Abstract
The commonly used “end diagnosis” phenotype that is adopted in linkage and association studies of complex traits is likely to represent an oversimplified model of the genetic background of a disease. This is also likely to be the case for common types of migraine, for which no convincingly associated genetic variants have been reported. In headache disorders, most genetic studies have used end diagnoses of the International Headache Society (IHS) classification as phenotypes. Here, we introduce an alternative strategy; we use trait components—individual clinical symptoms of migraine—to determine affection status in genomewide linkage analyses of migraine-affected families. We identified linkage between several traits and markers on chromosome 4q24 (highest LOD score under locus heterogeneity [HLOD] 4.52), a locus we previously reported to be linked to the end diagnosis migraine with aura. The pulsation trait identified a novel locus on 17p13 (HLOD 4.65). Additionally, a trait combination phenotype (IHS full criteria) revealed a locus on 18q12 (HLOD 3.29), and the age at onset trait revealed a locus on 4q28 (HLOD 2.99). Furthermore, suggestive or nearly suggestive evidence of linkage to four additional loci was observed with the traits phonophobia (10q22) and aggravation by physical exercise (12q21, 15q14, and Xp21), and, interestingly, these loci have been linked to migraine in previous studies. Our findings suggest that the use of symptom components of migraine instead of the end diagnosis provides a useful tool in stratifying the sample for genetic studies.
Migraine (MIM 157300) is the most common cause of chronic episodic severe headache, affecting 10%–12% of the adult population.1 The genetic component of migraine has been well established in family and twin studies.2–6 Several loci linked to common forms of migraine with significant evidence of linkage have been reported,7–15 yet, to date, no genes responsible for susceptibility to the common types of migraine, migraine without aura (MO) and migraine with aura (MA), have been described. Thus far, three genes, CACNA1A (MIM 601011), ATP1A2 (MIM 182340), and SCN1A (MIM 182389), which contribute to familial hemiplegic migraine (FHM1–3 [MIMs 141500, 602481, and 609634, respectively), a rare Mendelian subtype of MA, remain the only genes found in headache disorders.16–18
Most studies aiming to unravel the genetic basis of headache disorders use the end diagnoses of the International Classification of Headache Disorders (later referred to as “International Headache Society [IHS] classification”) as the phenotype when monitoring for linkage or association19,20 (appendix A). Although diagnosis-based phenotypes have been successfully used to identify predisposing loci and even genes in some complex traits,21–23 it is likely that they represent an oversimplified model of the genetic background of a disease, which in reality is complex and polygenic. In some complex diseases, the use of clinical traits (referred to as “quantitative traits,” “endophenotypes,” or “trait components”)—for example, lipid values, components of language deficits, or allergy-related phenotypes—provides an opportunity for a more refined analysis.24–28 These traits probably reflect features that are closer to the molecular background of the disorder than the clinical classification, which represents a consensus among clinicians. The development of alternative phenotypes and/or endophenotypes would be especially useful for headache disorders, for which diagnosis is typically based on the patient’s description of attacks and no laboratory, radiological, or any other objective, quantitative findings are used for diagnostics.
The diagnosis and classification of migraine and its subtypes are based on the fulfillment of symptomatic criteria, summarized in the IHS classification (appendixes B and C). Since the migraine diagnosis consists of a combination of traits and/or symptoms, a single affected individual has to present with several traits simultaneously; furthermore, the individual traits are not completely independent of each other (fig. 1). The clinical traits and trait groups forming the basis of the IHS classification provide a natural and clinically plausible way to monitor for possible trait correlations. Although these criteria work well in clinical settings, they still define a very broad syndrome. For example, the criteria classify both a severe, one-sided, and pulsating headache and a moderate, bilateral, and dull headache as migraine, if the other IHS-defined features are present. Allowance for variability is clearly needed for clinical purposes, but a more narrow definition, especially one that uses individual traits that can be quantified, might serve better in dissecting the underlying pathophysiological mechanisms of migraine. Since the use of one specific trait, motor weakness, led to the discovery of genes causing FHM,18 a similar approach might be useful in more common forms of migraine. A similar division of the features of migrainous headache could contribute to the selection of a more homogeneous study sample.
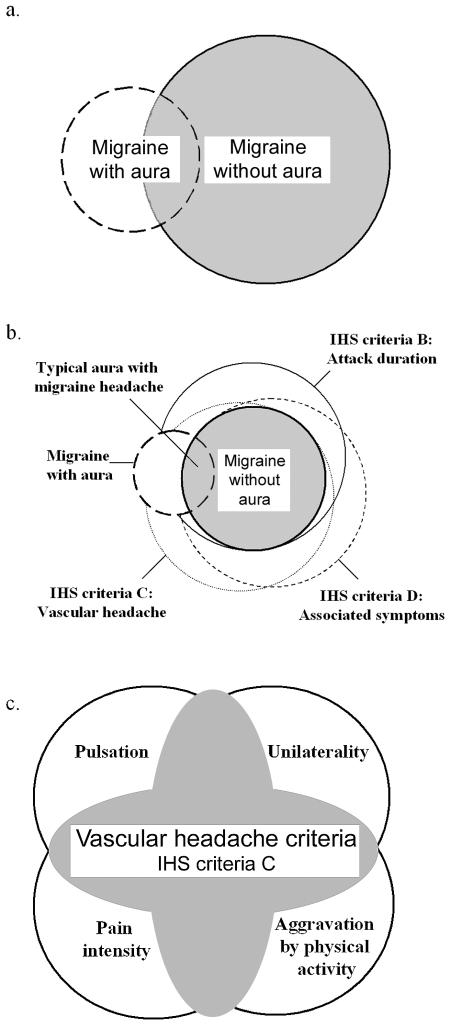
Figure 1. .
Schematic illustration of some relationships between diagnoses and traits in the 2004 IHS classification. Overlapping circles highlight the fact that each patient can have a unique combination of symptoms, and not all symptoms are needed to fulfill the criteria of the end diagnosis migraine. The circles and their overlapping surface areas do not represent population prevalence; the figure serves purely as an illustration. a, Relationship between MA and MO diagnostic groups indicating that some patients may be categorized as having purely MA type or purely MO type of attacks, but a considerable number of patients have both types. b, Relationships between traits in the IHS criteria, illustrating that, although criteria B–D are not shared by all patients with MA, those with typical aura with migraine headache have to fulfill the MO criteria as well. c, Breakdown of the vascular headache trait group. The shaded area indicates patients who fulfill IHS criteria C; at least two of the individual traits have to co-occur (e.g., pulsation and unilaterality).
In summary, since migraine is a syndrome instead of a clearly differentiated disease, we hypothesized that individual clinical components of migraine (i.e., traits such as pulsating pain and photophobia, among others) might represent reflections of specific rather than shared loci and thus independently contribute to susceptibility to migraine. To test this hypothesis, the individual traits and trait groups of the IHS criteria were used as phenotypes for genomewide linkage analyses. These analyses were performed using the same data set of 50 families as was used in our previous study, in which evidence of linkage to chromosome 4q24 was detected using end point diagnosis MA as the trait.7 By use of the trait-based approach introduced here, individuals from both the MA and the MO diagnosis groups could potentially be scored as affected. This reflects the sharing of the studied traits by migraine diagnoses 1.1 (migraine without aura [appendix B]) and 1.2.1 (typical aura with migraine headaches [appendix C]). Our hypothesis was that the shared components of the two IHS diagnoses might result in migraine phenotypes more reflective of the underlying biological processes than would a strict adherence to the end diagnoses. Here, the aim was to analyze which IHS traits provide best evidence of linkage to the 4q24 locus and whether additional susceptibility loci could be localized.
Material and Methods
Diagnoses
The study sample consisted of 438 genotyped individuals (186 men and 252 women) in 50 independent, multigenerational Finnish families and was the same set that was used in our previous genomewide screen.7 The ethics committee of Helsinki University Central Hospital approved the study protocol. The inclusion criterion for the families selected for the previous genomewide screen was a high prevalence of MA within the family. Various migraine features concerning the aura, headache, provoking factors, and prodromal symptoms were recorded by one neurologist (M.A.K.) on the basis of the validated Finnish Migraine Specific Questionnaire for Family Studies.29 Detailed selection and case-definition procedures have been reported elsewhere.7 In the previous study, 246 individuals received a diagnosis of MA, and 50 individuals received a diagnosis of MO. In the present study, the updated IHS classification (2004) was used. Under the new classification, 225 individuals fulfilled the IHS criteria shared between MO (1.1 in the IHS diagnoses) and typical aura with migraine headaches (1.2.1). This constitutes 49% of the study sample.
Formation of Phenotypes from the Individual Traits and Trait Groups
Altogether, nine individual traits (attack length, pulsation, unilaterality, aggravation by physical exercise, intensity of pain, photophobia, phonophobia, nausea, and vomiting) and five trait groups (IHS full criteria, pain criteria, associated symptoms, nausea and/or vomiting, and photo- and phonophobia) were analyzed independently as phenotypes in the linkage analysis. Table 1 summarizes the relative frequencies of the features of migrainous headache in the study sample as reported by the patients, defined by the IHS criteria, and used as traits in the analyses. The important distinction between the individual traits and the trait groups is that the individual traits reflect a distinct migraine feature, whereas the trait groups reflect a clinical consensus.
Table 1. .
Frequencies of Individual Trait Components and Trait Groups and the Sex Proportions of Those Affected, According to IHS Criteria,20 in the Study Sample
| Trait or Trait Group Corresponding to IHS Criteria |
n | Percentage of Total |
Percentage Male |
Percentage Female |
| Full criteriaa: | 225 | 52.3 | 26.2 | 73.8 |
| Attack length | 241 | 56.0 | 29.5 | 70.5 |
| Vascular headache criteriaa: | 322 | 74.9 | 33.9 | 66.1 |
| Unilateral headache | 327 | 76.0 | 34.3 | 65.7 |
| Pulsating headache | 235 | 54.7 | 27.2 | 72.8 |
| Intensity, moderate and/or severe: | 315 | 73.3 | 32.4 | 67.6 |
| Unbearable | 109 | 31.6 | 27.5 | 72.5 |
| Severe | 136 | 31.6 | 28.7 | 71.3 |
| Moderate | 70 | 16.3 | 47.1 | 52.9 |
| Aggravated by physical activity | 239 | 55.6 | 29.7 | 70.3 |
| Associated symptomsa: | 298 | 69.3 | 30.5 | 69.5 |
| Nausea and/or vomitinga: | 266 | 61.9 | 30.1 | 69.9 |
| Nausea | 266 | 61.9 | 30.1 | 69.9 |
| Vomiting | 173 | 40.2 | 31.8 | 68.2 |
| Photo- and phonophobiaa: | 229 | 53.3 | 25.8 | 74.2 |
| Photophobia | 265 | 61.6 | 30.6 | 69.4 |
| Phonophobia | 234 | 54.4 | 26.5 | 73.5 |
A trait group.
Since all individuals with vomiting also experienced nausea, the nausea and the nausea and/or vomiting categories were equal, and thus the nausea trait was not analyzed. In addition to the IHS traits, we also used the age at onset of migraine symptoms as a trait. Age was dichotomized to those individuals with the onset of migraine symptoms before age 20 years (211 individuals) and those with a later onset (64 individuals).
Genotyping
The process of the genomewide screen has been described in detail elsewhere.7 Briefly, this screen covered all autosomes and the X chromosome and used 350 polymorphic microsatellite markers, with an average of 11 cM between loci. In the fine-mapping phase, 14 new microsatellite markers were analyzed (9 on chromosome 17 and 5 on chromosome 18). For fine mapping, capillary electrophoresis, as employed by the MegaBACE 1000 DNA Sequencing System (GE Healthcare Bio-Sciences), was used to separate DNA fragments. Alleles were called by the MegaBACE Genetic Profiler 1.5 software (GE Healthcare Bio-Sciences).
Linkage Analyses
Parametric and nonparametric LOD scores were calculated using an affecteds-only strategy (i.e., all individuals not scored as affected were considered as unknown) for the 350 markers genotyped. For parametric analysis, a dominant mode of inheritance and locus heterogeneity was assumed. Each of the IHS traits and trait groups were analyzed, in turn, as the phenotype. All analyses were performed with the disease-gene frequency set at 0.001, under the assumption of autosomal dominant inheritance and a phenocopy proportion of 2.4%, reflecting the frequency of MA in the population,7,30 in accordance with the strategy proposed by Göring and Terwilliger31 and in line with our previous research. Allele frequencies were calculated from the genotypes of all individuals. The mistyping option of the SimWalk2 program32 was used to detect genotyping errors. In case of bilineal family structure, the affected married-in spouse, as well as the offspring, were treated as unknown. The initial linkage analysis was performed using a two-point approach—that is, by using a single marker and the trait. The components of the IHS migraine criteria were used as the phenotype. Two-point parametric linkage analysis was performed both under locus homogeneity and under locus heterogeneity by the computer programs LINKAGE33 and HOMOG.34 To lessen the dependence of our results on the assumption of a dominance model, we also completed an affected sib pair (ASP) analysis. The identity-by-descent (IBD) status of each ASP was estimated using the program Sibpair, which favors a recessive mode of inheritance. The ANALYZE utility program31 was used to conduct these analyses. Multipoint parametric and nonparametric analyses were performed for regions showing evidence of linkage in the parametric two-point analysis by use of the program GENEHUNTER,35 version 2.1_r5beta. Parametric linkage analysis was performed using the model presented above, while allowing for locus heterogeneity. The nonparametric statistic NPLall, which estimates the statistical significance of alleles shared IBD between all affected family members, was calculated also.
To address the multiple-testing issue and to determine the significance of the results, we applied the equation introduced by Kidd and Ott.36 They suggest that the lower limit of a significant result in a genomewide scan is 3 plus the base 10 logarithm of the number of independent tests performed. An addition of 0.3 to the significance limits is necessary to take into account the extra df from the heterogeneity LOD (HLOD) calculation. Since the traits we studied are not independent of each other, a correlation matrix of the phenotypes was constructed (table 2), and the software matSPD (see Web Resources) was used to calculate the overall correlation coefficient to determine the equivalent number of independent traits.37–39 This analysis showed that the original 14 traits (where the one duplicate trait, nausea, is eliminated by the analysis) and trait combinations correspond to just five independent variables; thus, the lower limit of significant evidence of linkage is 4.00. That is, because of the high correlation between the 14 traits, they behave statistically like five effectively independent traits. Similarly, the lower limit of suggestive evidence of linkage is 1.6 plus the base 10 logarithm of the number of tests plus 0.3, which equals 2.60. These limits are only slightly less conservative than the ones obtained using the most conservative correction based on the number of tests. Finally, to further determine the significance of the observed LOD scores, we simulated the marker with the best observed LOD score (D17S945) in 10,000 replicates of the pedigree set, assuming no linkage and using the program SIMULATE.40 The tested limits were the best observed LOD score (4.65) and the approximations of the significance limits (4.00 and 2.60). These three limits were reached zero, zero, and five times by chance in the simulated data sets, with the highest result 3.19, which is less conservative than our significance limit obtained from the trait correlation matrix.
Table 2. .
Correlation Matrix of the Analyzed Traits and Trait Groups[Note]
| Trait | a | b | c | d | e | f | g | h | i | j | k | l | m | n |
| a | 1.000 | .944 | .710 | .623 | .698 | .639 | .707 | .773 | .685 | .685 | .476 | .658 | .674 | .663 |
| b | .944 | 1.000 | .753 | .620 | .739 | .649 | .736 | .706 | .625 | .625 | .434 | .604 | .657 | .623 |
| c | .710 | .753 | 1.000 | .737 | .975 | .747 | .954 | .884 | .794 | .794 | .573 | .706 | .798 | .720 |
| d | .623 | .620 | .737 | 1.000 | .716 | .720 | .705 | .716 | .661 | .661 | .511 | .646 | .679 | .643 |
| e | .698 | .739 | .975 | .716 | 1.000 | .734 | .930 | .882 | .787 | .787 | .556 | .701 | .791 | .714 |
| f | .639 | .649 | .747 | .720 | .734 | 1.000 | .723 | .700 | .668 | .668 | .493 | .596 | .657 | .601 |
| g | .707 | .736 | .954 | .705 | .930 | .723 | 1.000 | .879 | .787 | .787 | .565 | .710 | .791 | .724 |
| h | .773 | .706 | .884 | .716 | .882 | .700 | .879 | 1.000 | .894 | .894 | .632 | .784 | .814 | .784 |
| i | .685 | .625 | .794 | .661 | .787 | .668 | .787 | .894 | 1.000 | 1.000 | .707 | .648 | .695 | .651 |
| j | .685 | .625 | .794 | .661 | .787 | .668 | .787 | .894 | 1.000 | 1.000 | .707 | .648 | .695 | .651 |
| k | .476 | .434 | .573 | .511 | .556 | .493 | .565 | .632 | .707 | .707 | 1.000 | .481 | .458 | .490 |
| l | .658 | .604 | .706 | .646 | .701 | .596 | .710 | .784 | .648 | .648 | .481 | 1.000 | .880 | .982 |
| m | .674 | .657 | .798 | .679 | .791 | .657 | .791 | .814 | .695 | .695 | .458 | .880 | 1.000 | .860 |
| n | .663 | .623 | .720 | .643 | .714 | .601 | .724 | .784 | .651 | .651 | .490 | .982 | .860 | 1.000 |
Note.— Correlations were determined across individuals and are not dependent on relatedness of cases. Traits are as follows: a = full criteria; b = attack length; c = vascular headache criteria; d = unilaterality; e = pulsation; f = pain intensity; g = aggravation by physical exercise; h = associated symptoms; i = nausea and/or vomiting; j = nausea; k = vomiting; l = photo- and phonophobia; m = photophobia; n = phonophobia.
Results
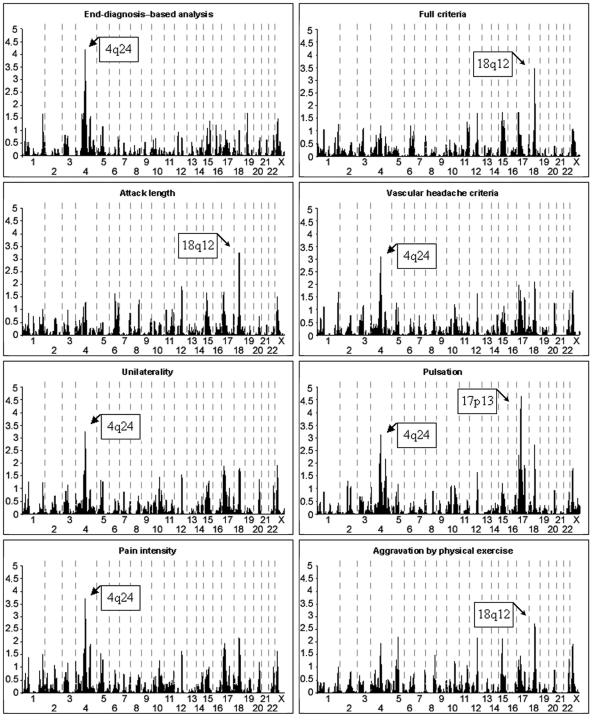
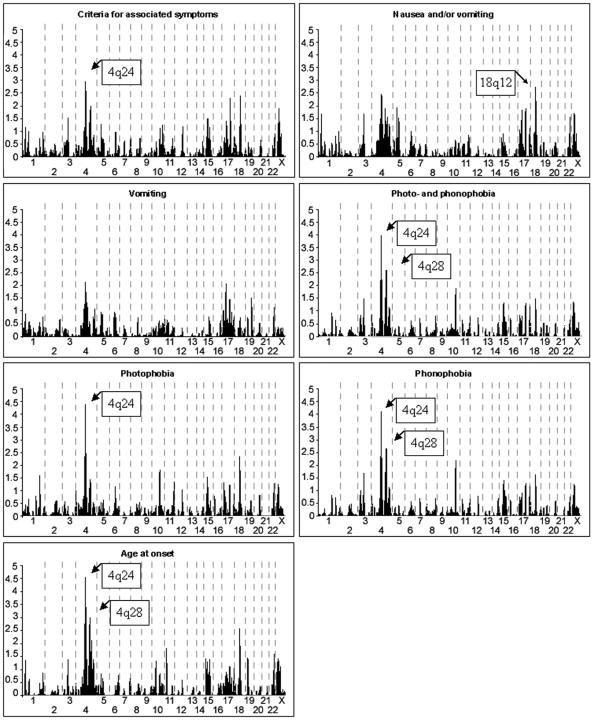
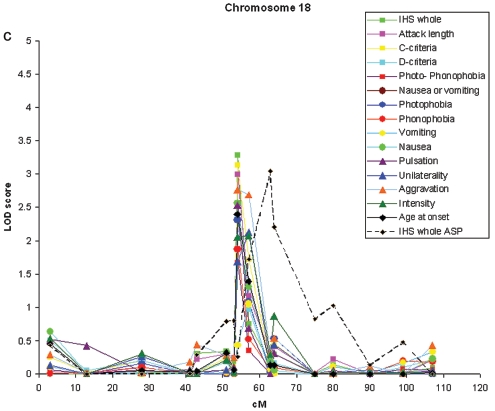
Linkage to 13 migraine-related traits was analyzed in a genomewide scan of 438 individuals from 50 migraine-affected families enriched for MA. Significant evidence of linkage was observed with the individual traits pulsation (17p13), photophobia (4q24), phonophobia (4q24), and age at onset (4q24) (fig. 2 and table 3). With the trait combinations, suggestive evidence of linkage was observed with photo- and phonophobia (4q24) and IHS full criteria (18q12), a combination of the shared traits of diagnoses 1.1 and 1.2.1 of the IHS 2004 classification that includes individuals classified with MA and/or MO as their end diagnosis. Table 3 shows the significant and suggestive two-point LOD scores found.
Figure 2. .
Results of the parametric two-point linkage analysis under heterogeneity for genomewide screen with the end diagnosis MA, the trait components and trait groups, and age at onset used to determine affection status. The lower significance limits are 4.00 for significant evidence and 2.60 for suggestive evidence of linkage. Arrows denote markers with significant or suggestive results.
Table 3. .
Locations and Two-Point LOD Scores for Traits or Trait Groups Providing a LOD Score >2.60 in the Genomewide Screen in at Least One Analysis
| Resultsunder LocusHomogeneity |
Resultsunder LocusHeterogeneity |
||||
| Marker (Location) and Trait or Trait Group |
LOD Score |
P | LOD Score |
P | ASP Analysis LOD Score |
| D4S2380 (4q22): | |||||
| Age at onset | 1.86 | .001713 | 1.96 | .005482 | 2.93 |
| D4S1647 (4q24): | |||||
| Age at onset | 4.53 | .000002 | 4.53 | .000015 | 4.02 |
| Photophobia | 4.39 | .000003 | 4.39 | .000020 | 2.20 |
| Phonophobia | 4.10 | .000007 | 4.10 | .000040 | 2.81 |
| Photo- and phonophobiaa | 3.97 | .000010 | 3.97 | .000054 | 2.76 |
| Intensity | 3.71 | .000018 | 3.71 | .000097 | 2.60 |
| Unilaterality | 3.25 | .000055 | 3.25 | .000281 | 2.88 |
| Pulsation | 3.13 | .000073 | 3.14 | .000362 | 3.49 |
| Vascular headachea | 3.09 | .000081 | 3.09 | .000406 | 2.30 |
| Associated symptomsa | 2.98 | .000106 | 2.98 | .000524 | 2.28 |
| Nausea | 2.46 | .000382 | 2.46 | .001734 | 3.28 |
| Vomiting and/or nauseaa | 2.46 | .000382 | 2.46 | .001734 | 3.28 |
| Attack length | 1.18 | .009874 | 1.24 | .028772 | 2.61 |
| D4S2394 (4q28): | |||||
| Age at onset | 2.99 | .000233 | 2.99 | .001094 | .25 |
| D4S1520 (4q31): | |||||
| Phonophobia | 2.66 | .000257 | 2.66 | .001199 | .82 |
| Photo- and phonophobiaa | 2.62 | .000257 | 2.62 | .001199 | 1.06 |
| D17S945 (17p13): | |||||
| Pulsation | 4.65 | .000002 | 4.65 | .000011 | .82 |
| D18S877 (18q12): | |||||
| IHS full criteriaa | 1.06 | .013573 | 3.29 | .000256 | .26 |
| Vascular headachea | .92 | .019779 | 3.14 | .000362 | .42 |
| Attack length | .95 | .018236 | 3.00 | .000500 | .09 |
| Aggravation by physical exercise | 1.24 | .008432 | 2.77 | .000849 | .03 |
| D18S862 (18q21): | |||||
| Aggravation by physical exercise | 1.75 | .002264 | 2.69 | .001021 | 1.21 |
| D18S1364 (18q22): | |||||
| IHS full criteriaa | .03 | .364661 | .17 | .338041 | 3.04 |
| Attack length | .18 | .181291 | .29 | .256431 | 2.71 |
A trait group.
MA Locus on 4q24
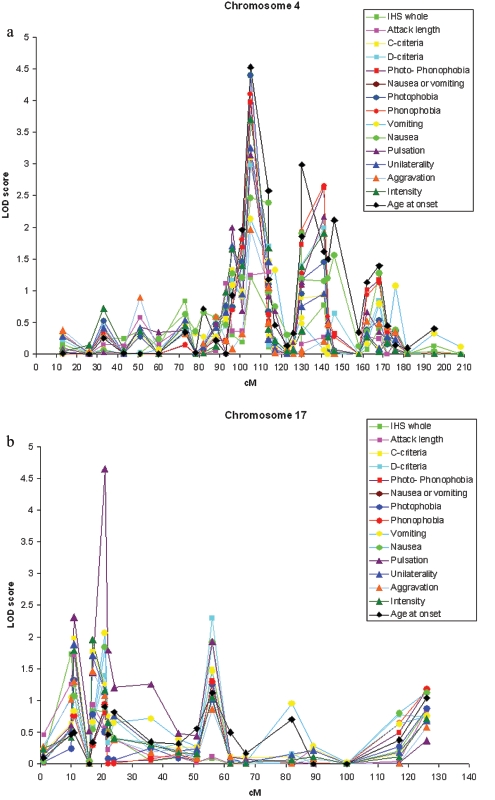
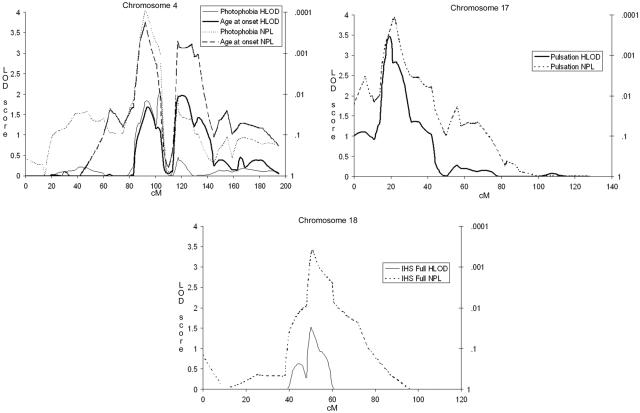
Our previously reported and replicated MA locus on 4q247,15 showed significant evidence of linkage to photophobia (two-point HLOD 4.39) and phonophobia (4.10) and suggestive evidence of linkage to pain intensity (3.71), unilaterality (3.25), and pulsation (3.14) as well as the photo- and phonophobia trait group (3.97) and the associated symptom trait group (2.98) (fig. 3a) with marker D4S1647. With the trait photophobia, GeneHunter multipoint parametric (HLOD 2.05) and nonparametric (NPLall 4.13) analyses positioned the peak at 103 cM and 92 cM, respectively (fig. 4a).
Figure 3. .
Parametric two-point HLOD scores for chromosome 4 (a), chromosome 17 (b), and chromosome 18 (c) for all trait components and age at onset.
Figure 4. .
Multipoint parametric HLOD scores (solid lines) and nonparametric NPLall scores (dotted lines) for chromosome 4 and phonophobia (a), chromosome 17 and pulsation (b), and chromosome 18 and IHS full criteria (c).
New Locus on 17p13.1
Significant evidence of linkage was found between the pulsation trait and marker D17S945, where a two-point HLOD score of 4.65 was detected (fig. 3b). This encouraging result triggered us to fine map the region with nine additional markers, providing a map interval of 1.7 cM. These markers supported the existence of a susceptibility locus in this region. GeneHunter multipoint parametric (HLOD 3.46) and nonparametric (NPLall 3.94) tests positioned the peak at 19.4 and 22.0 cM for the pulsation trait (fig. 4b). Other IHS traits showed, at best, nearly suggestive evidence of linkage to this locus, the highest of which was between the trait vomiting and marker D17S945 (HLOD of 2.07).
Loci Providing Suggestive Evidence of Linkage
The second new locus was detected on 18q12.1 with marker D18S877 (fig. 3c) and the IHS full criteria (two-point HLOD 3.29). With this marker, individual traits showed suggestive evidence of linkage, with vascular criteria (3.14), attack length (3.00), and aggravation by physical exercise (2.77) showing the highest LOD scores. In addition, vomiting and nausea showed nearly suggestive evidence of linkage at this locus (HLOD 2.66). Neither of the adjacent markers of the 10-cM marker set (D18S453, 12 cM proximal, and D18S535, 11.5 cM distal) provided supportive evidence of linkage with any trait. In spite of this, we decided to fine map this locus with five additional markers, providing a map interval of 3.2 cM. Two markers supported a locus in this region, with suggestive (D18S56 and aggravation by physical exercise) and nominal (D18S1107 and attack length) evidence of linkage. GeneHunter multipoint parametric analysis positioned the peak at 50.0 cM, with an HLOD of 1.53 (fig. 4c) with the IHS full criteria. At this position, however, the NPLall score was 3.40 (at 51.0 cM), suggesting that perhaps the parametric dominant model was not a good fit for this trait. By use of the ASP method and the IHS full criteria, marker D18S1102, 8 cM distal of the parametric two-point HLOD peak, provided an ASP LOD score of 3.04. Adjacent markers D18S56 and D18S535 provided ASP LOD scores of 1.72 and 2.20, respectively.
In addition to the locus on 4q24, a second region on 4q, 4q28-q31, indicated suggestive evidence of linkage with multiple traits and age at onset. Among the traits, the highest evidence of linkage was observed between marker D4S1520 and the traits phonophobia (HLOD 2.66) and photo- and phonophobia (HLOD 2.62). Furthermore, several markers around marker D4S1520 showed nearly suggestive evidence of linkage with the same traits.
Loci Providing Nominal Evidence of Linkage
In addition to the chromosomal loci on 4q24, 4q28, 17p13, and 18q12, four other loci with nearly suggestive evidence of linkage were observed on 10q22, 12q21, 15q14, and Xp21. Marker D10S2327 produced a two-point HLOD score of 2.27 with phonophobia. On chromosome 12q21, marker D12S1064 produced an HLOD score of 2.17 with the aggravation by physical exercise trait. However, none of the surrounding markers supported linkage to these loci. On 15q14, aggravation by physical exercise produced a two-point HLOD score of 2.14 with marker ACTC. On Xp21, aggravation by physical exercise produced an HLOD of 1.92, associated symptoms an HLOD of 1.91, and unilaterality an HLOD of 1.90 with marker DXS9896. At these two loci, a number of adjacent markers also provided nominal evidence of linkage to several traits (three for 15q14 and seven for Xp21).
Age at Onset
In addition to the IHS traits, we analyzed the age at onset of migraine as a trait. Age was dichotomized to consider those patients whose migraine symptoms began before age 20 years as affected and the others as unknown. The analysis showed a significant two-point HLOD score of 4.52 at 4q24 (D4S1647). One other marker (D4S2394) with suggestive evidence of linkage was found, on 4q28 (HLOD 2.99), with adjacent markers providing nearly suggestive evidence of linkage, corroborating the second locus on chromosome 4. In analyzing for late onset (at age >20 years), no markers showed evidence of linkage reaching the suggestive level.
Stratification by Sex
In a study of migraine in the Icelandic population,15 linkage results were improved considerably when the sexes were analyzed separately. Thus, we analyzed the data by treating all females and males, in turn, as unknown for all traits for the chromosomes yielding suggestive or significant results. On the 4q24 locus, no significant differences between the sexes were found. At the 17p13 locus, the pulsation trait showed a two-point HLOD score of 2.02 for females (n=174) and 2.72 for males (n=67). At this locus, no other trait exceeded that score in males, but the intensity trait reached a LOD score of 2.34 in females. On the 18q12 locus, the IHS full criteria produced a nearly significant two-point LOD score of 3.79 for females (n=167) but a LOD of only 1.95 for males (n=63). At this locus, several other traits produced suggestive results (in males, 3.41 for the pain criteria, 3.11 for nausea, and 2.92 for intensity; in females, 2.76 for photophobia), but, with all traits, the sex difference was smaller than the one observed with IHS full criteria. In addition, attack length produced a LOD score of 3.35 in females and 3.23 in males. No other significant differences between the sex-specific phenotypes were found for the two loci.
Discussion
Although introduction of the IHS criteria has done much to ease the diagnostics of migraine disorders and has had a major impact on clinical trials and epidemiological research in this field, none of the features occur in all patients who meet a strict definition of IHS migraine, and no single symptom is required for diagnosis. In other words, although there is considerable correlation between individual symptoms (table 2), migraine is a symptom complex with variable symptom profiles, and individuals presenting with dissimilar symptoms can equally satisfy the same diagnosis (appendixes B and C). In fact, controversy exists about whether MO and MA are actually two separate entities.41,42 Furthermore, some individuals may not completely satisfy the IHS criteria but are nonetheless considered affected in a clinical setting. We therefore hypothesized that examination of individual symptoms and their subgroupings (i.e., trait components) would provide considerably greater power than use of the IHS end diagnosis to identify genes underlying migraine susceptibility.
The trait-component analysis identified several traits that also show linkage to the previously reported MA locus on chromosome 4q24. Interestingly, the restratification revealed one novel locus (17p13) with significant evidence of linkage to the pulsation trait and two other loci with suggestive evidence of linkage to the traits age at onset (4q28) and IHS full criteria (18q12).
These findings suggest that dissecting the end diagnosis into intermediate phenotypes and/or traits might help to dissect the genetic basis of headache disorders and thus help to stratify study samples into less heterogeneous groups. Here, we were able to identify new loci to which only a subset of migraine-associated symptoms were linked. This supports the well-accepted hypothesis in complex traits that specific gene variants in different loci contribute in different combinations to the individual susceptibility.43,44 However, this can be verified and further studied only after specific alleles within these loci have been identified. Trait-based approaches have been used in several complex traits, such as asthma,45 schizophrenia,46 autism,26 and hyperlipidemias.27 The migraine end diagnosis is composed of a number of possibly biologically diverse components; thus, it is plausible to hypothesize different biological processes behind these components. Also, migraine has a distinct set of diagnostic criteria that provide a good starting point for this type of analysis.19,20 In addition, some biochemical evidence for such an approach has been found in another common primary headache disorder. In tension-type headache, patients with the pulsating subtype seem to have higher calcitonin gene–related peptide levels than those of patients with nonpulsating headache.47
Prompted by the differences in the results of the linkage analysis between individual traits and the end diagnosis MA,7 we analyzed the differences between the affection groups contributing to the peaks at 17p13 and 18q12. Of the individuals in the set pointing to the locus at 17p13, there were 66 individuals with pulsating headache who were coded as unknown in the original study, either because of their lack of aura symptoms or because the migraine was inherited in a bilineal fashion. Conversely, there were 51 individuals with nonpulsating headache who were coded as affected in the original study. Combined, this means a total difference of 49.7% (117 of 235) in the pulsation group classified as affected in this study compared with our previous genomewide scan. Similarly, there were 62 affected individuals in the set pointing to the 18q12 locus who were coded as unaffected in the previous study because of a non-MA diagnosis or bilineal family structure and, conversely, 61 individuals who did not fulfill the full shared IHS criteria who were coded as affected in the original study. This resulted in a 52% (123 of 225) combined difference between the group showing the 18q12 locus and the end diagnosis group.7 Most of the difference between these groups is the result of the addition of patients with MO fulfilling the IHS full criteria. In contrast, from the total of 246 individuals with MA, 62 had an attack length of <4 or >72 h. This causes them to fall outside of the 1.2.1 (typical aura with migraine headache) diagnostic group, which is new to the IHS 2004 classification. One could speculate that the change in the classification eliminated excess heterogeneity from the MA group and thus revealed the 18q12 locus.
It is likely that analyzing the trait components of headache is feasible only in highly selected samples—for example, in the present study, in which families with a high incidence of IHS migraine were considered. For instance, if a gene for pulsating headache exists, it will most likely be a small component of the total genetic load that predisposes a patient to the migraine syndrome. In the general population, the coexistence of this pulsating factor and other contributing factors is likely to be rare, but, in study samples already enriched for the presence of migraine, both elements may have a chance to co-occur and predispose the patients in the family to a migraine headache that pulsates (“beats with the heart”). Thus, as for other complex traits, the highly selected families with migraine may be better for identifying some migraine susceptibility genes and thus make identification of those genes a realistic goal. This, in the end, could even help to validate the clinical decisions that form the basis of the current migraine criteria.
It is interesting that, even though several traits provided evidence of linkage at the same locus, individual traits within the main IHS categories provided evidence of linkage at different loci. For example, the traits in the associated-symptoms category (nausea and/or vomiting and photo- and phonophobia) provided evidence of linkage at different loci; vomiting and nausea showed nearly suggestive evidence of linkage on chromosome 18q12, and photo- and phonophobia on chromosome 4q24. Similarly, intensity and pulsation, both traits of the vascular headache criteria, provided evidence of linkage on different chromosomes: 4q24 and 17p13, respectively. This could suggest that, even if the grouping of IHS symptoms is logical and practical in clinical practice, it may not reflect the genetic background of the susceptibility of individual traits. This is not very surprising in light of what is known about the heterogeneity of the clinical findings in Mendelian forms of migraine. In FHM-affected families, family members with the same underlying mutation can have different phenotypes, ranging from severe hemiplegic symptoms to MO to having no migraine at all.18 In other complex traits, the idea of using subgroups of clinical classifications (e.g., DSM-IV in schizophrenia) is under active study, even though the approach has yet to provide support for stratifying the sample for susceptibility classification. However, much of the trait relationship between susceptibility loci remains speculative as long as the underlying allelic variants are not identified; because the linkage information is provided by relatively few meioses, there is a possibility that some of these trait differences weaken or disappear once association analyses are performed.
In further analyses, it was found that, at the 18q12 locus, a few large families provided clearly negative LOD scores (three families had LOD scores ranging from −1.65 to −2.17), likely contributing to the large difference between the LOD scores under locus homo- and heterogeneity. By analyzing the data without those families, the difference between the scores decreases considerably, with the homogeneity LOD score rising closer to the level of the HLOD score. We also observed a large difference between the sex-specific LOD scores, with a considerably higher female score. The reason for this difference is not clear, as it might reflect a true sex difference at this locus or merely follow from the smaller sample size of males. The sex-specific analysis is a relevant option in migraine because of the high difference in prevalence between the sexes.1
At present, several loci, 4q21-q24,7,15 5q21,11 6p12.2-p21.1,14 11q24,8 14q21.2-q22.3,13 and 15q11-q13,9 with significant evidence of linkage to common forms of migraine have been reported, with only the chromosome 4q21-q24 region detected in two independent studies. Interestingly, the most significant new locus identified in this study, on 17p13, has shown nominal evidence of linkage both in our previous study7 and in an Australian study.10 It remains to be seen whether this locus harbors a specific variant(s) contributing to the sensation of pulsating pain. Our results also seem to corroborate several other previous findings. The locus on 18q12 has been implicated in an Icelandic study,15 in which the same marker (D18S877) gave a LOD score of 1.50. That study was conducted using 103 families with MO-affected members, which ties well with our approach and results. Also, a marker 13 cM away (D18S53) gave a LOD score of 2.30 in an Australian study.10 The general observation in migraine, for which different studies provide different loci, is typical for complex traits for which finding a causative variation has proven difficult, such as hypertension25 and schizophrenia.48
Furthermore, it is interesting to note that several of our findings agree with those from an empirical clustering approach called “latent class analysis” (LCA), introduced in a recent article by Nyholt et al.49 and applied to migraine symptom data in genome scans in two separate collections of migraine-affected Australian families.10,11 LCA is a statistical method, closely analogous to cluster analysis, for finding subtypes of related individuals (latent classes) from multivariate categorical data.50 Briefly, a latent class cluster model describes the relationship between a set of observed variables and an unobserved, latent variable. The categories of this latent variable are called “latent classes,” or clusters. Therefore, LCA groupings consist of individuals with different numbers and combinations of symptoms and thus reflect a measure of severity, different from the traditional end point diagnosis based on strict IHS diagnostic criteria and also different from the individual-trait analysis used here. The LCA approaches migraine from a different viewpoint than does our trait-component analysis, as the LCA classes are, in effect, new classifications combining features from both end diagnosis and trait components, formed on the basis of heritability estimates. The important point, however, with respect to our approach, is that the LCA analysis49 named a typical trait most correlated with each class in a manner approaching the present analysis, and it is interesting to note that several results from these two analyses agreed with each other.
Interestingly, the Australian LCA study of twins11 implicated a locus on 10q22 (nonparametric multipoint LOD score of 2.13), which was attained with marker D10S2327, which also gave a two-point LOD score of 2.78 in this study. They also reported this marker to be “most associated with phonophobia and photophobia,”11(p.500) and it is intriguing that the same marker and same clinical trait produce suggestive results in two different populations. Our 12q21 peak marker D12S1064 had a nominal LOD score of 0.74 in our previous study,7 which also reported the Xp21 peak marker DXS9896 (LOD 1.08). Marker ACTC on chromosome 15 lies next to a genomic region containing three GABA receptor genes that were linked to migraine in an Italian study of 10 families with MA.9 As can be expected, many loci reported in other studies (table 4) did not show evidence of linkage beyond the nominal level. Similarly, no linkage to the FHM1,18 FHM2,16 and FHM317 loci on chromosomes 19p13, 1q23, and 2q24 was found in this study. Traits with the highest evidence of linkage at these loci were age at onset (LOD score 0.53), photo- and phonophobia (0.93), and photophobia (0.62), for the FHM1, FHM2, and FHM3 loci, respectively. In our earlier 2002 study, none of the three loci showed evidence of linkage.7 The newly identified regions contain several counterparts to the known FHM genes. Other interesting candidates for additional research include genes participating in energy metabolism and neurotransmitter release.
Table 4. .
Results of Previous Genomewide Scans in Migraine with Suggestive or Significant LOD Scores and the Corresponding Results of the Present Study
| Chromosomal Arm | Study Reference | Studied Phenotype | Marker | LOD Score | LOD Score in This Study | Trait in This Study |
| 3q | 10 | LCA-severea | D3S1311 | 2.28b | 1.23 | Intensity |
| 4q | 15 | Loose MOc | D4S1534 | 2.87b | 2.00 | Pulsation |
| 4q | 15 | Females-only loose MOc | D4S2409 | 4.08b | 2.00 | Pulsation |
| 4q | 7 | MA | D4S1647 | 4.20d | 4.53 | Age at onset |
| 5q | 11 | LCA-severea | D5S2501 | 3.03b | .03 | Intensity |
| 6p | 14 | MA and MO | D6S452 | 5.41d | .83 | Vomiting |
| 10q | 11 | LCA-severea | D10S2327 | 2.32b | 2.27 | Phonophobia |
| 11q | 8 | MA | D11S4464 | 4.24d | .53 | Nausea and/or vomiting |
| 14q | 13 | MO | D14S978 | 3.70d | .18 | Attack length |
| 18p | 10 | LCA-severea | D18S53 | 2.30b | .15 | Aggravation by physical exercise |
| 18q | …e | MA and MO | D18S877 | … | 3.43 | IHS full criteria |
LCA refers to latent class analysis, as presented elsewhere.49
Nonparametric multipoint results.
Refers to a reduced end diagnosis; in the study,15 it included patients who did not fulfill either vascular headache criteria or associated symptom criteria of the IHS classification.
Parametric two-point results.
A marker in the present study, with a distance of 13 cM to the D18S53 marker in the study by Lea et al.10
Further studies performed with these traits are needed to confirm whether trait analysis can reclassify patients into more-homogeneous groups. The findings here suggest that trait information collected to establish the IHS-based diagnosis can provide an additional tool for stratifying a migraine study sample. This should be applicable not only to family studies but also to case-control studies. It is hoped that subsequent studies will confirm the validity of this approach.
Acknowledgments
This study was supported by the Sigrid Juselius Foundation, the Academy of Finland (200923 [to A.P.] and 00213 [to M.W.]), the Helsinki University Central Hospital, the EuroHead (LSHM-CT-2004-504837), the GenomEUtwin project (QLG2-CT-2002-01254), the Oxnard Foundation, the Helsinki Biomedical Graduate School (to V.A.), the Biomedicum Helsinki Foundation, the Finnish Cultural Foundation, the Finnish Neurology Foundation, the Nordic Center of Excellence for Disease Genetics, the Center of Excellence for Complex Disease Genetics of the Academy of Finland, and the National Institutes of Health (RO1 NS37675 [to A.P.]). Finally, we thank the Finnish migraine patients for their invaluable participation in this study.
Appendix A: Migraine Subtypes According to the International Classification of Headache Disorders, 2nd Edition, 200420
-
1.1
Migraine without aura
-
1.2 Migraine with aura
-
1.2.1 Typical aura with migraine headache
-
1.2.2 Typical aura with nonmigraine headache
-
1.2.3 Typical aura without headache
-
1.2.4 Familial hemiplegic migraine
-
1.2.5 Sporadic hemiplegic migraine
-
1.2.6 Basilar-type migraine
-
1.2.1
-
1.3 Childhood periodic syndromes that are commonly precursors of migraine
-
1.3.1 Cyclical vomiting
-
1.3.2 Abdominal migraine
-
1.3.3 Benign paroxysmal vertigo of childhood
-
1.3.1
-
1.4
Retinal migraine
-
1.5 Complications of migraine
-
1.5.1 Chronic migraine
-
1.5.2 Status migrainosus
-
1.5.3 Persistent aura without infarction
-
1.5.4 Migrainous infarction
-
1.5.5 Migraine-triggered seizure
-
1.5.1
-
1.6 Probable migraine
-
1.6.1 Probable migraine without aura
-
1.6.2 Probable migraine with aura
-
1.6.3 Probable chronic migraine
-
1.6.1
Appendix B: IHS Criteria for Migraine without Aura, 1988 and 200419,20
1.1 Migraine without aura
-
A.
At least five attacks fulfilling criteria B–D
-
B.
Headache attacks lasting 4–72 h (untreated or unsuccessfully treated)
-
C. Headache has at least two of the following characteristics:
-
1. Unilateral location
-
2. Pulsating quality
-
3. Moderate or severe intensity (inhibits or prohibits daily activities)
-
4. Aggravation by walking stairs or similar routine physical activity
-
1.
-
D. During headache, at least one of the following:
-
1. Nausea and/or vomiting
-
2. Photophobia and phonophobia
-
1.
-
E. At least one of the following:
-
1. History, physical- and neurological examinations do not suggest secondary cause of headache
-
2. History, physical- and neurological examinations do suggest such disorder, but it is ruled out by appropriate investigations
-
3. Such disorder is present, but migraine attacks do not occur for the first time in close temporal relation to the disorder
-
1.
Appendix C: Comparison of the Criteria for IHS Diagnoses 1.2 Migraine with Aura of the 1988 Classification and the New 1.2.1 Typical Aura with Migraine Headache of the 2004 Classification
1.2 Migraine with aura (1988)
-
A.
At least two attacks fulfilling criterion B
-
B. At least three of the following four characteristics:
-
1. One or more fully reversible aura symptoms indicating focal cerebral cortical and/or brain stem dysfunction
-
2. At least one aura symptom develops gradually over >4 min, or ⩾2 symptoms occur in succession
-
3. No aura symptom lasts >60 min. If more than one aura symptom is present, accepted duration is proportionally increased
-
4. Headache follows aura with a free interval of <60 min (it may also begin before or simultaneously with the aura)
-
1.
-
C. At least one of the following:
-
1. History, physical- and neurological examinations do not suggest secondary cause of headache
-
2. History, physical- and neurological examinations do suggest such disorder, but it is ruled out by appropriate investigations
-
3. Such disorder is present, but migraine attacks do not occur for the first time in close temporal relation to the disorder
-
1.
1.2.1 Typical aura with migraine headache (2004)
-
A.
At least two attacks fulfilling criteria B–D
-
B. Aura consisting of at least one of the following, but no motor weakness:
-
1. Fully reversible visual symptoms including positive features (e.g., flickering lights, spots, or lines) and/or negative features (i.e., loss of vision)
-
2. Fully reversible sensory symptoms including positive features (i.e., pins and needles) and/or negative features (i.e., numbness)
-
3. Fully reversible dysphasic speech disturbance
-
1.
-
C. At least two of the following:
-
1. Homonymous visual symptoms (note: additional loss or blurring of central vision may occur) and/or unilateral sensory symptoms
-
2. At least one aura symptom develops gradually over ⩾5 min, and/or different aura symptoms occur in succession over ⩾5 min
-
3. Each symptom lasts ⩾5 and ⩽60 min
-
1.
-
D.
Headache fulfilling criteria B–D for 1.1 Migraine without aura begins during the aura or follows aura within 60 min
-
E.
Not attributed to another disorder (note: history and physical and neurological examinations do not suggest any of the disorders listed in groups 5–12, or history and/or physical and/or neurological examinations do suggest such disorder but it is ruled out by appropriate investigations, or such disorder is present but attacks do not occur for the first time in close temporal relation to the disorder)
Web Resources
The URLs for data presented herein are as follows:
- matSpD, http://genepi.qimr.edu.au/general/daleN/matSpD/
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for migraine, CACNA1A, ATP1A2, SCN1A, FHM1, FHM2, and FHM3)
References
- 1.Russell MB, Rasmussen BK, Thorvaldsen P, Olesen J (1995) Prevalence and sex-ratio of the subtypes of migraine. Int J Epidemiol 24:612–618 [DOI] [PubMed] [Google Scholar]
- 2.Honkasalo ML, Kaprio J, Winter T, Heikkila K, Sillanpaa M, Koskenvuo M (1995) Migraine and concomitant symptoms among 8167 adult twin pairs. Headache 35:70–78 10.1111/j.1526-4610.1995.hed3502070.x [DOI] [PubMed] [Google Scholar]
- 3.Larsson B, Bille B, Pederson NL (1995) Genetic influence in headaches: a Swedish twin study. Headache 35:513–519 10.1111/j.1526-4610.1995.hed3509513.x [DOI] [PubMed] [Google Scholar]
- 4.Gervil M, Ulrich V, Kyvik KO, Olesen J, Russell MB (1999) Migraine without aura: a population-based twin study. Ann Neurol 46:606–611 [DOI] [PubMed] [Google Scholar]
- 5.Ulrich V, Gervil M, Kyvik KO, Olesen J, Russell MB (1999) Evidence of a genetic factor in migraine with aura: a population-based Danish twin study. Ann Neurol 45:242–246 [DOI] [PubMed] [Google Scholar]
- 6.Russell MB, Iselius L, Olesen J (1996) Migraine without aura and migraine with aura are inherited disorders. Cephalalgia 16:305–309 10.1046/j.1468-2982.1996.1605305.x [DOI] [PubMed] [Google Scholar]
- 7.Wessman M, Kallela M, Kaunisto MA, Marttila P, Sobel E, Hartiala J, Oswell G, Leal SM, Papp JC, Hamalainen E, Broas P, Joslyn G, Hovatta I, Hiekkalinna T, Kaprio J, Ott J, Cantor RM, Zwart JA, Ilmavirta M, Havanka H, Farkkila M, Peltonen L, Palotie A (2002) A susceptibility locus for migraine with aura, on chromosome 4q24. Am J Hum Genet 70:652–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cader ZM, Noble-Topham S, Dyment DA, Cherny SS, Brown JD, Rice GP, Ebers GC (2003) Significant linkage to migraine with aura on chromosome 11q24. Hum Mol Genet 12:2511–2517 10.1093/hmg/ddg252 [DOI] [PubMed] [Google Scholar]
- 9.Russo L, Mariotti P, Sangiorgi E, Giordano T, Ricci I, Lupi F, Chiera R, Guzzetta F, Neri G, Gurrieri F (2005) A new susceptibility locus for migraine with aura in the 15q11-q13 genomic region containing three GABA-A receptor genes. Am J Hum Genet 76:327–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lea RA, Nyholt DR, Curtain RP, Ovcaric M, Sciascia R, Bellis C, Macmillan J, Quinlan S, Gibson RA, McCarthy LC, Riley JH, Smithies YJ, Kinrade S, Griffiths LR (2005) A genome-wide scan provides evidence for loci influencing a severe heritable form of common migraine. Neurogenetics 6:67–72 10.1007/s10048-005-0215-6 [DOI] [PubMed] [Google Scholar]
- 11.Nyholt DR, Morley KI, Ferreira MA, Medland SE, Boomsma DI, Heath AC, Merikangas KR, Montgomery GW, Martin NG (2005) Genomewide significant linkage to migrainous headache on chromosome 5q21. Am J Hum Genet 77:500–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nyholt DR, Curtain RP, Griffiths LR (2000) Familial typical migraine: significant linkage and localization of a gene to Xq24-28. Hum Genet 107:18–23 10.1007/s004390050004 [DOI] [PubMed] [Google Scholar]
- 13.Soragna D, Vettori A, Carraro G, Marchioni E, Vazza G, Bellini S, Tupler R, Savoldi F, Mostacciuolo ML (2003) A locus for migraine without aura maps on chromosome 14q21.2-q22.3. Am J Hum Genet 72:161–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlsson A, Forsgren L, Nylander PO, Hellman U, Forsman-Semb K, Holmgren G, Holmberg D, Holmberg M (2002) Identification of a susceptibility locus for migraine with and without aura on 6p12.2-p21.1. Neurology 59:1804–1807 [DOI] [PubMed] [Google Scholar]
- 15.Björnsson A, Gudmundsson G, Gudfinnsson E, Hrafnsdottir M, Benedikz J, Skuladottir S, Kristjansson K, Frigge ML, Kong A, Stefansson K, Gulcher JR (2003) Localization of a gene for migraine without aura to chromosome 4q21. Am J Hum Genet 73:986–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Fusco M, Marconi R, Silvestri L, Atorino L, Rampoldi L, Morgante L, Ballabio A, Aridon P, Casari G (2003) Haploinsufficiency of ATP1A2 encoding the Na+/K+ pump α2 subunit associated with familial hemiplegic migraine type 2. Nat Genet 33:192–196 10.1038/ng1081 [DOI] [PubMed] [Google Scholar]
- 17.Dichgans M, Freilinger T, Eckstein G, Babini E, Lorenz-Depiereux B, Biskup S, Ferrari MD, Herzog J, van den Maagdenberg AM, Pusch M, Strom TM (2005) Mutation in the neuronal voltage-gated sodium channel SCN1A in familial hemiplegic migraine. Lancet 366:371–377 10.1016/S0140-6736(05)66786-4 [DOI] [PubMed] [Google Scholar]
- 18.Ophoff RA, Terwindt GM, Vergouwe MN, van Eijk R, Oefner PJ, Hoffman SM, Lamerdin JE, Mohrenweiser HW, Bulman DE, Ferrari M, Haan J, Lindhout D, van Ommen GJ, Hofker MH, Ferrari MD, Frants RR (1996) Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell 87:543–552 10.1016/S0092-8674(00)81373-2 [DOI] [PubMed] [Google Scholar]
- 19.International Headache Society (1988) Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Headache Classification Committee of the International Headache Society. Cephalalgia 8:1–96 10.1046/j.1468-2982.1988.0801001.x [DOI] [PubMed] [Google Scholar]
- 20.Headache Classification Subcommittee of the International Headache Society (2004) The International Classification of Headache Disorders: 2nd edition. Cephalalgia Suppl 1 24:9–160 10.1111/j.1468-2982.2003.00824.x [DOI] [PubMed] [Google Scholar]
- 21.Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, Achkar JP, Brant SR, Bayless TM, Kirschner BS, Hanauer SB, Nunez G, Cho JH (2001) A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 411:603–606 10.1038/35079114 [DOI] [PubMed] [Google Scholar]
- 22.Edwards AO, Ritter R 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA (2005) Complement factor H polymorphism and age-related macular degeneration. Science 308:421–424 10.1126/science.1110189 [DOI] [PubMed] [Google Scholar]
- 23.Bottini N, Musumeci L, Alonso A, Rahmouni S, Nika K, Rostamkhani M, MacMurray J, Meloni GF, Lucarelli P, Pellecchia M, Eisenbarth GS, Comings D, Mustelin T (2004) A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat Genet 36:337–338 10.1038/ng1323 [DOI] [PubMed] [Google Scholar]
- 24.Bouzigon E, Dizier MH, Krahenbuhl C, Lemainque A, Annesi-Maesano I, Betard C, Bousquet J, Charpin D, Gormand F, Guilloud-Bataille M, Just J, Le Moual N, Maccario J, Matran R, Neukirch F, Oryszczyn MP, Paty E, Pin I, Rosenberg-Bourgin M, Vervloet D, Kauffmann F, Lathrop M, Demenais F (2004) Clustering patterns of LOD scores for asthma-related phenotypes revealed by a genome-wide screen in 295 French EGEA families. Hum Mol Genet 13:3103–3113 10.1093/hmg/ddh340 [DOI] [PubMed] [Google Scholar]
- 25.Hamet P, Merlo E, Seda O, Broeckel U, Tremblay J, Kaldunski M, Gaudet D, Bouchard G, Deslauriers B, Gagnon F, Antoniol G, Pausova Z, Labuda M, Jomphe M, Gossard F, Tremblay G, Kirova R, Tonellato P, Orlov SN, Pintos J, Platko J, Hudson TJ, Rioux JD, Kotchen TA, Cowley AW Jr (2005) Quantitative founder-effect analysis of French Canadian families identifies specific loci contributing to metabolic phenotypes of hypertension. Am J Hum Genet 76:815–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alarcon M, Cantor RM, Liu J, Gilliam TC, Geschwind DH (2002) Evidence for a language quantitative trait locus on chromosome 7q in multiplex autism families. Am J Hum Genet 70:60–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pajukanta P, Nuotio I, Terwilliger JD, Porkka KV, Ylitalo K, Pihlajamaki J, Suomalainen AJ, Syvanen AC, Lehtimaki T, Viikari JS, Laakso M, Taskinen MR, Ehnholm C, Peltonen L (1998) Linkage of familial combined hyperlipidaemia to chromosome 1q21-q23. Nat Genet 18:369–373 10.1038/ng0498-369 [DOI] [PubMed] [Google Scholar]
- 28.Paunio T, Tuulio-Henriksson A, Hiekkalinna T, Perola M, Varilo T, Partonen T, Cannon TD, Lonnqvist J, Peltonen L (2004) Search for cognitive trait components of schizophrenia reveals a locus for verbal learning and memory on 4q and for visual working memory on 2q. Hum Mol Genet 13:1693–1702 10.1093/hmg/ddh184 [DOI] [PubMed] [Google Scholar]
- 29.Kallela M, Wessman M, Färkkilä M (2001) Validation of a migraine specific questionnaire for use in family studies. Eur J Neurol 8:61–66 10.1046/j.1468-1331.2001.00165.x [DOI] [PubMed] [Google Scholar]
- 30.Hovatta I, Kallela M, Farkkila M, Peltonen L (1994) Familial migraine: exclusion of the susceptibility gene from the reported locus of familial hemiplegic migraine on 19p. Genomics 23:707–709 10.1006/geno.1994.1563 [DOI] [PubMed] [Google Scholar]
- 31.Göring HH, Terwilliger JD (2000) Linkage analysis in the presence of errors III: marker loci and their map as nuisance parameters. Am J Hum Genet 66:1298–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sobel E, Lange K (1996) Descent graphs in pedigree analysis: applications to haplotyping, location scores, and marker-sharing statistics. Am J Hum Genet 58:1323–1337 [PMC free article] [PubMed] [Google Scholar]
- 33.Lathrop GM, Lalouel JM (1984) Easy calculations of lod scores and genetic risks on small computers. Am J Hum Genet 36:460–465 [PMC free article] [PubMed] [Google Scholar]
- 34.Ott J (1983) Linkage analysis and family classification under heterogeneity. Ann Hum Genet 47:311–320 [DOI] [PubMed] [Google Scholar]
- 35.Kruglyak L, Daly MJ, Reeve-Daly MP, Lander ES (1996) Parametric and nonparametric linkage analysis: a unified multipoint approach. Am J Hum Genet 58:1347–1363 [PMC free article] [PubMed] [Google Scholar]
- 36.Kidd KK, Ott J (1984) Power and sample-size in linkage studies. Cytogenet Cell Genet 37:510–511 [Google Scholar]
- 37.Nyholt DR (2004) A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet 74:765–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J, Ji L (2005) Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity 95:221–227 10.1038/sj.hdy.6800717 [DOI] [PubMed] [Google Scholar]
- 39.Cheverud JM (2001) A simple correction for multiple comparisons in interval mapping genome scans. Heredity 87:52–58 10.1046/j.1365-2540.2001.00901.x [DOI] [PubMed] [Google Scholar]
- 40.Ott J (1989) Computer-simulation methods in human linkage analysis. PNAS 86:4175–4178 10.1073/pnas.86.11.4175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blau JN (1995) Migraine with aura and migraine without aura are not different entities. Cephalalgia 15:186–190 10.1046/j.1468-2982.1995.015003186.x [DOI] [PubMed] [Google Scholar]
- 42.Russell MB, Ulrich V, Gervil M, Olesen J (2002) Migraine without aura and migraine with aura are distinct disorders: a population-based twin survey. Headache 42:332–336 10.1046/j.1526-4610.2002.02102.x [DOI] [PubMed] [Google Scholar]
- 43.Gottesman II, Gould TD (2003) The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiat 160:636–645 10.1176/appi.ajp.160.4.636 [DOI] [PubMed] [Google Scholar]
- 44.Shoulders CC, Jones EL, Naoumova RP (2004) Genetics of familial combined hyperlipidemia and risk of coronary heart disease. Hum Mol Genet 13:R149–R160 10.1093/hmg/ddh069 [DOI] [PubMed] [Google Scholar]
- 45.Laitinen T, Daly MJ, Rioux JD, Kauppi P, Laprise C, Petays T, Green T, Cargill M, Haahtela T, Lander ES, Laitinen LA, Hudson TJ, Kere J (2001) A susceptibility locus for asthma-related traits on chromosome 7 revealed by genome-wide scan in a founder population. Nat Genet 28:87–91 10.1038/88319 [DOI] [PubMed] [Google Scholar]
- 46.Egan MF, Goldberg TE (2003) Intermediate cognitive phenotypes associated with schizophrenia. Methods Mol Med 77:163–197 [DOI] [PubMed] [Google Scholar]
- 47.Ashina M, Bendtsen L, Jensen R, Schifter S, Jansen-Olesen I, Olesen J (2000) Plasma levels of calcitonin gene-related peptide in chronic tension-type headache. Neurology 55:1335–1340 [DOI] [PubMed] [Google Scholar]
- 48.Owen MJ, Craddock N, O’Donovan MC (2005) Schizophrenia: genes at last? Trends Genet 21:518–525 10.1016/j.tig.2005.06.011 [DOI] [PubMed] [Google Scholar]
- 49.Nyholt DR, Gillespie NG, Heath AC, Merikangas KR, Duffy DL, Martin NG (2004) Latent class and genetic analysis does not support migraine with aura and migraine without aura as separate entities. Genet Epidemiol 26:231–244 10.1002/gepi.10311 [DOI] [PubMed] [Google Scholar]
- 50.Rindskopf D, Rindskopf W (1986) The value of latent class analysis in medical diagnosis. Stat Med 5:21–27 [DOI] [PubMed] [Google Scholar]