscle actin filaments. Results are representative of three independent experiments. B, SDS-PAGE analysis of in vitro–translated proteins. The same amount of in vitro–translated protein reaction of wild-type and mutant POF1B was run on an SDS-PAGE gel, to measure the yield of produced proteins. C, Quantification of the radioactive signal in the pellet after the nonmuscle actin–binding assay. A triple asterisk (***) indicates P<.005.
Discussion
The molecular mechanisms that lead to POF are poorly understood. Most frequently, POF cases are idiopathic or sporadic.3 Previous studies have described familial cases of POF with a dominant mutation or an association with X-chromosome abnormalities.19,20 A major obstacle to the identification of new genes responsible for POF is the lack of familial cases with full pedigrees.21 In the present study, we show that a homozygous point mutation in POF1B, a gene mapping to the distal end of the POF2 critical interval on X chromosome, may be linked to the pathogenesis of POF. We show that each affected 46,XX daughter in a Lebanese family carries a homozygous R329Q variant in exon 10 of POF1B at np 1132, whereas unaffected females (one healthy daughter and the mother) are heterozygous for this variant. This variant alters the binding of the POF1B protein to nonmuscle actin.
POF1B and POF
POF1B, a gene found only in vertebrates, was discovered elsewhere to be interrupted by an X;1 balanced translocation in a patient with secondary amenorrhea.15 Recently, a mutation analysis study performed on Italian patients with POF showed that, among several polymorphisms, 1 of 223 patients carried the heterozygous R329Q mutation in the POF1B gene, which was also found in 3 of 900 healthy controls. The authors concluded that there was no significant association between POF1B and POF.16 The lack of an association in the Italian study could be due either to population differences or to the fact that most of the Italian patients had secondary amenorrhea, whereas our patients had primary amenorrhea. Therefore, the etiology of each type is likely to be different.
Our data suggest that, in a homozygous state, R329Q is associated with POF. Since the mother and one daughter, both unaffected by POF, were heterozygous, and since there could be consanguinity in this family, a recessive mode of inheritance is likely. In the control group—women from the same ethnicity as our familial case—we identified four heterozygous mutations in 92 individuals. This high frequency of this genotype in Lebanon increases the likelihood of the presence of R329Q at the homozygous state. Although there is no public health information about the incidence of POF in the Lebanese population available at this time, we speculate that there is a relatively high rate of the condition in that country. Sporadic and familial cases should be investigated, in a large number of Lebanese women, for the presence of R329Q variants.
POF1B Protein Function
Although POF1B is one of the candidate genes for POF, its function remains unknown. Protein-function predictions suggest that POF1B shares homology with the myosin tail portion of the human myosin protein. Also, predictions support the hypothesis that the presence of the R329Q mutation affects a phosphorylation site represented by a triplet of amino acids: serine-leucine-arginine, normally recognized by the PKC.
Myosin plays an important role in cytokinesis. At the time of division, filaments of actin and myosin form a contractile ring, a structure that is fundamental and required for normal cell division.22–27 Also, it is well known that phosphorylation events are essential for myosin protein function. In mammalian smooth muscle and nonmuscle systems, myosin ATPase activity, which regulates the motor function of the protein, is highly dependent on phosphorylation events.28 Given the high degree of homology of POF1B and the myosin tail of the human myosin protein, we speculate that POF1B is a protein involved in actin-filaments interaction and that the R329Q variant leads to a lack of phosphorylation at the serine-leucine-arginine site, which causes the loss of function of the POF1B protein.
To test the hypothesis that the POF1B mutant binds actin filaments with less affinity than the wild type, we assessed the capacity of both wild-type POF1B and the mutant to bind nonmuscle-actin filaments in vitro. Our data demonstrated that the variant POF1B binds nonmuscle actin with less affinity than the wild type, suggesting that the R329Q variant affects the binding of POF1B with actin.
Molecular Mechanisms of POF1B in POF
Between E16.5 and P5 (during the POF1B expression time frame), while germ cells within germline cysts enter meiosis and progress through the stages of pachytene and diplotene, massive germ-cell apoptosis occurs.29 In mice, as in humans, this natural process of female germ-cell loss occurs to eliminate abnormal cells.30–32 The disruption of POF1B binding to actin filaments may have various consequences for ovarian-follicle formation. The reported interval of expression of the POF1B mRNA in mouse precludes its involvement in primordial-germ-cell migration, since it is first detected at day 16.5 post coitum. Furthermore, at this particular time interval, synaptonemal-complex assembly has occurred, and oocytes are in the pachytene stage. The interval of expression is consistent with a role of the protein in the transition from pachytene to diplotene and dictyate arrest. The period from day 16.5 to birth is characterized by massive germ-cell attrition through programmed cell death, due to either failure of completion of meiosis 1 or premature initiation of metaphase 1.33 On the basis of the above and the homology of POF1B to myosin, we speculate that POF1B could play a role in the pairing of meiotic chromosomes and that alteration in its function could lead to cell death and a drastic reduction in the final number of oocytes created during ovarian development.
Alternatively, POF1B could be involved in the regulation of germ-cell apoptosis through a role in cytoskeletal dynamics. Alterations in the actin cytoskeleton have been recently implicated in release of reactive oxygen species from mitochondria and germ-cell apoptosis.34 POF1B may act as an antiapoptosis factor, to slow down the process of germ-cell loss. As a consequence, loss of function of POF1B could lead to exaggerated germ-cell apoptosis and POF. The precise molecular mechanisms of action of POF1B in POF will be elucidated by animal models targeting POF1B in ovarian germ cells.
Acknowledgments
We thank Dr. Fleming for her thoughtful suggestions. Microarray studies were performed within the UCLA DNA Microarray Facility. We thank Zugen Chen, for help in array performance, and Allen Day, for computational support.
Web Resources
The URL for data presented herein is as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for diaphanous and POF1B)
References
- 1.McKinlay S, Jefferys M, Thompson B (1972) An investigation of the age at menopause. J Biosoc Sci 4:161–173 [DOI] [PubMed] [Google Scholar]
- 2.Wyshak G, Frisch RE (1982) Evidence for a secular trend in age of menarche. N Engl J Med 306:1033–1035 [DOI] [PubMed] [Google Scholar]
- 3.Coulam CB, Adamson SC, Annegers JF (1986) Incidence of premature ovarian failure. Obstet Gynecol 67:604–606 [PubMed] [Google Scholar]
- 4.Vegetti W, Marozzi A, Manfredini E, Testa G, Alagna F, Nicolosi A, Caliari I, Taborelli M, Tibiletti MG, Dalpra L, Crosignani PG (2000) Premature ovarian failure. Mol Cell Endocrinol 161:53–57 10.1016/S0303-7207(99)00224-5 [DOI] [PubMed] [Google Scholar]
- 5.de Moraes-Ruehsen M, Jones GS (1967) Premature ovarian failure. Fertil Steril 18:440–461 [DOI] [PubMed] [Google Scholar]
- 6.Wheatcroft N, Weetman AP (1997) Is premature ovarian failure an autoimmune disease? Autoimmunity 25:157–165 [DOI] [PubMed] [Google Scholar]
- 7.Bione S, Toniolo D (2000) X chromosome genes and premature ovarian failure. Semin Reprod Med 18:51–57 10.1055/s-2000-13475 [DOI] [PubMed] [Google Scholar]
- 8.Santoro N (2001) Research on the mechanisms of premature ovarian failure. J Soc Gynecol Investig 8:S10–S12 10.1016/S1071-5576(00)00097-6 [DOI] [PubMed] [Google Scholar]
- 9.Christin-Maitre S, Vasseur C, Portnoi MF, Bouchard P (1998) Genes and premature ovarian failure. Mol Cell Endocrinol 145:75–80 10.1016/S0303-7207(98)00172-5 [DOI] [PubMed] [Google Scholar]
- 10.Therman E, Laxova R, Susman B (1990) The critical region on the human Xq. Hum Genet 85:455–461 [DOI] [PubMed] [Google Scholar]
- 11.Prueitt RL, Ross JL, Zinn AR (2000) Physical mapping of nine Xq translocation breakpoints and identification of XPNPEP2 as a premature ovarian failure candidate gene. Cytogenet Cell Genet 89:44–50 10.1159/000015560 [DOI] [PubMed] [Google Scholar]
- 12.Prueitt RL, Chen H, Barnes RI, Zinn AR (2002) Most X;autosome translocations associated with premature ovarian failure do not interrupt X-linked genes. Cytogenet Genome Res 97:32–38 10.1159/000064052 [DOI] [PubMed] [Google Scholar]
- 13.Bione S, Sala C, Manzini C, Arrigo G, Zuffardi O, Banfi S, Borsani G, Jonveaux P, Philippe C, Zuccotti M, Ballabio A, Toniolo D (1998) A human homologue of the Drosophila melanogaster diaphanous gene is disrupted in a patient with premature ovarian failure: evidence for conserved function in oogenesis and implications for human sterility. Am J Hum Genet 62:533–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gersak K, Meden-Vrtovec H, Peterlin B (2003) Fragile X premutation in women with sporadic premature ovarian failure in Slovenia. Hum Reprod 18:1637–1640 10.1093/humrep/deg327 [DOI] [PubMed] [Google Scholar]
- 15.Riva P, Magnani I, Fuhrmann Conti AM, Gelli D, Sala C, Toniolo D, Larizza L (1996) FISH characterization of the Xq21 breakpoint in a translocation carrier with premature ovarian failure. Clin Genet 50:267–269 [DOI] [PubMed] [Google Scholar]
- 16.Bione S, Rizzolio F, Sala C, Ricotti R, Goegan M, Manzini MC, Battaglia R, Marozzi A, Vegetti W, Dalpra L, Crosignani PG, Ginelli E, Nappi R, Bernabini S, Bruni V, Torricelli F, Zuffardi O, Toniolo D (2004) Mutation analysis of two candidate genes for premature ovarian failure, DACH2 and POF1B. Hum Reprod 19:2759–2766 10.1093/humrep/deh502 [DOI] [PubMed] [Google Scholar]
- 17.Miller SA, Dykes DD, Polesky HF (1988) A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 16:1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lander ES, Botstein D (1987) Homozygosity mapping: a way to map human recessive traits with the DNA of inbred children. Science 236:1567–1570 [DOI] [PubMed] [Google Scholar]
- 19.Sala C, Arrigo G, Torri G, Martinazzi F, Riva P, Larizza L, Philippe C, Jonveaux P, Sloan F, Labella T, Toniolo D (1997) Eleven X chromosome breakpoints associated with premature ovarian failure (POF) map to a 15-Mb YAC contig spanning Xq21. Genomics 40:123–131 10.1006/geno.1996.4542 [DOI] [PubMed] [Google Scholar]
- 20.Vegetti W, Grazia Tibiletti M, Testa G, de Lauretis Y, Alagna F, Castoldi E, Taborelli M, Motta T, Bolis PF, Dalpra L, Crosignani PG (1998) Inheritance in idiopathic premature ovarian failure: analysis of 71 cases. Hum Reprod 13:1796–1800 10.1093/humrep/13.7.1796 [DOI] [PubMed] [Google Scholar]
- 21.Simpson JL, Rajkovic A (1999) Ovarian differentiation and gonadal failure. Am J Med Genet 89:186–200 [DOI] [PubMed] [Google Scholar]
- 22.Glotzer M (1997) Cytokinesis. Curr Biol 7:R274–R276 10.1016/S0960-9822(06)00135-7 [DOI] [PubMed] [Google Scholar]
- 23.Glotzer M (1997) The mechanism and control of cytokinesis. Curr Opin Cell Biol 9:815–823 10.1016/S0955-0674(97)80082-8 [DOI] [PubMed] [Google Scholar]
- 24.Mabuchi I (1986) Biochemical aspects of cytokinesis. Int Rev Cytol 101:175–213 [DOI] [PubMed] [Google Scholar]
- 25.Mabuchi I, Okuno M (1977) The effect of myosin antibody on the division of starfish blastomeres. J Cell Biol 74:251–263 10.1083/jcb.74.1.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson DN, Spudich JA (2000) Dynacortin, a genetic link between equatorial contractility and global shape control discovered by library complementation of a Dictyostelium discoideum cytokinesis mutant. J Cell Biol 150:823–838 10.1083/jcb.150.4.823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson DN, Spudich JA (2000) Towards a molecular understanding of cytokinesis. Trends Cell Biol 10:228–237 10.1016/S0962-8924(00)01747-5 [DOI] [PubMed] [Google Scholar]
- 28.Tan JL, Ravid S, Spudich JA (1992) Control of nonmuscle myosins by phosphorylation. Annu Rev Biochem 61:721–759 10.1146/annurev.bi.61.070192.003445 [DOI] [PubMed] [Google Scholar]
- 29.Pepling ME, Spradling AC (2001) Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol 234:339–351 10.1006/dbio.2001.0269 [DOI] [PubMed] [Google Scholar]
- 30.Baker TG (1972) Gametogenesis. Acta Endocrinol Suppl (Copenh) 166:18–41 [DOI] [PubMed] [Google Scholar]
- 31.Krakauer DC, Mira A (1999) Mitochondria and germ-cell death. Nature 400:125–126 10.1038/22026 [DOI] [PubMed] [Google Scholar]
- 32.Morita Y, Tilly JL (1999) Oocyte apoptosis: like sand through an hourglass. Dev Biol 213:1–17 10.1006/dbio.1999.9344 [DOI] [PubMed] [Google Scholar]
- 33.De Felici M, Klinger FG, Farini D, Scaldaferri ML, Iona S, Lobascio M (2005) Establishment of oocyte population in the fetal ovary: primordial germ cell proliferation and oocyte programmed cell death. Reprod Biomed Online 10:182–191 [DOI] [PubMed] [Google Scholar]
- 34.Gourlay CW, Ayscough KR (2005) The actin cytoskeleton: a key regulator of apoptosis and ageing? Nat Rev Mol Cell Biol 6:583–589 10.1038/nrm1682 [DOI] [PubMed] [Google Scholar]