Abstract
Over the past decade there has been great interest in the therapeutic potential of brain cooling for epilepsy, stroke, asphyxia and other neurological diseases. However, there is still no consensus regarding the neurophysiological effect(s) of brain cooling. We employed standard physiological techniques and 2-photon microscopy to directly examine the effect of temperature on evoked neurotransmitter release in rat hippocampal slices. We observed a monotonic decline in extracellular synaptic potentials and their initial slope over the temperature range 33–20°C, when the slices were cooled to a new set point in less than 5 s. Imaging the fluorescent synaptic marker FM1-43 with 2-photon microscopy showed that the same cooling protocol dramatically reduced transmitter release between 33 and 20°C. Cooling also reduced the terminal FM1-43 destaining that was induced by direct depolarization with elevated K+, indicating that axonal conduction block cannot account for our observations. The temperature dependence of FM1-43 destaining correlated well with the effect of temperature on field potential slope, compatible with a presynaptic explanation for our electrophysiological observations. Optical measurement of FM1-43 dissociation from cell membranes was not affected by temperature, and rapid cooling of slices loaded with FM1-43 did not increase their fluorescence. Our experiments provide visible evidence that a major neurophysiological effect of cooling in the mammalian brain is a reduction in the efficacy of neurotransmitter release. This presynaptic effect may account for some of the therapeutic benefits of cooling in epilepsy and possibly stroke.
There has been a resurgence in interest in the therapeutic potential of brain cooling for epilepsy, stroke, neonatal asphyxia and other devastating neurological diseases. Recent in vivo observations have shown that focal cooling can rapidly terminate very robust ictal-like discharges in the rodent neocortex (Yang & Rothman, 2001; Yang et al. 2002). Additional observations on epileptic human neocortex exposed during surgery have demonstrated that application of cold physiological saline solution can abruptly abort paroxysmal electrical activity, supporting the hypothesis that rapid cooling may be able to suppress focal human seizures as well (Sartorius & Berger, 1998; Karkar et al. 2002). There is convincing evidence that prolonged moderate hypothermia can protect experimental animals from brain ischaemia, and the use of cooling to improve human stroke outcomes is being actively explored (Colbourne et al. 2003; De Georgia et al. 2004). Newer information also suggests that asphyxiated infants suffer fewer neurological complications if subjected to cranial cooling (Gluckman et al. 2005).
Despite this renewed attention to cooling, there are relatively few studies that have utilized modern neurobiological techniques to unravel the underlying mechanisms of brain cooling. Many of the older neurophysiological studies have focused on possible postsynaptic mechanisms. A large number of in vitro studies found that moderate hypothermia altered postsynaptic voltage-gated channels (Schiff & Somjen, 1985; Thompson et al. 1985; Shen & Schwartzkroin, 1988). Newer work suggests that hypothermia can also affect ion pumps, which could account for some of the effects of lowered brain temperature (Volgushev et al. 2000b; Aihara et al. 2001).
There is limited electrophysiological evidence that cooling acts presynaptically to inhibit end-plate potentials at the frog neuromuscular junction (Katz & Miledi, 1965). Consistent with this, some newer, albeit indirect, studies of mammalian transmitter release have found diminished release probability at central synapses during cooling (Volgushev et al. 2004). However, there is no consensus on the effect of cooling on neurotransmitter release. Furthermore, several recent rigorous, in vitro studies have disagreed on the magnitude and direction of the temperature dependence of release probability (Hardingham & Larkman, 1998; Allen & Stevens, 1994; Pyott & Rosenmund, 2002; Fernandez-Alfonso & Ryan, 2004).
Focal seizures induced by microinjection of 4-aminopyridine, which augments neurotransmitter release, are rapidly terminated by cortical cooling (Heuser et al. 1979; Tapia & Sitges, 1982; Perreault & Avoli, 1991; Yang & Rothman, 2001). This suggested that cooling might work presynaptically by diminishing transmitter release. We therefore decided to characterize the influence of rapid cooling on synaptic transmission in rat hippocampal slices, and then use fluorescent imaging techniques to directly test the hypothesis that cooling inhibits vesicular neurotransmitter release in intact brain slices.
Methods
Hippocampal slice electrophysiology
Male, 4–6 week old Sprague–Dawley rats were anaesthetized with halothane and rapidly decapitated using a protocol approved by the Washington University Animal Studies Committee. The brains were removed, and briefly immersed in ice-cold artificial cerebrospinal fluid (ACSF) containing (mm): 124 NaCl, 5 KCl, 2 CaCl2, 2 MgSO4, 1.25 NaH2PO4, 22 NaHCO3 and 10 glucose, continuously bubbled with a 95%O2/5% CO2 gas mixture. They were then placed on their dorsal surface on ACSF-dampened filter paper. The cerebellum and brain stem were removed with a scalpel, and the portion of the brain anterior to the optic chiasm was removed with one coronal cut. The flat frontal surface was then rotated down, and the ventral surface was placed against an agarose block in a vibratome pan (Pelco, St Louis, MO, USA). The pan was filled with oxygenated ice-cold ACSF, and the vibratome well was filled with ice water. We cut 500 μm transverse slices using the highest setting of blade vibration amplitude and an excursion speed that allowed the blade to pass through the brain in 20–30 s. The hemispheres of each slice were separated, and incubated in a submerged oxygenated holding chamber at 25°C for at least 1 h, before transfer to a submerged recording chamber for electrophysiology or imaging.
The initial electrophysiological recordings were made in a submerged chamber (Warner Instruments, Hamden, CT, USA) perfused with oxygenated ACSF (95% O2/5% CO2) flowing at 2 ml min−1 and maintained at 33°C by heating the inflow. In order to achieve rapid cooling, we inserted a thermoelectric device (4 × 4 mm; TE Technology, Traverse City, MI, USA) into a custom-machined chamber bottom, so that its top surface was flush with the chamber. This allowed the slice to make direct contact with the cold surface of the thermoelectric device. The bottom surface of the thermoelectric device was glued to a large brass heat sink, which quickly dissipated heat when the thermoelectric device was activated. Temperature at the slice–thermoelectric interface was directly monitored with a miniature thermocouple (Omega, Stamford, CT, USA) whose output was fed into a temperature controller (CN1001; Omega) that regulated the DC power supply for the thermoelectric device.
Recording microelectrodes were made from 1.2 o.d./0.68 i.d. (mm) borosilicate glass (WPI, Sarasota, FL, USA) and had resistances of 4–6 MΩ when filled with ACSF. A constant current stimulator (WPI 305) delivered pulses of 1 Hz and 50 μs in CA3 stratum radiatum through a bipolar tungsten microelectrode (David Kopf Instruments, Tujunga, CA, USA). The current was adjusted to produce a half-maximal response. CA1 field potentials were fed into a conventional DC amplifier (Axoclamp 2 A; Axon Instruments, Union City, CA, USA), digitized at 1 kHz, and stored on a personal computer using a commercially available A/D converter and software (Digidata 1200 and pClamp 9; Axon Instruments). The field potentials were analysed off-line to determine peak magnitude and maximum rate of rise.
The 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and (+)–MK-801 hydrogen maleate were purchased from Tocris (St Louis, MO, USA) and RBI (Natick, MA, USA), respectively). The remainder of the specialty chemicals were from Sigma-Aldrich.
2-Photon imaging of FM1-43 destaining
Our imaging technique was very similar to a previously described protocol (Zakharenko et al. 2001). Individual slices were placed into a submerged chamber, which was mounted on an adjustable platform below the lens of our 2-photon microscope (Zeiss LSM 510 NLO). We used glass micropipettes, identical to those used for extracellular recording, to pressure inject the fluorescent vesicular label FM1-43 (25 μm in ACSF; Molecular Probes, Eugene, OR, USA) into a section of CA1 70–150 μm below the slice surface. This indicator only fluoresces when bound to membrane. We maintained slice temperature at 33°C for FM1-43 loading, even though we assessed release at lower temperatures. When we measured release at different temperatures, the initial observation was taken at the same temperature as the subsequent measurements. After the first minute of pressure application, the FM1-43 was loaded into terminals by stimulating stratum radiatum for 2 min at 10 Hz. Pressure was maintained for another 30 s after stimulation to facilitate endocytosis. The slices were then perfused with ACSF containing β-cyclodextrin sulfobutyl ether (ADVASEP-7, 0.1 mm; Cydex, Overland Park, KS, USA) to remove the FM1-43 that failed to load into the presynaptic compartment (Zakharenko et al. 2001). ADVASEP was present during destaining to prevent FM1-43 reuptake into nerve terminals. During destaining, d-(−)-2-amino-5-phosphonovalerate (50 μm; Sigma) was added to the ACSF to prevent excessive stimulation of postsynaptic N-methyl-d-aspartate receptors and the development of long-term potentiation or dendritic injury.
With the slice in the same chamber and the same stimulating electrodes in place, we used the fluorescence elicited by 2-photon (900 nm) laser excitation (Chameleon; Coherent, Santa Clara, CA, USA) to monitor the effect of cooling on FM1-43 destaining. A water immersion lens (×40 IR Achroplan, 0.8 NA; Zeiss, Thornwood, NY, USA) with ×3 digital zoom was used for the imaging, and four slices were tested at each temperature. The slices were stimulated at 1 Hz and z-stacks (512 × 512 pixels, 0.11 μm per pixel in the horizontal plane, five planes per stack, each separated by 1 μm) were obtained every minute and analysed off-line to quantify loss of fluorescence over 15 min. The laser power was 6 mW and the pixel dwell time was 6.4 μs. We subtracted a background correction, obtained after applying an additional 1200 stimulation pulses (10 Hz) at the end of the experiment, from all of the prior intensities in each layer of each z-stack, to adjust for nonreleasable FM1-43.
We quantified FM1-43 destaining by two different methods. First, we visually identified individual fluorescent puncta or clusters of several puncta that could be individually tracked in a single z-section over time. The fluorescence intensity of each of these regions, which correspond to synaptic boutons, were individually measured, normalized and plotted over time, after background subtraction (Zakharenko et al. 2001). Second, we measured the average background-corrected fluorescence intensity in five separate, 100-square-pixel (11 μm) regions of three z-sections at the first time point. Each z-series obtained at subsequent times was aligned with the first z-series by shifting each image in three dimensions. We normalized all fluorescence values to the initial fluorescence intensity in the same stack section and region. The values from each section in the entire stack were averaged at every time point to obtain the normalized intensity in each slice. All of the image analysis was done with commercial software (MetaMorph; Universal Imaging, Downington, PA, USA).
In one set of experiments we examined the temperature dependency of FM1-43 destaining induced by elevated extracellular K+. After loading terminals using the stimulation protocol described above, we perfused slices with ACSF, modified to contain (mm): 70 sodium gluconate and 60 KCl, instead of the standard concentrations of NaCl and KCl. The gluconate was used to prevent the swelling that would be anticipated in the presence of high extracellular K+ and Cl− (Rothman, 1985). FM1-43 destaining was quantified in these experiments, as above, except that we measured the corrected fluorescence intensity in one, 100-square-pixel region of the three best z-sections, instead of five regions.
Temperature dependence of FM 1–43 affinity
We examined the possibility that FM1-43 affinity, and therefore vesicular destaining, was directly influenced by temperature, by imaging its dissociation from the surface of mixed cultures of cortical neurones and glia at 22 and 31°C (Hasbani et al. 2001). These experiments exploit the lack of fluorescence of unbound FM1-43. Other investigators have used a similar technique to compare unbinding of different styrylpyridinium dyes (Ryan et al. 1996). FM1-43 (15 μm) was pressure ejected for 30 ms from a micropipette (tip diameter approximately 1 μm) positioned a few micrometres above the culture surface, while the chamber (volume <0.2 ml) was perfused with Hanks' balanced salt solution (2 ml min−1). For several experiments, the inflow tubing (0.5 mm diameter) was positioned 2 mm opposite the FM1-43 micropipette. This allowed the perfusate (linear flow 40 mm min−1) to rapidly exchange medium above the area of the culture exposed to dye, minimizing rebinding of dye. The perfusate was heated by an inline heater placed immediately next to the chamber. We used an inverted, scanning confocal microscope (LSM 5 Pascal; Zeiss) and ×20 Plan-Neofluar lens (Zeiss) to monitor the destaining of FM 1–43. Image acquisition (488 nm excitation; 512 × 512 pixels at 1.6 μs pixel−1) began several seconds prior to the FM1-43 application and continued every 500 ms for 40–60 s. We calculated the FM1-43 destaining rate by plotting the fluorescence decay from the point of peak intensity within a region of interest.
Statistics
All errors and error bars in text, legends and figures represent standard deviation. Statistical significance was verified by t test or ANOVA followed by tests for multiple comparisons.
Results
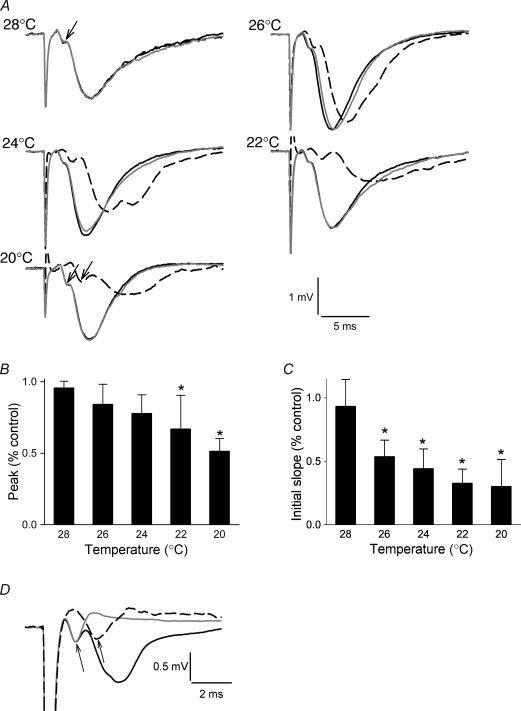
When we stepped the slices from a control temperature of 33°C, to preset temperatures between 28 and 20°C for 1 min, we observed a monotonic decline in the peak and initial rate of rise of the field potential (Fig. 1). The latency to field potential onset also lengthened. The effect of cooling was completely reversible within a few minutes (Fig. 1A). These observations are all consistent with earlier results suggesting that cooling reduces synaptic potentials.
Figure 1. Effect of cooling on evoked responses.
A, the black control traces in each of the frames represent the average of 30 evoked responses sampled every minute (0.017 Hz) at 33°C before cooling to the specified temperature. The dashed traces show the effect of cooling to the specified temperature for 1 min and are single evoked responses. The reduced magnitude and rate of rise of the field potentials are evident. There was complete or almost complete recovery after stepping back to 33°C (grey trace in all figures). The recovery trace is an average of 10 evoked responses, also sampled at 0.017 Hz. Arrows indicate presynaptic fibre volleys, which were delayed, but not reduced, by cooling. B, cooling led to a monotonic decrease in the peak of the evoked response. C, the initial field potential slope was also reduced by cooling. Each bar represents the average of single responses in five separate slices, normalized to the control response at 33°C (*P < 0.05 compared to 33°C, ANOVA followed by Dunnett's Method). D, the presynaptic fibre volley amplitude is not affected by cooling. Control CA1 field potential (black) superimposed upon field potentials obtained in the presence of CNQX, MK-801 and picrotoxin at 33°C (grey) and 20°C (dashed). Cooling prolonged the latency, but did not reduce magnitude of the volley (arrows). Each trace represents a single evoked response.
We were concerned that cooling might block axonal conduction in stratum radiatum, and secondarily reduce synaptic potentials by preventing terminal invasion by action potentials. In order to investigate this possibility, we recorded in the presence of CNQX (30 μm), (+)–MK-801 hydrogen maleate (10 μm) and picrotoxin (PTXN; 100 μm) to eliminate synaptic responses produced by activation of AMPA, NMDA and GABAA receptors, which obscure the presynaptic fibre bundle volley. We found that cooling from 33 to 20°C had no effect on the magnitude of the volley (0.59 ± 0.22 mV at 33°C vs. 0.58 ± 0.18 mV at 20°C; P = 0.9, n = 4 slices), although it did prolong its latency (1.6 ± 0.3 ms vs. 2.6 ± 0.5 ms; P = 0.01 by t test) and duration (Fig. 1D).
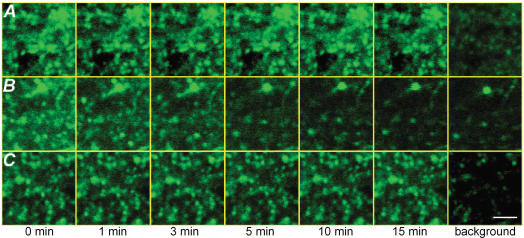
While much of the previous research on the neurophysiology of cooling has emphasized postsynaptic effects, the magnitude and rapidity of the effects we observed suggested that cooling could be reducing transmitter release. We used the fluorescent vesicular label FM1-43 to directly test this hypothesis in our hippocampal slices (Cochilla et al. 1999). Slices (n = 4 for all temperatures) were placed in the perfusion chamber on top of the thermoelectric device. The Schaffer collaterals were stimulated for 2 min at 10 Hz to facilitate presynaptic loading of FM1-43 that had been pressure-injected into CA1 stratum radiatum; excess dye was bound to ADVASEP and washed away (Zakharenko et al. 2001). The slices were imaged using a multiphoton laser scanning microscope. We could easily resolve multiple fluorescent puncta in the distribution of Schaffer collateral terminals in CA1. Almost all of these destained after 1200 (10 Hz) stimuli, indicating that they labelled a functional presynaptic vesicular compartment (Fig. 2).
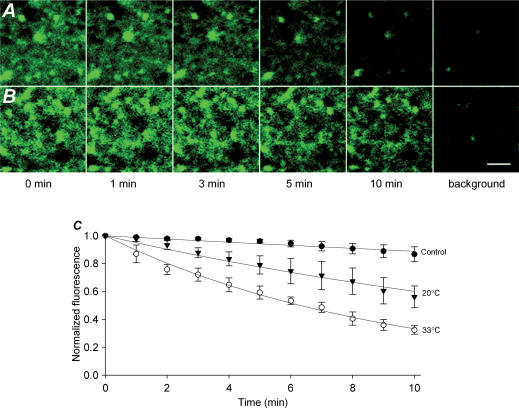
Figure 2. Cooling reduces transmitter exocytosis.
A, single 512 × 512 pixel image plane showing FM1-43 fluorescence imaged over a 15 min period at 33°C in the absence of electrical stimulation. The final image in each series is the background (maximal destaining) obtained after an additional 10 Hz stimulation for 2 min. B, another image plane viewed over 15 min of 1 Hz electrical stimulation at 33°C. At 15 min almost all FM1-43 fluorescence has been eliminated and the image is similar to background. C, stimulation at 1 Hz causes very little FM1-43 release at 20°C. Most of the dye finally destained after 10 Hz stimulation for 2 min, indicating that it was in a functional compartment. Scale, 5 μm.
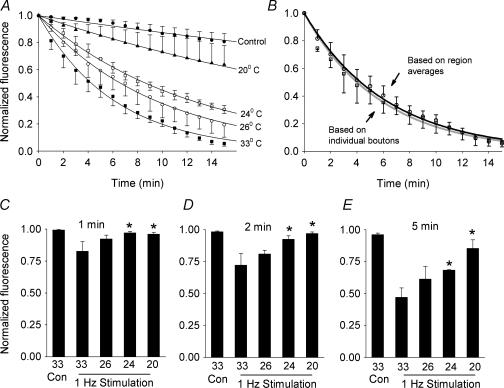
We first measured the fluorescence decay averaged over 100-square-pixel regions. The fluorescence intensity decayed very slowly (k = 0.01 min−1) when the slices were maintained at 33°C, but not stimulated (Figs 2A and 3A). When stimulated at 1 Hz at 33°C, there was a rapid exponential decay in fluorescence over 15 min (k = 0.16 min−1) (Figs 2B and 3A). An additional 1200 stimuli did not appreciably increase destaining. However, when slices were stimulated at 20°C, the fluorescence decay was significantly reduced (k = 0.03 min−1), indicating that presynaptic release was reduced (Figs 2C and 3A). Intermediate temperatures (26 and 24°C) produced a more moderate effect on transmitter release (k = 0.10 and 0.08 min−1, respectively) (Fig. 3A).
Figure 3. Cooling has a graded effect on transmitter release.
A, normalized FM1-43 destaining at temperatures from 33 to 20°C fitted to single exponentials. The control trace shows intensity from unstimulated slices at 33°C. The data demonstrate clear temperature dependence of release. Each curve represents the average normalized intensities from four slices. B, comparison of two methods of quantifying destaining at 33°C. The black line is an exponential fit to the average intensity measurements of the same 100 pixel regions of interest in four slices over 15 min (open squares). The grey line shows the average intensity over time of 50 individual boutons or clusters in the same four slices (open circles). C–E, with 1 Hz stimulation, there is significantly less FM1-43 destaining at 1 (C), 2 (D) and 5 (E) min at 20 and 24 C than at 33 C with stimulation; *P < 0.001 by Student–Newman–Keuls. In addition, there is no significant difference between destaining at 1 and 2 min at 20 and 24 C and unstimulated destaining at 33°C (P > 0.05).
In four slices in which destaining had already been quantified in 100-square-pixel regions at 33°C, the fluorescence of 50 individual puncta or distinct clusters was sequentially tracked (Fig. 3B). We found that serially measuring the fluorescence intensity of regions yielded destaining rates identical to those observed by tracking individual boutons (0.16 ± 0.03 min−1vs. 0.18 ± 0.04 min−1; P = 0.4, NS). We therefore used the former, more straightforward, method in our destaining calculations.
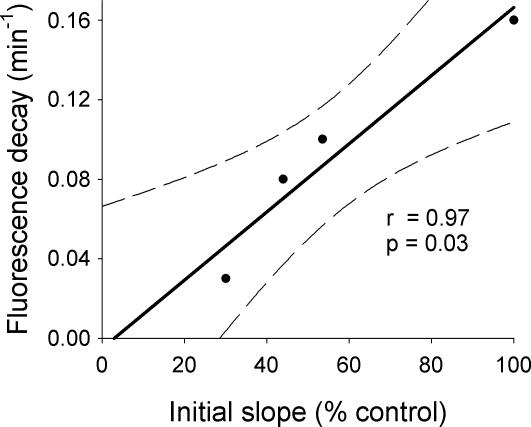
When the 1 and 2 min time points were individually assessed, there was no significant difference in the normalized fluorescence between the unstimulated slices at 33°C and the slices stimulated at 20 and 24°C (Fig. 3C–E). However, at 1, 2 and 5 min, there was a significant reduction in release at 20 and 24°C, compared with stimulated release at 33°C (Fig. 3C–E). These are the temperatures associated with acute seizure control in our prior experiments (Yang & Rothman, 2001; Yang et al. 2002). The temperature dependence of the FM1-43 destaining also correlated closely with the temperature dependence of the field potential initial slope observed in the electrophysiology experiments described above (Fig. 4). Moreover, for the temperature range 33–24°C, the Q10 of the destaining time constant, 2.5, was close to the Q10 of the field potential slope, 2.2. While these correlations do not establish that diminished transmitter release explains the EPSP reduction, they are consistent with this hypothesis.
Figure 4. FM1-43 destaining correlates with the reduction of initial slope.
When the fraction of the field potential slope, preserved after cooling (from Fig. 1C), is plotted against the FM1-43 destaining rate at different temperatures (from Fig. 3A), there is excellent correlation. We used a value of 100 for the slope at 33°C, because all values were normalized to this temperature. The regression line was extended through the axes and is bounded by 95% confidence limits.
Even though we found that the fibre volley was preserved in the cooled slices, we were concerned that the orthodromic action potential might not propagate normally to the CA3 axon terminals. We therefore bypassed the action potential trigger for transmitter release by directly depolarizing the nerve terminals with elevated extracellular potassium (60 mm). We still observed a robust temperature dependence of the FM1-43 destaining, indicating that transmitter release was directly diminished by cooling (Fig. 5). At all time points between 1 and 10 min, FM1-43 destaining was significantly reduced at 20°C compared with 33°C. The time course is not identical to electrical stimulation, due to the latency inherent in perfusing the slice with elevated K+.
Figure 5. Potassium-induced neurotransmitter release is also temperature dependent.
A, when exposed to 60 mm extracellular K+ at 33°C, hippocampal terminals loaded with FM1-43 destain almost completely. There is a longer latency to onset of destaining, compared with electrical stimulation, attributable to the rate of perfusion through the slice. The final image shows the background fluorescence taken after an additional 1200 pulses to maximally destain. B, under identical conditions, except for temperature reduction to 20°C, FM1-43 destaining is much slower. Scale, 5 μm. C, graph of normalized FM1-43 fluorescence over time. Destaining with high K+ at 20°C is significantly reduced compared with 33°C (P < 0.05 at all time points). The top line is replotted from Fig. 4 and shows rate of destaining at 33°C without stimulation.
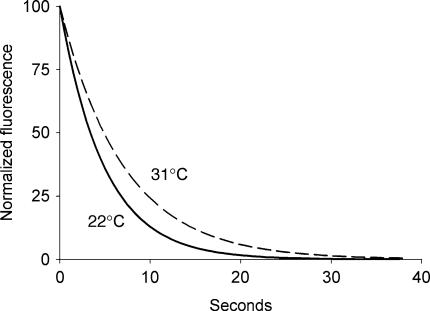
We performed two additional sets of control experiments to examine the possibility that the physical dissociation of FM1-43 from biological membranes is itself temperature dependent, because this could account for the slower destaining at lower temperatures. First, FM1-43 was briefly (30 ms) applied to the external surface of mixed neurone–glia cultures, and the fluorescence that appeared when dye bound to the outer plasma membranes was rapidly imaged with a scanning confocal microscope. After peaking within a few seconds, the fluorescence intensity diminished rapidly as the dye unbound and rapidly washed away in the perfusion system. After normalizing the data, we fit the four most rapid decays seen at 22 and 31°C to single exponentials and found no significant effect of temperature (k = 0.24 ± 0.11 s−1 at 22°C vs. 0.14 ± 0.03 s−1 at 31°C; P > 0.1 by t test) (Fig. 6). These are close to two previously published values for FM1-43 that were obtained in a similar manner (Ryan et al. 1996; Klingauf et al. 1998). Second, we loaded slices with FM1-43 in our conventional manner at 33°C and used the 2-photon microscope to follow fluorescence without stimulation. This eliminated possible dye application artifact. When the fluorescence intensity was remeasured within a minute after the slice temperature had been abruptly lowered to 20°C, the fluorescence intensity (four separate image planes per slice) increased by only 2.7 ± 0.5% (n = 3 slices). This indicates that cooling has only a tiny effect on the off- rate of FM1-43 binding to membranes.
Figure 6. The rate of FM1-43 destaining from isolated membrane is not temperature sensitive.
A single exponential fit to the four most rapid fluorescence decays when FM1-43 was briefly applied to the surface of cultured neurones and glia at 22°C (continuous line) and 31°C (dashed line). The fluorescence decay was not significantly different at the two temperatures.
Discussion
While neurophysiologists have been investigating the effects of brain cooling for over a century, the experiments described above may be the most direct demonstration of the temperature dependence of evoked neurotransmitter release in an intact mammalian preparation, because they optically isolate pre- and postsynaptic components (Brooks, 1983). Moreover, these experiments were performed over a temperature range relevant to epilepsy and provide a basic correlate to recent observations on experimental epilepsy (Yang & Rothman, 2001; Yang et al. 2002).
We confirmed that cooling diminishes field potentials, as has been reported by several other investigators over the past quarter century (Schiff & Somjen, 1985; Volgushev et al. 2000a, b; Aihara et al. 2001). A variety of mechanisms have been proposed for this finding. The most often suggested is inhibition of ion pumps, leading to postsynaptic depolarization and a reduction of the driving force for excitatory postsynaptic potentials (EPSPs) (Schiff & Somjen, 1985). There is also credible evidence supporting a direct effect of cooling on voltage-gated channels (Thompson et al. 1985; Shen & Schwartzkroin, 1988). While there could be a temperature dependence of the postsynaptic glutamate currents, experimental data indicate that receptor occupancy actually decreases at higher temperature (Tong & Jahr, 1994).
Using our thermoelectric cooling apparatus, we did not observe the increase in evoked potentials in the mid-20°C range, which has been described by other groups (Volgushev et al. 2000a; Aihara et al. 2001). The thermoelectric cooling is much more rapid than the methods employed by these other investigators, so it is unlikely to induce the postsynaptic changes that increase the evoked response. In a separate set of experiments, we have verified that slower cooling, using a perfusion system, does augment evoked responses at 28°C. For the present study, we decided to focus on the rapid cooling protocol, which provides some mechanistic information relevant to our prior epilepsy observations (Yang & Rothman, 2001).
Electrophysiological experiments initiated by Katz and colleagues over 50 years ago suggested that presynaptic mechanisms could account for the inhibitory effect of cooling at the frog neuromuscular junction (Fatt & Katz, 1952; Katz & Miledi, 1965). Our FM1-43 experiments provide more graphic evidence utilizing an entirely different technique, which supports a presynaptic mechanism for cooling to reduce synaptic transmission in the mammalian central nervous system. There was a clear, temperature-dependent effect on FM1-43 destaining over the range 33–20°C, which correlated well with the reduction in initial field potential slope and amplitude. This effect was readily apparent below 24°C, in good agreement with our observation that this seems to be the temperature threshold for seizure termination (Yang & Rothman, 2001; Yang et al. 2002).
There are other previously mentioned effects of low temperature on synaptic transmission, so this is unlikely to be a unitary explanation for hypothermia terminating seizures (Schiff & Somjen, 1985; Thompson et al. 1985). However, the excellent correlation between the temperature dependence of the reduction in evoked response slope, a measure of peak synaptic current, and FM1-43 destaining rate is consistent with a dominant presynaptic effect of cooling. Furthermore, the Q10 for these two processes, 2.5 and 2.2, are extremely close to each other, and to the Q10 derived from intracellular recording of EPSP slope in hippocampal CA3 pyramidal neurones, 2.4 (calculated from Fig. 5E in Aihara et al. 2001).
Our results indicate that cooling must be reducing the amount of transmitter release. We have shown that cooling does not block action potential propagation into distal axons, and that even in the absence of action potentials, cooling reduces the destaining resulting from direct terminal depolarization. We have not identified the precise location in the transmitter release pathway that is affected by cooling, and note that there are multiple steps that could be individually or cumulatively vulnerable to temperature reductions. First, it is conceivable that cooling influences some step prior to vesicle release. For example, FM1-43 destaining would diminish if cooling inhibited action potential propagation into axon terminals. The preservation of presynaptic fibre volley amplitude in our cooled field potential recordings largely refutes this possibility (Fig. 1). In fact, the prolongation of the fibre volley might be expected to increase exocytosis. Second, cooling could diminish transmitter release by reducing Ca2+ entry into presynaptic terminals. Third, the sequence of protein binding steps that regulate vesicle docking, fusion, and pore formation may be highly temperature dependent and slowed by cooling (Koh & Bellen, 2003). Acting at one or more of these steps, cooling could change the ratio of fully emptying and endocytosed vesicles to those that are immediately reused (e.g. ‘kiss and run’), or it could diminish the fraction of vesicles available within the readily releasable pool (Stevens & Williams, 2000; Aravanis et al. 2003; Rizzoli & Betz, 2004; Sudhof, 2004). The resolution of the FM1-43 methodology used in the present study cannot distinguish among these alternative mechanisms, all of which are compatible with our central hypothesis.
It is unlikely that our results are influenced by terminal depolarization produced by block of presynaptic ion pumps. While terminal depolarization could result from cooling and lead to diminished transmitter release, the time course of the effects observed in the current experiments and in our prior epilepsy experiments appear too rapid to be explained by pump inhibition (Yang et al. 2002).
We are aware that some investigators have suggested that cooling actually increases the amount of vesicular release, but diminishes peak synaptic responses because the release is desynchronized (Pyott & Rosenmund, 2002). Our findings do not support this hypothesis. We did observe a prolongation of the presynaptic fibre volley, which could correlate with reduced release synchrony during cooling. However, the diminished destaining we see with cooling is inconsistent with increased transmitter release. It is possible, as carefully discussed by the authors of the study, that the effects of cooling on glutamate desensitization and pharmacological variables complicate their quantitative analysis of transmitter release.
Two additional confounding issues in our own study relate to the use of FM1-43. First, there is concern that FM1-43 may not be a reliable marker of the presynaptic neurotransmitter compartment. This possibility appears remote: the imaging characteristics of the dye in slices were consistent with labelling synaptic terminals and repetitive physiological stimulation largely eliminated the dye. We corrected for the small amount of nonspecific staining by subtracting a background prior to any of our calculations. While the kinetics of FM1-43 destaining will not exactly mirror the time course of neurotransmitter release, the dye does provide a measure of presynaptic activity. Second, it is possible that the actual rate of FM1-43 dissociation from the internal vesicular membrane is temperature dependent. We specifically investigated this possibility in two separate experiments. When we applied FM1-43 to the outside surface of cultured cells, there was no evidence of temperature dependence of FM1-43 dissociation over the temperature range of our experiments. While the surface of neurone–glia cultures is not identical to the vesicular membrane, other investigators who have utilized a similar preparation believe that it provides a reasonable estimate of off-rates of styrylpyridinium dyes (Ryan et al. 1996; Klingauf et al. 1998). In addition, we observed only a small increase in vesicular fluorescence when temperature was abruptly lowered after FM1-43 loading into unstimulated slices. We would have expected to find a larger increase in FM1-43 fluorescence if cooling reduced the off-rate of FM1-43 binding to vesicular membrane, accounting for diminished destaining at lower temperature.
Our observations at least partially explain the dramatic ability of cooling to terminate seizures. It is not clear that these results can account for any of the neuroprotective benefits of brain cooling in stroke and asphyxia. In those insults, modest 2–3°C reductions in systemic temperature are associated with significantly improved outcomes (Colbourne et al. 1997, 2000, 2003; Gluckman et al. 2005). While small temperature decrements in this range lead to insignificant reductions (or even increases) in central synaptic potentials, they may still be capable of reducing transmitter release that can aggravate neuronal injury in a variety of experimental stroke and trauma protocols (Bruno et al. 1994). There are likely to be more elusive effects of hypothermia that may contribute to its antiepileptic and neuroprotective properties. Identifying these is a worthwhile goal, because they may aid in the design of rational therapy for serious neurological illnesses.
Acknowledgments
We are grateful to Kelvin Yamada, MD, for help with slice physiology methods, to Mark Goldberg, MD, for use of his cultures, and to Drs John Heuser and Robert Wilkinson for experimental suggestions that greatly improved the paper. The experiments described in this paper were supported by the Alafi Family Foundation, the NINDS (R01 NS42936 and R21 NS045652 to S.M.R., and P01 NS NS32636 to Mark Goldberg, MD), and Citizens United for Research in Epilepsy (S.M.R.).
References
- Aihara H, Okada Y, Tamaki N. The effects of cooling and rewarming on the neuronal activity of pyramidal neurons in guinea pig hippocampal slices. Brain Res. 2001;893:36–45. doi: 10.1016/s0006-8993(00)03285-6. [DOI] [PubMed] [Google Scholar]
- Allen C, Stevens CF. An evaluation of causes for unreliability of synaptic transmission. Proc Natl Acad Sci U S A. 1994;91:10380–10383. doi: 10.1073/pnas.91.22.10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravanis AM, Pyle JL, Tsien RW. Single synaptic vesicles fusing transiently and successively without loss of identity. Nature. 2003;423:643–647. doi: 10.1038/nature01686. [DOI] [PubMed] [Google Scholar]
- Brooks VB. Study of brain function by local, reversible cooling. Rev Physiol Biochem Pharmacol. 1983;95:1–109. [Google Scholar]
- Bruno VM, Goldberg MP, Dugan LL, Giffard RG, Choi DW. Neuroprotective effect of hypothermia in cortical cultures exposed to oxygen–glucose deprivation or excitatory amino acids. J Neurochem. 1994;63:1398–1406. doi: 10.1046/j.1471-4159.1994.63041398.x. [DOI] [PubMed] [Google Scholar]
- Cochilla AJ, Angleson JK, Betz WJ. Monitoring secretory membrane with FM1-43 fluorescence. Annu Rev Neurosci. 1999;22:1–10. doi: 10.1146/annurev.neuro.22.1.1. [DOI] [PubMed] [Google Scholar]
- Colbourne F, Grooms SY, Zukin RS, Buchan AM, Bennett MV. Hypothermia rescues hippocampal CA1 neurons and attenuates down-regulation of the AMPA receptor GluR2 subunit after forebrain ischemia. Proc Natl Acad Sci U S A. 2003;100:2906–2910. doi: 10.1073/pnas.2628027100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbourne F, Sutherland G, Corbett D. Postischemic hypothermia. A critical appraisal with implications for clinical treatment. Mol Neurobiol. 1997;14:171–201. doi: 10.1007/BF02740655. [DOI] [PubMed] [Google Scholar]
- Corbett D, Hamilton M, Colbourne F. Persistent neuroprotection with prolonged postischemic hypothermia in adult rats subjected to transient middle cerebral artery occlusion. Exp Neurol. 2000;163:200–206. doi: 10.1006/exnr.2000.7369. [DOI] [PubMed] [Google Scholar]
- De Georgia MA, Krieger DW, Abou-Chebl A, Devlin TG, Jauss M, Davis SM, Koroshetz WJ, Rordorf G, Warach S. Cooling for Acute Ischemic Brain Damage (COOL AID): a feasibility trial of endovascular cooling. Neurology. 2004;63:312–317. doi: 10.1212/01.wnl.0000129840.66938.75. [DOI] [PubMed] [Google Scholar]
- Fatt P, Katz B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952;117:109–128. [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Alfonso T, Ryan TA. The kinetics of synaptic vesicle pool depletion at CNS synaptic terminals. Neuron. 2004;41:943–953. doi: 10.1016/s0896-6273(04)00113-8. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, Polin RA, Robertson CM, Thoresen M, Whitelaw A, Gunn AJ. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- Hardingham NR, Larkman AU. Rapid report: the reliability of excitatory synaptic transmission in slices of rat visual cortex in vitro is temperature dependent. J Physiol. 1998;507:249–256. doi: 10.1111/j.1469-7793.1998.249bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbani MJ, Schlief ML, Fisher DA, Goldberg MP. Dendritic spines lost during glutamate receptor activation reemerge at original sites of synaptic contact. J Neurosci. 2001;21:2393–2403. doi: 10.1523/JNEUROSCI.21-07-02393.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser JE, Reese TS, Dennis MJ, Jan Y, Jan L, Evans L. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J Cell Biol. 1979;81:275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkar KM, Garcia PA, Bateman LM, Smyth MD, Barbaro NM, Berger M. Focal cooling suppresses spontaneous epileptiform activity without changing the cortical motor threshold. Epilepsia. 2002;43:932–935. doi: 10.1046/j.1528-1157.2002.03902.x. [DOI] [PubMed] [Google Scholar]
- Katz B, Miledi R. The effect of temperature on the synaptic delay at the neuromuscular junction. J Physiol. 1965;181:656–670. doi: 10.1113/jphysiol.1965.sp007790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingauf J, Kavalali ET, Tsien RW. Kinetics and regulation of fast endocytosis at hippocampal synapses. Nature. 1998;394:581–585. doi: 10.1038/29079. [DOI] [PubMed] [Google Scholar]
- Koh TW, Bellen HJ. Synaptotagmin I, a Ca2+ sensor for neurotransmitter release. Trends Neurosci. 2003;26:413–422. doi: 10.1016/S0166-2236(03)00195-4. [DOI] [PubMed] [Google Scholar]
- Perreault P, Avoli M. Physiology and pharmacology of epileptiform activity induced by 4-aminopyridine in rat hippocampal slices. J Neurophysiol. 1991;65:771–785. doi: 10.1152/jn.1991.65.4.771. [DOI] [PubMed] [Google Scholar]
- Pyott SJ, Rosenmund C. The effects of temperature on vesicular supply and release in autaptic cultures of rat and mouse hippocampal neurons. J Physiol. 2002;539:523–535. doi: 10.1113/jphysiol.2001.013277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzoli SO, Betz WJ. The structural organization of the readily releasable pool of synaptic vesicles. Science. 2004;303:2037–2039. doi: 10.1126/science.1094682. [DOI] [PubMed] [Google Scholar]
- Rothman SM. The neurotoxicity of excitatory amino acids is produced by passive chloride influx. J Neurosci. 1985;5:1483–1489. doi: 10.1523/JNEUROSCI.05-06-01483.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan TA, Smith SJ, Reuter H. The timing of synaptic vesicle endocytosis. Proc Natl Acad Sci U S A. 1996;93:5567–5571. doi: 10.1073/pnas.93.11.5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorius CJ, Berger MS. Rapid termination of intraoperative stimulation-evoked seizures with application of cold Ringer's lactate to the cortex – Technical note. J Neurosurg. 1998;88:349–351. doi: 10.3171/jns.1998.88.2.0349. [DOI] [PubMed] [Google Scholar]
- Schiff SJ, Somjen GG. The effects of temperature on synaptic transmission in hippocampal tissue slices. Brain Res. 1985;345:279–284. doi: 10.1016/0006-8993(85)91004-2. [DOI] [PubMed] [Google Scholar]
- Shen KF, Schwartzkroin PA. Effects of temperature alterations on population and cellular activities in hippocampal slices from mature and immature rabbit. Brain Res. 1988;475:305–316. doi: 10.1016/0006-8993(88)90619-1. [DOI] [PubMed] [Google Scholar]
- Stevens CF, Williams JH. ‘Kiss and run’ exocytosis at hippocampal synapses. Proc Natl Acad Sci U S A. 2000;97:12828–12833. doi: 10.1073/pnas.230438697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- Tapia R, Sitges M. Effect of 4-aminopyridine on transmitter release in synaptosomes. Brain Res. 1982;250:291–299. doi: 10.1016/0006-8993(82)90423-1. [DOI] [PubMed] [Google Scholar]
- Thompson SM, Masukawa LM, Prince DA. Temperature dependence of intrinsic membrane properties and synaptic potentials in hippocampal CA1 neurons in vitro. J Neurosci. 1985;5:817–824. doi: 10.1523/JNEUROSCI.05-03-00817.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong G, Jahr CE. Block of glutamate transporters potentiates postsynaptic excitation. Neuron. 1994;13:1195–1203. doi: 10.1016/0896-6273(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Volgushev M, Kudryashov I, Chistiakova M, Mukovski M, Niesmann J, Eysel UT. Probability of transmitter release at neocortical synapses at different temperatures. J Neurophysiol. 2004;92:212–220. doi: 10.1152/jn.01166.2003. [DOI] [PubMed] [Google Scholar]
- Volgushev M, Vidyasagar TR, Chistiakova M, Eysel UT. Synaptic transmission in the neocortex during reversible cooling. Neuroscience. 2000a;98:9–22. doi: 10.1016/s0306-4522(00)00109-3. [DOI] [PubMed] [Google Scholar]
- Volgushev M, Vidyasagar TR, Chistiakova M, Yousef T, Eysel UT. Membrane properties and spike generation in rat visual cortical cells during reversible cooling. J Physiol. 2000b;522:59–76. doi: 10.1111/j.1469-7793.2000.0059m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XF, Duffy DW, Morley RE, Rothman SM. Neocortical seizure termination by focal cooling: temperature dependence and automated seizure detection. Epilepsia. 2002;43:240–245. doi: 10.1046/j.1528-1157.2002.33301.x. [DOI] [PubMed] [Google Scholar]
- Yang X-F, Rothman SM. Focal cooling rapidly terminates experimental neocortical seizures. Ann Neurol. 2001;49:721–726. doi: 10.1002/ana.1021. [DOI] [PubMed] [Google Scholar]
- Zakharenko SS, Zablow L, Siegelbaum SA. Visualization of changes in presynaptic function during long-term synaptic plasticity. Nat Neurosci. 2001;4:711–717. doi: 10.1038/89498. [DOI] [PubMed] [Google Scholar]