Abstract
This study determined the effects of α1-adrenergic receptor (α1-AR) stimulation by phenylephrine (PE) on L-type Ca2+ current (ICa,L) in cat atrial myocytes. PE (10 μm) reversibly increased ICa,L (51.3%; n = 40) and shifted peak ICa,L activation voltage by −10 mV. PE-induced stimulation of ICa,L was blocked by each of 1 μm prazocin, 10 μml-NIO, 10 μm W-7, 10 μm ODQ, 2 μm H-89 or 10 μm LY294002, and was unaffected by 10 μm chelerythrine or incubating cells in pertussis toxin (PTX). PE-induced stimulation of ICa,L also was inhibited by each of 10 μm ryanodine or 5 μm thapsigargin, by blocking IP3 receptors with 2 μm 2-APB or 10 μm xestospongin C or by intracellular dialysis of heparin. In field-stimulated cells, PE increased intracellular NO (NOi) production. PE-induced NOi release was inhibited by each of 1 μm prazocin, 10 μml-NIO, 10 μm W-7, 10 μm LY294002, 2 μm H-89, 10 μm ryanodine, 5 μm thapsigargin, 2 μm 2-APB or 10 μm xestospongin C, and unchanged by PTX. PE (10 μm) increased phosphorylation of Akt, which was inhibited by LY294002. Confocal microscopy showed that PE stimulated NOi release from subsarcolemmal sites and this was prevented by 2 mm methyl-β-cyclodextrin, an agent that disrupts caveolae formation. PE also increased local, subsarcolemmal SR Ca2+ release via IP3-dependent signalling. Electron micrographs of atrial myocytes show peripheral SR cisternae in close proximity to clusters of caveolae. We conclude that in cat atrial myocytes PE acts via α1-ARs coupled to PTX-insensitive G-protein to release NOi, which in turn stimulates ICa,L. PE-induced NOi release requires stimulation of both PI-3K/Akt and IP3-dependent Ca2+ signalling. NO stimulates ICa,L via cGMP-mediated cAMP-dependent PKA signalling. IP3-dependent Ca2+ signalling may enhance local SR Ca2+ release required to activate Ca2+-dependent eNOS/NOi production from subsarcolemmal caveolae sites.
α1-Adrenergic receptor (α1-AR) stimulation plays important roles in the regulation of cardiac contraction, cell growth, hypertrophy and cardioprotection (Li et al. 1997). Classically, α1-ARs are coupled via pertussis toxin (PTX)-insensitive G-proteins (Gq), although coupling to Gi-protein has been reported (Steinberg et al. 1985; Han et al. 1989; Keung & Karliner, 1990; Perez et al. 1993). In general, α1-AR stimulation activates phospholipase C (PLC) to hydrolyse phosphatidylinositol 4,5-bisphosphate (PIP2), leading to the production of diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). DAG activates protein kinase C (PKC) and in cardiac muscle IP3 enhances intracellular Ca2+ release (Nosek et al. 1986; Fabiato, 1986; Zima & Blatter, 2004). Atrial myocytes express functional IP3 receptors (IP3R type-2) at 6–10 times higher levels than in ventricular myocytes and IP3Rs colocalize with ryanodine receptors in the subsarcolemmal space (Lipp et al. 2000; Mackenzie et al. 2002). In permeabilized cat atrial myocytes, exposure to IP3 stimulates local Ca2+ release, i.e. Ca2+ sparks, from the sarcoplasmic reticulum (SR) (Zima & Blatter, 2004). Although IP3 signalling has been implicated in atrial excitation–contraction coupling and/or atrial arrhythmias (Lipp et al. 2000; Mackenzie et al. 2002; Zima & Blatter, 2004), the functional role of IP3 signalling in atrial muscle is still not clear.
In general, phenylephrine (PE), an α1-AR agonist, exerts positive inotropic effects in adult ventricular muscle (Hartmann et al. 1988; Hescheler et al. 1988; Ertl et al. 1991; Terzic et al. 1992) as well as in rat (Ertl et al. 1991; Jahnel et al. 1994) and human (Schumann et al. 1978; Skomedal et al. 1985; Jahnel et al. 1992a) atrial muscle. The majority of studies indicate that the positive inotropic effect of α1-AR stimulation is not primarily mediated via increases in ICa,L (Ertl et al. 1991). However, in guinea pig ventricular myocytes PE acts via PKC signalling to stimulate ICa,L and contractile amplitude (Woo & Lee, 1999). Also, in rat ventricular myocytes PE can increase ICa,L, possibly via PKC activation when the intracellular environment is not disturbed by intracellular dialysis (Zhang et al. 1998). In neonatal rat ventricular myocytes (Liu et al. 1994), PE increases ICa,L (Liu et al. 1994) and chronic exposure to PE in culture induces hypertrophy and increases ICa,L (Gaughan et al. 1998). PE may increase myofilament Ca2+ sensitivity (Terzic et al. 1992) possibly via PKC activation. In rat atria, PE increases intracellular Ca2+ uptake via cAMP-dependent stimulation of voltage-dependent Ca2+ channels (Jahnel et al. 1994), and possibly in part via secondary changes in Na+/Ca2+ exchange (Jahnel et al. 1991, 1992b, 1994). In ventricular muscle, the positive inotropic effect of α1-AR stimulation does not correlate with increases in cellular cAMP levels (Schumann et al. 1975; Brodde et al. 1978; Bogoyevitch et al. 1993). Clearly, the cellular mechanisms underlying α1-AR stimulation in heart are diverse and not entirely understood.
In cat atrial myocytes, stimulation of β2-ARs (Dedkova et al. 2002) or muscarinic receptors (Wang et al. 1998; Dedkova et al. 2003) stimulates Gi-mediated release of intracellular NO (NOi). In human (Kirstein et al. 1995) and cat (Wang et al. 2002) atrial myocytes, NO stimulates ICa,L via cGMP-induced inhibition of phosphodiesterase (PDE) type III activity to enhance endogenous cAMP-dependent PKA activity. The purpose of the present study was to determine whether α1-AR stimulation regulates ICa,L in cat atrial myocytes and if so, whether NOi signalling plays a role. The results indicate that α1-ARs act via PTX-insensitive G-proteins to increase ICa,L via NOi signalling. Moreover, α1-AR stimulation activates NOi release via PI-3K/Akt and IP3-dependent signalling mechanisms. IP3-dependent Ca2+ signalling may enhance local subsarcolemmal SR Ca2+ release required to activate Ca2+/calmodulin (CaM)-dependent eNOS. A portion of this work has been presented in abstract form (Lipsius et al. 2003).
Methods
Adult cats of either sex were anaesthetized with sodium pentobarbital (50 mg kg−1, i.p.). Once fully anaesthetized, a bilateral thoracotomy was performed, and the heart was rapidly excised and mounted on a Langendorff perfusion apparatus. After the heart was enzymatically (collagenase; type II, Worthington Biochemical Corp., Lakewood, NJ, USA) digested, atrial myocytes were isolated as previously reported (Wu et al. 1991). Thirty-three hearts were used to isolate atrial myocytes. The animal protocols used in this study were approved by and in accordance with the Institutional Animal Care and Use Committee of Loyola University of Chicago, Stritch School of Medicine. The number of animals used in this study was limited to a minimum.
Atrial myocytes used for electrophysiological studies were transferred to a small tissue bath (0.3 ml) on the stage of an inverted microscope (Nikon Diaphot) and superfused with a Hepes-buffered modified Tyrode solution containing (mm): NaCl 145, KCl 4, MgCl2 1, CaCl2 2, Hepes 5, glucose 11 and titrated with NaOH to a pH of 7.4. Solutions were perfused by gravity and electrophysiology experiments were performed at 35 ± 1°C. In general, voltage and ionic currents were recorded using a nystatin (150 μg ml−1)-perforated patch whole-cell recording method. The internal pipette solution contained (mm): caesium glutamate 100, KCl 40, MgCl2 1.0, Na2-ATP 4, EGTA 0.5, Hepes 5 and titrated with KOH to pH 7.2. CsCl (5 mm) also was added to all external solutions to block K+ conductances. In one series of experiments, a ruptured patch recording method was used to dialyse heparin intracellularly. The internal pipette solution contained (mm): caesium glutamate 100, CsCl 40, MgCl2 1, Na2-ATP 4, EGTA 0.5, Hepes 10, and titrated with CsOH to pH 7.2. A single suction pipette recorded either voltage (bridge mode) or ionic currents (discontinuous voltage clamp mode) using an Axoclamp 2A amplifier (Axon Instruments, Union City, CA, USA). Computer software (pCLAMP; Axon Instruments) was used to deliver voltage protocols, acquire and analyse data. L-type Ca2+ current (ICa,L) was activated by depolarizing pulses from a holding potential of −40 mV to 0 mV for 200 ms every 5 s. Peak ICa,L amplitude was measured in relation to steady-state current.
Immunoblots were used to analyse PE-induced phosphorylation of Akt (protein kinase B) using phospho-Akt antibody (Ser473). Isolated atrial cells were treated with either control medium (M199), 10 μm PE, or PE plus 10 μm LY294002 before harvesting. Cells were incubated with LY294002 for 15 min followed by a 2 min exposure to PE.
Measurements of intracellular NO (NOi) production were obtained by incubating cells with the fluorescent NO-sensitive dye 4,5-diaminofluorescein (DAF-2) (Kojima et al. 1998; Nakatsubo et al. 1998), as previously described (Wang et al. 2002; Dedkova et al. 2003). Cells were exposed to the membrane-permeant DAF-2 diacetate ([DAF-2 DA]= 5 μm; Calbiochem, San Diego, CA, USA) for 10 min in 1 ml standard Tyrode solution. Cells were subsequently washed for 10 min in Tyrode solution containing 100 μml-arginine. Solutions were perfused by gravity and NOi measurements were performed at room temperature. DAF-2 fluorescence was excited at 480 nm (F480) and emitted cellular fluorescence was recorded at 540 nm. Single cell fluorescence signals were recorded with a photomultiplier tube (model R2693, Hamamatsu Corp.) by masking individual cells with an iris positioned in the emission path. Changes in cellular DAF-2 fluorescence intensities (F) in each experiment were normalized to the level of fluorescence recorded prior to stimulation (Fo), and changes in [NO]i are expressed as F/Fo. DAF-2 is not sensitive to changes in physiological [Ca2+] (Suzuki et al. 2002). Activation of DAF-2 by NO is irreversible and therefore fluorescence intensity remains constant even if NOi levels decrease. In the experiments designed to measure NOi, solutions contained 100 μml-arginine. l-Arginine was omitted when l-NIO was used to block endothelial NO synthase (eNOS). Changes in NOi induced by PE were measured at 5 min of exposure, unless stated otherwise. Cells were field-stimulated at 1 Hz by 3 ms duration suprathreshold rectangular voltage pulses delivered through a pair of extracellular platinum electrodes.
Fast 1-dimensional (1-D) linescan imaging of local intracellular Ca2+ release, i.e. Ca2+ sparks, was performed at room temperature using a confocal laser scanning unit (Bio-Rad Radiance 2100) attached to an inverted microscopy (Nikon TE2000-u) with a × 40 oil-immersion objective lens (Plan fluor, n.a. = 1.3, Nikon). Fluo-4 (fluo-4/AM; 20 μm, incubation time 20 min) was excited with the 488 nm line of an argon ion laser and emitted fluorescence was collected at > 515 nm. The scan line was positioned parallel with the longitudinal axis of the cell within the subsarcolemmal space and was scanned repetitively at 1.4 ms intervals. Linescan profiles are presented as background-subtracted F/Fo. Ca2+ spark frequency was measured with custom-made software (Spark Laboratory; generously provided by Dr J. Puglisi) and is expressed as the number of observed Ca2+ sparks s−1 (100 μm)−1 of scanned distance in the linescan mode.
Two-dimensional (2-D) imaging was performed at room temperature using a confocal scanning unit (LSM 410, Carl Zeiss, Germany) attached to an inverted microscope (Axiovert 100, Zeiss) fitted with a × 40 oil-immersion objective lens (Plan-Neofluar, n.a. = 1.3, Zeiss). Atrial myocytes were loaded with the NO-sensitive indicator DAF-2 as described above. DAF-2 fluorescence was excited with a 488 nm line of an argon laser and the emitted fluorescence was collected at wavelengths > 515 nm.
Drugs and chemicals in this study include: phenylephrine, prazocin, LY294002, pertussis toxin (PTX), l-N5-1-iminoethylornithine (l-NIO), N-(6-aminohexyl)-5-chloro-1-naphthalene sulphonamide hydrochloride (W-7), 1H-[1,2,4] oxadiazolo [4,3-α] quinoxaline-1-one (ODQ), H-89, ryanodine, 2-aminoethoxydiphenyl borate (2-APB), xestospongin C, heparin, thapsigargin, chelerythrine, methyl-β-cyclodextrin, spermine/NO (all from Sigma Chemical Co., St Louis, MO, USA), and 4,5-diaminofluorescein diacetate (DAF-2 DA) (Calbiochem). Inhibition of Gi-protein by PTX (3.5 μg ml−1 at 36°C for at least 3 h) was confirmed by the ability of PTX to block ACh-induced increases in K+ conductance.
Measurements of ICa,L, NOi and Ca2+ sparks involving one cell or two groups of cells were analysed using either paired or unpaired Student's t test for significance at P < 0.05. Data from Western blots and Ca2+ sparks involving multiple values were evaluated using ANOVA and Student-Newman-Keuls test for significance at P < 0.05.
Results
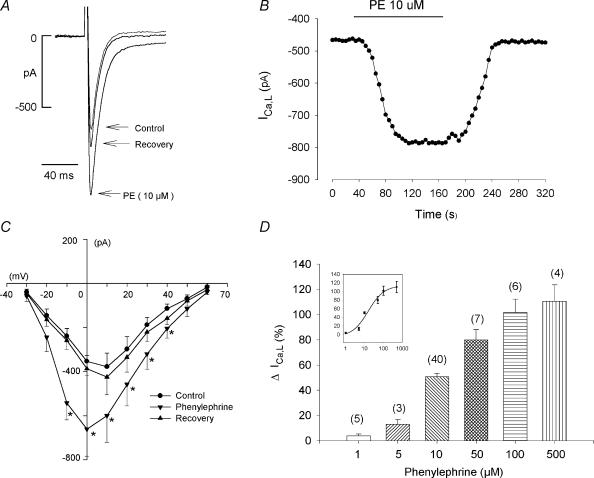
Figure 1A shows the typical effect of 10 μm phenylephrine (PE) to increase L-type Ca2+ current (ICa,L) in an atrial myocyte. PE had no effect on holding current at −40 mV and reversibly increased peak ICa,L by 52%. In a total of 40 cells, 10 μm PE increased ICa,L by 51 ± 3% (P < 0.001). Figure 1B shows peak ICa,L amplitude recorded from another cell plotted against time throughout a typical experiment. PE-induced stimulation of ICa,L amplitude reach steady-state in approximately 1 min, remained constant during exposure to PE and reversed to control upon withdrawal of PE. In Fig. 1C the current–voltage (I–V) relationships show that 10 μm PE reversibly increased peak ICa,L from −30 to +40 mV and elicited a −10 mV shift in the voltage at which maximum ICa,L was activated compared to control, without affecting the reversal potential (n = 6). Figure 1D shows that PE elicited a dose-dependent stimulation of ICa,L with an EC50 of 13.6 μm.
Figure 1. Effects of phenylephrine (PE; 10 μm) on ICa,L.
A, PE reversibly increased peak ICa,L. B, consecutive measurements of peak ICa,L amplitude before, during and after exposure to PE shows the time course of PE-induced stimulation of ICa,L. C, the current–voltage relationship shows that PE reversibly increased ICa,L from −30 to +40 mV and shifted the voltage of maximum ICa,L activation by −10 mV without affecting the reversible potential. D, dose–response relationship of PE (1–500 μm) to stimulate ICa,L. The inset shows a sigmoidal dose–response relationship fitted with a Boltzmann equation. The numbers in parentheses indicate the number of cells tested in each experiment. *P < 0.05.
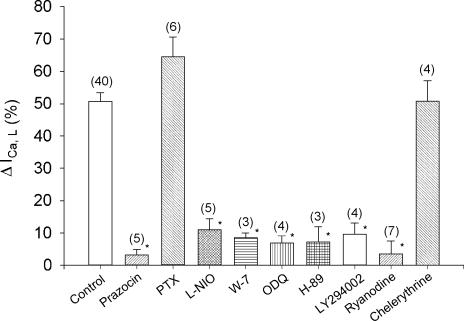
In the following experiments we used a variety of specific pharmacological agents to analyse the signalling mechanisms responsible for PE-induced stimulation of ICa,L. All experiments were performed by comparing the effects of 10 μm PE in the absence (control) and presence (test) of each drug in cells isolated from the same hearts. ICa,L was activated by clamp steps from −40 to 0 mV for 200 ms. Figure 2 summarizes the results. For clarity, all control responses to 10 μm PE (51 ± 3%, n = 40) are grouped together (open bar). Pre-treatment of cells with 1 μm prazocin, a specific α-AR blocking agent, abolished the effects of PE to stimulate ICa,L (control, 43 ± 8%versus prazosin, 3 ± 2%; P < 0.05). Propranolol (0.05 μm) failed to affect PE-induced stimulation of ICa,L (control, 34 ± 3%versus propranolol, 37 ± 3%, n = 4). To determine whether the effects of PE were mediated via PTX-insensitive G-protein, cells were incubated in PTX (see Methods). As summarized in Fig. 2, PTX failed to prevent PE-induced stimulation of ICa,L and in fact somewhat enhanced the effects of PE (control, 59 ± 5%versus PTX, 66 ± 6%), although the increase did not reach statistical significance. In both human (Kirstein et al. 1995) and cat (Wang et al. 1998) atrial myocytes NO stimulates ICa,L. Because NO is produced by Ca2+/calmodulin (CaM)-dependent eNOS, we examined PE-induced stimulation of ICa,L in the absence and presence of l-NIO, a specific eNOS inhibitor (Rees et al. 1990) and W-7, a specific CaM inhibitor (Hidaka et al. 1981). As summarized in Fig. 2, compared with control PE-induced stimulation of ICa,L was significantly inhibited by 10 μml-NIO (control, 42 ± 4%versusl-NIO, 11 ± 3%; P < 0.05) and by 10 μm W-7 (control, 51 ± 10%versus W-7, 9 ± 1%; P < 0.05). In atrial myocytes NO acts via cGMP-induced inhibition of phosphodiesterase (PDE) type III to stimulate endogenous cAMP-dependent PKA signalling (Kirstein et al. 1995; Wang et al. 1998). In the present study, PE-induced stimulation of ICa,L was essentially abolished by 10 μm ODQ (control, 40 ± 5%versus 7 ± 2%; P < 0.05), a specific inhibitor of soluble guanylate cyclase (Brunner et al. 1996) and by 2 μm H-89 (control, 46 ± 4%versus H-89, 7 ± 4%; P < 0.05), an inhibitor of cAMP-dependent PKA activity (Chijiwa et al. 1990). Additional experiments showed that 2 μm H-89 also prevented the effects of PE to shift the voltage of maximum ICa,L activation to more negative values (n = 4; not shown), indicating that this effect of PE is mediated by cAMP-dependent PKA signalling. In endothelial (Dimmeler et al. 1999) and cardiac (Vila Petroff et al. 2001; Wang et al. 2002) cells, NO release is mediated via phosphoinositol-3′ kinase (PI-3K) signalling. In the present study, preincubation (30 min) of atrial myocytes with 10 μm LY294002, a specific inhibitor of PI-3K (Vlahos et al. 1994), inhibited PE-induced stimulation of ICa,L (control, 55 ± 11%versus 10 ± 3%; P < 0.05). Because eNOS is Ca2+ dependent we determined the role of intracellular Ca2+ release by exposing cells to 10 μm ryanodine. This concentration of ryanodine locks the SR Ca2+ release channel in the open position, and thereby depletes SR Ca2+ content, preventing SR Ca2+ release (Fill & Copello, 2002). Ryanodine alone did not inhibit basal ICa,L amplitude. However, ryanodine pretreatment abolished PE-induced stimulation of ICa,L (control, 39 ± 5%versus ryanodine, 4 ± 4%; P < 0.05). Similar results were obtained with 5 μm thapsigargin, an agent that depletes SR Ca2+ content by inhibiting SR Ca2+ uptake (control, 40 ± 8%versus thapsigargin, 12 ± 5%; P < 0.05, n = 7). Finally, because PE is known to activate PKC activity we tested the effects of 4 μm chelerythrine, a non-selective PKC inhibitor (Herbert et al. 1990). Chelerythrine failed to prevent PE-induced stimulation of ICa,L (control, 52 ± 8%versus chelerythrine, 51 ± 6%). None of the drugs used in the experiments summarized in Fig. 2 had any significant direct effects on basal ICa,L amplitude. Together, these findings indicate that PE acts via α1-ARs coupled to PTX-insensitive G-protein and PI-3K signalling to activate Ca2+/CaM-dependent eNOS activity. NO acts via cGMP-mediated inhibition of PDE type III and subsequent stimulation of endogenous cAMP-dependent PKA signalling to stimulate ICa,L, as previously reported (Wang et al. 1998). PE does not act via PKC to stimulate ICa,L in cat atrial myocytes.
Figure 2. Pharmacological analysis of the signalling mechanisms responsible for PE-induced stimulation of ICa,L.
Each experiment was performed by testing PE in the absence (control) and presence (test) of each drug in myocytes isolated from the same hearts. The control values from each experiment (n = 40) are grouped together for clarity. Compared with control (open bar), prazocin (1 μm), l-NIO (10 μm), W-7 (10 μm), ODQ (10 μm), H-89 (2 μm), LY294002 (10 μm) and ryanodine (10 μm) each significantly inhibited PE-induced stimulation of ICa,L. Incubation of cells in PTX or exposure to chelerythrine (4 μm) failed to affect PE-induced stimulation of ICa,L. The numbers in parentheses indicate the number of cells tested in each experiment. *P < 0.05.
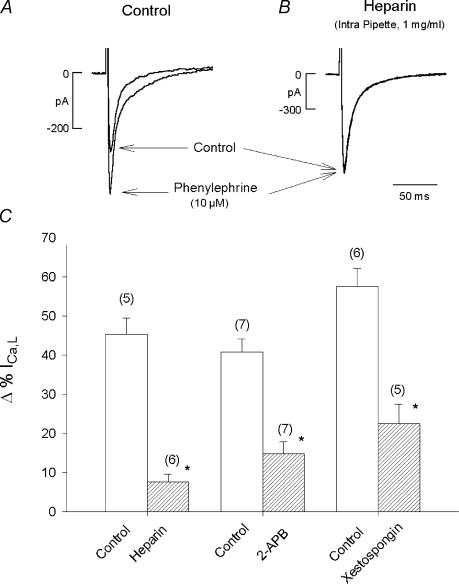
The fact that ryanodine and thapsigargin prevented PE-induced stimulation of ICa,L indicates that Ca2+ release from the SR is essential, presumably to stimulate Ca2+-dependent eNOS activity. In addition, PE activates IP3 signalling and IP3 receptor (IP3R) stimulation enhances SR Ca2+ release in atrial myocytes (Lipp et al. 2000; Mackenzie et al. 2002; Zima & Blatter, 2004). We therefore examined the role of IP3 signalling by testing the effects of PE to stimulate ICa,L in the absence and presence of three different putative IP3R blockers: heparin, 2-APB (Maruyama et al. 1997) and xestospongin C (Gafni et al. 1997). Figure 3A and B shows original traces of ICa,L recorded from two different atrial myocytes using a ruptured patch method. In control (Fig. 3A), 10 μm PE elicited a typical increase in ICa,L. In Fig. 3B, the recording pipette contained 1 mg ml−1 heparin, a potent IP3R blocker. With intracellular dialysis of heparin, PE failed to stimulate ICa,L. As summarized in the bar graph (Fig. 3C) PE-induced stimulation of ICa,L was essentially abolished by heparin (control, 45 ± 4%versus heparin, 8 ± 2%; P < 0.05). Additional experiments used a perforated (nystatin) patch method to determine the effects of 2 μm 2-APB and 10 μm xestospongin, which are both membrane permeant. Both acute exposure to 2-APB (control, 41 ± 3%versus 2-APB, 15 ± 3%; P < 0.05) and incubating cells in xestospongin C (2 h) (control, 58 ± 5%versus xestospongin C, 23 ± 5%; P < 0.05) significantly inhibited PE-induced stimulation of ICa,L. Together, these findings suggest that IP3-dependent signalling participates in PE-induced stimulation of ICa,L.
Figure 3. Inhibition of IP3 signalling inhibits PE-induced stimulation of ICa,L.
A, original records showing the effects of PE to stimulate ICa,L recorded with a ruptured patch method. PE elicited a typical increase in ICa,L amplitude. B, another atrial myocyte in which ICa,L was recorded during intracellular dialysis of heparin (1 mg ml−1) contained within the pipette solution. PE failed to increase ICa,L amplitude. In two additional series of experiments, PE was tested in the absence and presence of 2 μm 2-APB or 10 μm xestospongin C (incubation 2 h) using a perforated patch recording method. C, summary of the inhibitory effects of heparin, 2 μm 2-APB or 10 μm xestospongin C. Each IP3 receptor blocking agent significantly inhibited PE-induced stimulation of ICa,L. Numbers in parentheses indicate the number of cells tested in each experiment. *P < 0.05.
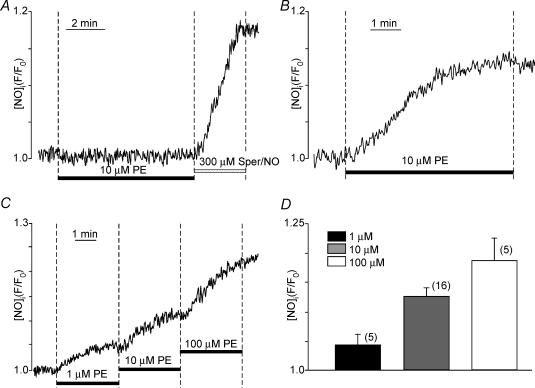
The present results suggest that PE acts via NO signalling to stimulate ICa,L. We therefore used fluorescence microscopy and the NO-sensitive indicator DAF-2 to directly measure PE-induced NOi production. Figure 4A shows that PE was unable to release NOi in quiescent cells, i.e. not field stimulated. In the same cell, exposure to the NO donor spermine/NO (300 μm) significantly increased NOi. Similar results were obtained in a total of three cells. Figure 4B shows that in another atrial myocyte field stimulated at 1 Hz, PE increased NOi release. The obligatory requirement of field stimulation suggests the importance of Ca2+ influx and/or intracellular Ca2+ release for receptor-mediated NOi production. Figure 4C shows that PE elicited a dose-dependent (1, 10, 100 μm) increase in NOi release in atrial myocytes field stimulated at 1 Hz. Figure 4C summarizes the dose-dependent PE-induced stimulation of NOi release.
Figure 4. Effects of PE to stimulate NOi release in atrial myocytes.
A; PE (10 μm) is unable to stimulate NOi release in a quiescent atrial myocyte. Exposure to 300 μm spermine/NO (an NO donor) increases NOi. B; PE (10 μm) stimulates NOi release in an atrial myocyte field stimulated at 1 Hz. C; PE (1, 10, 100 μm) elicits a dose-dependent increase in NOi release. D; summary of dose-dependent PE-induced stimulation of NOi. The numbers in parentheses indicate the number of cells tested in each experiment.
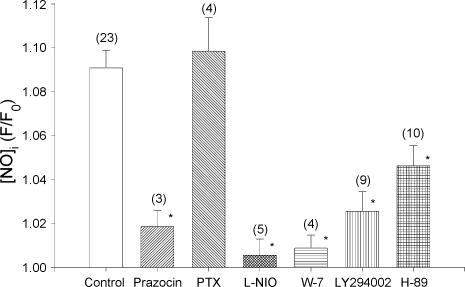
Figure 5 summarizes the results of experiments in which we studied the signalling mechanisms underlying PE-induced NOi production by using many of the same pharmacological agents that we used to study PE-induced stimulation of ICa,L. Again, control and test cells were obtained from the same hearts and control values are grouped together for clarity. Compared with control responses, 10 μm PE-induced NOi release was abolished by the specific α-AR antagonist prazocin (1 μm) and unaffected by incubating cells in PTX. Non-selective inhibition of β-ARs by 0.1 μm propranolol did not affect PE-induced NOi release (n = 3; data not shown). As expected, PE-induced NOi production was abolished by inhibition of eNOS with 10 μml-NIO and by inhibition of CaM by 10 μm W-7. Additionally, inhibition of PI-3K signalling by 10 μm LY294002 significantly inhibited PE-induced NOi production. In the presence of either l-NIO or LY294002, 100 μm spermine/NO elicited a typical increase in DAF-2 fluorescence, indicating that neither l-NIO nor LY294002 blocked PE-induced stimulation of NOi by somehow interfering with DAF-2 fluorescence (data not shown). These findings indicate that PE acts via α1-ARs coupled to PI-3K signalling to stimulate Ca2+/CaM-dependent eNOS and the production of NOi. Moreover, the fact that interventions which inhibit PE-induced NOi release also inhibit PE-induced stimulation of ICa,L strongly suggests that NOi release is responsible for stimulation of ICa,L. This is consistent with reports that exogenous NO stimulates ICa,L in both human (Kirstein et al. 1995) and cat (Wang et al. 1998) atrial myocytes.
Figure 5. Pharmacological analysis of the signalling mechanisms responsible for PE-induced stimulation of NOi release.
Each experiment was performed by testing PE in the absence (control) and presence (test) of each drug on myocytes isolated from the same hearts. The control values from each experiment (n = 23) are grouped together for clarity. Compared with control (open bar) prazocin (1 μm), l-NIO (10 μm), W-7 (10 μm) and LY294002 (10 μm) each significantly inhibited PE-induced stimulation of NOi release. H-89 (2 μm) blocked approximately 50% of PE-induced NOi production. Incubation of cells in PTX had no effect on PE-induced NOi production. The numbers in parentheses indicate the number of cells tested in each experiment. *P < 0.05.
Does NO-induced stimulation of ICa,L (and subsequent CICR) elicited by PE contribute to further stimulation of NOi production? The present work as well as our previous studies (Wang et al. 1998) indicates that NO stimulates ICa,L via cGMP/cAMP-dependent PKA activity. We therefore determined the effects of PE to stimulate NOi when NO-mediated stimulation of ICa,L is blocked by 2 μm H-89. As summarized in Fig. 5, inhibition of cAMP-dependent PKA significantly attenuated (−51.8% of control) but did not prevent PE-induced stimulation of NOi. These findings suggest that PE elicits the release of NOi, which acts via cAMP/PKA signalling to stimulate ICa,L (and CICR), which in turn stimulates additional Ca2+-dependent eNOS/NOi production. As described below, the PE-induced release of NOi which is independent of cAMP/PKA signalling, is mediated via IP3-dependent Ca2+ signalling.
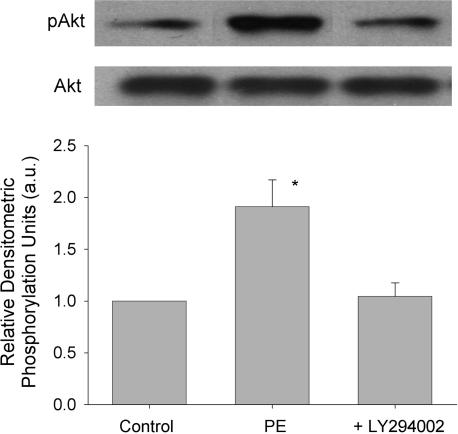
In endothelial cells, PI-3K acts via phosphorylation of Akt (protein kinase B) to activate eNOS activity (Fulton et al. 1999). We therefore determined whether PE phosphorylates Akt via a PI-3K-dependent mechanism in atrial myocytes. The Western blots in Fig. 6 show that 10 μm PE significantly increased phosphorylation of Akt (Ser473) 1.7 ± 0.1-fold compared with control (normalized to 1) and that 10 μm LY294002 blocked PE-induced phosphorylation of Akt. The graph shows mean ±s.e.m. values obtained in nine experiments. These results, together with those presented in Fig. 5, suggest that PE-induced activation of PI-3K/Akt signalling is required for stimulation of NOi production.
Figure 6. PE acts via PI-3K-dependent signalling to phosphorylate Akt.
The Western blots show phosphorylated (pAkt; Ser473) (upper) and total (lower) Akt in the absence and presence of 10 μm LY294002. Compared with control 10 μm PE significantly increased phosphorylation of Akt. Prior exposure to 10 μm LY294002 blocked PE-induced Akt phosphorylation. The graph summarizes the normalized data obtained in 9 separate experiments. *P < 0.05.
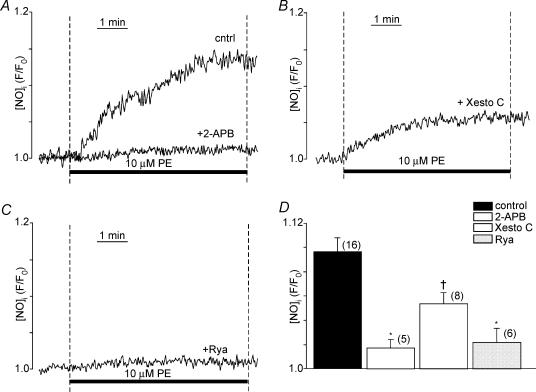
The present findings indicate that PE-induced stimulation of ICa,L is dependent on NOi release and IP3 signalling. Therefore, we next determined whether PE-induced NOi release is dependent on IP3 signalling. Figure 7A shows recordings from three different atrial myocytes where compared with control responses, PE (10 μm)-induced stimulation of NOi was significantly inhibited by 10 μm xestospongin C and abolished by 2 μm 2-APB. In another atrial myocyte (Fig. 7B), prior exposure to 10 μm ryanodine also abolished PE-induced NOi release. Similar experiments using 5 μm thapsigargin to deplete SR Ca2+ stores inhibited PE-induced NOi production by 80% (control, 1.091 ± 0.008 versus thapsigargin, 1.018 ± 0.007; P < 0.001, n = 6). Figure 7D summarizes the effects of 2-APB, xestospongin C, and ryanodine compared with control responses (filled bar) to10 μm PE. Together, these results suggest that PE-induced NOi release is dependent on IP3-mediated Ca2+ signalling. Moreover, because similar interventions blocked PE-induced stimulation of ICa,L they further support the idea that PE acts via NOi signalling to stimulate ICa,L. It may be argued that 2-APB (Bootman et al. 2002) as well as xestospongin C or heparin exerts non-specific effects. However, in permeabilized cat atrial myocytes heparin or 2-APB prevented direct IP3-induced increases in basal intracellular [Ca2+] and Ca2+ spark frequency (Zima & Blatter, 2004).
Figure 7. Inhibition of IP3 receptor signalling or SR Ca2+ release inhibits PE-induced stimulation of NOI.
A and B, compared to control (cntrl) PE (10 μm)-induced stimulation of NOi release was blocked by 2 μm 2-APB (A) and markedly inhibited by 2 h incubation in 10 μm xestospongin C (+ Xesto C) (B). C, prior exposure to 10 μm ryanodine blocked PE-induced stimulation of NOi release. D, summary of the effects of 2-APB, xestospongin C, and ryanodine compared to control (filled bar) effects of PE to increase NOi. The numbers in parentheses indicate that number of cells tested in each experiment. *P < 0.01; †P < 0.05.
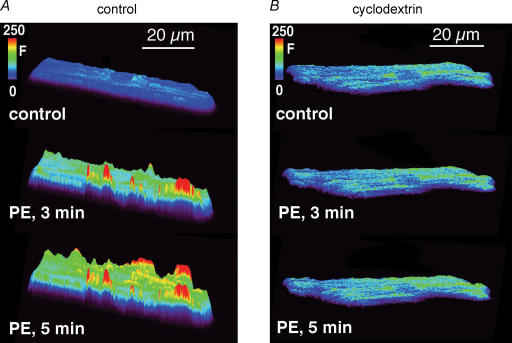
In Fig. 8 we used confocal fluorescence microscopy to directly visualize the spatial pattern of NOi release induced by PE. In Fig. 8A, compared with control, after 3 min of exposure to 10 μm PE, NOi was increased primarily along the cell periphery. After 5 min of PE exposure additional NOi release sites along the periphery are evident, as are smaller increases of NOi within the cell interior. This spatial pattern of NOi release was evident in a total of five atrial myocytes. In cardiac cells, eNOS is localized within caveolae (Feron et al. 1996). We therefore determined whether PE acts to release NOi from caveolae by testing PE in cells previously incubated (1 h) in 2 mm methyl-β-cyclodextrin (cyclodextrin), an agent that disrupts caveolae formation (Smart & Anderson, 2002). As shown in Fig. 8B, PE failed to increase NOi release in cyclodextrin-treated cells. Similar results were obtained in five atrial myocytes. These findings indicate that PE acts via IP3-mediated Ca2+ release to stimulate NOi production from subsarcolemmal regions.
Figure 8. 2-Dimensional surface plots obtained from atrial myocytes showing the spatial patterns of NOi production induced by 10 μm PE.
A, compared with control, after 3 min of exposure to PE, NOi production increased at discrete sites primarily along the cell periphery. At 5 min of PE exposure NOi production along the cell periphery was further increased at additional sites along the cell periphery with smaller increases of NOi within the cell interior. B, another atrial myocyte was incubated (1 h) in 2 mm methyl-β-cyclodextrin (cyclodextrin) to disrupt caveolae formation. Exposure to 10 μm PE failed to increase NOi.
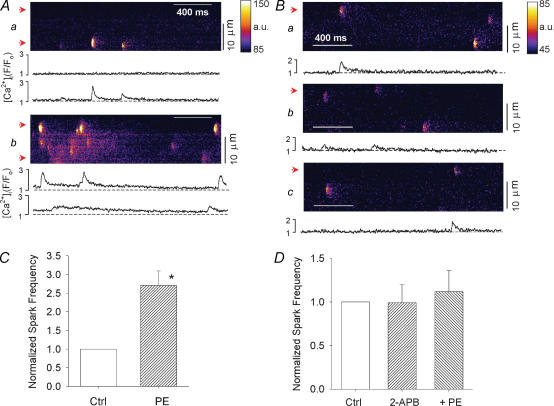
The previous findings raise the question of whether PE increases subsarcolemmal SR Ca2+ release that is mediated via IP3 signalling. We therefore used confocal laser scanning microscopy to determine whether PE increases local subsarcolemmal SR Ca2+ release, i.e. Ca2+ sparks, and whether this response is sensitive to inhibition of IP3R signalling. A repetitively scanned line was positioned parallel with the longitudinal axis of quiescent atrial myocytes within the subsarcolemmal space. Because the cells are quiescent, PE is unable to release NOi (see Fig. 4A). Therefore any increase in subsarcolemmal SR Ca2+ release cannot be due to NO-mediated stimulation of Ca2+ influx via stochastic opening of ICa,L channels. In Fig. 9 each panel shows confocal line scan images of spontaneous subsarcolemmal Ca2+ spark activity and the traces below each panel show local subcellular changes in [Ca2+]i within the subsarcolemmal space that correspond with the positions of the arrows at the left margin of each panel. Compared with control (Fig. 9Aa), exposure to 10 μm PE (2 min) (Fig. 9Ab) increased subsarcolemmal Ca2+ spark activity and raised baseline [Ca2+]i. In another atrial myocyte, compared with control (Fig. 9Ba), exposure to 2 μm 2-APB (Fig. 9Bb) had little effect on Ca2+ spark activity and prevented PE-induced increases in Ca2+ sparks (Fig. 9Bc). The graphs (Fig. 9C and D) summarize the results normalized to control. PE significantly increased Ca2+ spark frequency (170%) (Fig. 9C) and 2-APB prevented PE-induced stimulation of Ca2+ sparks (Fig. 9D). Although PE raised baseline [Ca2+]i in some cells, the change did not reach statistical significance. These findings indicate that in cat atrial myocytes, PE increases subsarcolemmal SR Ca2+ release mediated by IP3-dependent signalling.
Figure 9. Confocal laser linescan images of local SR Ca2+ release, i.e. Ca2+ sparks, recorded from quiescent atrial myocytes.
Each panel shows confocal line scan images of spontaneous subsarcolemmal Ca2+ spark activity. The traces below each panel show local subcellular changes in [Ca2+]i within the subsarcolemmal space that correspond with the arrows at the left margin of each panel. A, compared with control (a), PE (10 μm) increased Ca2+ spark activity (b). B, compared with control (a), 2 μm 2-APB had little effect on Ca2+ spark activity (b) but blocked PE-induced increases in Ca2+ sparks (c). C, normalized Ca2+ spark frequency (sparks s−1 (100 μm)−1); compared with control (Ctrl) PE significantly increased Ca2+ spark frequency (170%; n = 6). D, compared with control (Crtl), 2-APB blocked the effect of PE to increase Ca2+ spark frequency (n = 9). *P < 0.05.
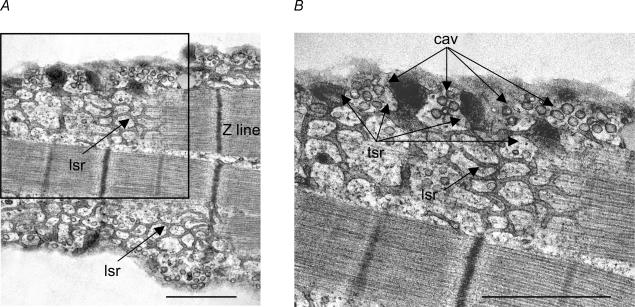
Together, the present findings suggest that IP3-dependent SR Ca2+ signalling mediates activation of eNOS contained within caveolae. Figure 10A shows an electron micrograph of a cat atrial myocyte sectioned longitudinally. The cell was sectioned just below the sarcolemma and reveals sarcomere units (Z line) and an extensive network of longitudinal sarcoplasmic reticulum (lsr) throughout the cell interior that is connected to numerous electron-dense terminal SR cisternae located at intervals within the subsarcolemmal space. These terminal cisternae are the peripheral SR Ca2+ release sites. The subsarcolemmal space also contains numerous caveolae. The inset shown in Fig. 10A is magnified in Fig. 10B and shows that closely interspersed between each terminal SR (tsr) Ca2+ release site are grape-like clusters of caveolae (Cav), the sites of eNOS and NOi release. This ultrastructural architecture is compatible with localized NOi release along the cell periphery that is mediated by local SR Ca2+ release.
Figure 10. Electron micrographs of an atrial myocyte cut parallel with the longitudinal axis of the cell.
A, micrograph shows sarcomere units (Z lines) and an extensive network of longitudinal sarcoplasmic reticulum (lsr) throughout the cell interior. B, higher magnification of inset in A shows that the longitudinal sarcoplasmic reticulum is connected to electron dense regions at the cell periphery which are terminal SR (tsr) Ca2+ release sites. Between each terminal SR Ca2+ release site are grape-like clusters of caveolae (Cav). Calibration bars = 1.0 μm.
Discussion
The present study shows, for the first time, that PE stimulates NOi release in atrial myocytes. PE-induced release of NOi required that atrial cells be electrically stimulated. This is similar to our previous findings in which NOi release induced by β2-AR (Wang et al. 2002; Dedkova et al. 2002) or muscarinic (Dedkova et al. 2003) receptor stimulation also required that atrial myocytes be electrically stimulated. In beating neonatal or quiescent adult rat ventricular myocytes PE has no effect on NOi release (Kanai et al. 1997). As demonstrated previously (Dedkova et al. 2003), cat atrial cells require voltage-activated Ca2+ influx for receptor-mediated stimulation of NOi production. However, as reported here and in our previous studies (Dedkova et al. 2002; Dedkova et al. 2003), basal Ca2+ influx and/or SR Ca2+ release elicited by electrical stimulation alone (without receptor stimulation) is not sufficient to stimulate NOi release. This is in contrast to ventricular myocytes in which electrical stimulation is sufficient to release NOi (Kaye et al. 1996; Dedkova et al. 2004). In the present study, ryanodine or thapsigargin, agents that interfere with SR Ca2+ release, inhibited PE-induced stimulation of both NOi release and ICa,L. Because neither drug inhibits basal ICa,L amplitude, i.e. Ca2+ influx, the present findings indicate that SR Ca2+ release and not Ca2+ influx per se is the critical Ca2+ source for PE-induced activation of Ca2+-dependent eNOS activity.
In atrial myocytes, α1-AR stimulation mediates IP3 production and IP3 enhances SR Ca2+ release (Nosek et al. 1986; Fabiato, 1986; Zima & Blatter, 2004). The present results indicate, for the first time, that PE-induced stimulation of NOi and subsequent stimulation of ICa,L are dependent on IP3 signalling. Moreover, PE stimulates local subsarcolemmal SR Ca2+ release, i.e. Ca2+ sparks, through IP3-dependent signalling. These findings are consistent with reports that in atrial myocytes IP3 stimulates SR Ca2+ release and may enhance Ca2+-induced Ca2+ release (CICR) (Lipp et al. 2000; Mackenzie et al. 2002; Zima & Blatter, 2004) and that IP3Rs are colocalized with subsarcolemmal SR Ca2+ release channels, i.e. ryanodine receptors (Lipp et al. 2000; Mackenzie et al. 2002). On the other hand, stimulation of rat atrial myocytes with endothelin or exposure to membrane-permeant IP3 increased SR Ca2+ release more in central than peripheral regions of the cell (Mackenzie et al. 2004).
In cardiac cells, eNOS (Feron et al. 1996) as well as α1-ARs, Gq and PLC are colocalized with caveolin-3 within caveolae (Fujita et al. 2001). As shown in the present experiments, PE activated local release of NOi from subsarcolemmal sites and this local NOi release was prevented by disruption of caveolae formation. These findings are similar to our previous study in which ACh exposure and withdrawal increase NOi release from subsarcolemmal caveolae sites in atrial myocytes (Dedkova et al. 2003). Moreover, our electron micrographs clearly show that the ultrastructure of cat atrial myocytes exhibit peripheral SR Ca2+ release sites located in close proximity to abundant subsarcolemmal caveolae. Together, the present findings suggest that IP3-dependent Ca2+ signalling mediates NOi release from subsarcolemmal caveolae sites.
Several of the present findings, however, make it unlikely that Ca2+ released via IP3Rs directly stimulates eNOS/NOi production. First, in cat atrial myocytes IP3R-dependent Ca2+ release events are 75–80% smaller in amplitude and their rise time is approximately 2-fold longer than average Ca2+ spark events (Zima & Blatter, 2004). Moreover, in quiescent atrial cells PE stimulates both PI-3K/Akt signalling and local IP3-dependent SR Ca2+ release, yet fails to stimulate NOi production. In addition, PE-induced NOi release requires voltage-activated Ca2+ influx and SR Ca2+ release. Finally, a significant portion of PE-induced NOi production is stimulated by cAMP/PKA signalling, indicative of a critical role for CICR. It therefore seems likely that IP3-dependent Ca2+ signalling acts indirectly by enhancing release of a localized SR Ca2+ pool that is stimulated by extracellular Ca2+ influx via ICa,L (CICR) and targeted for Ca2+-dependent eNOS activity in subsarcolemmal caveolae sites. Speculation leads to the possibility that the voltage-activated Ca2+ influx channel also may be localized to caveolae closely apposed to ryanodine receptors (Löhn et al. 2000).
In the present study, PE-induced stimulation of both ICa,L and NOi release were PTX-insensitive, consistent with coupling of α1-ARs to Gq signalling. In fact, PE-induced stimulation of ICa,L was somewhat enhanced in cells incubated in PTX, suggesting that Gi may, to some extent, modulate α1-AR stimulation. PKC signalling is not involved in PE-induced stimulation of either ICa,L or NOi release in cat atrial myocytes. This is in contrast to findings in guinea pig (Woo & Lee, 1999) and rat (Zhang et al. 1998) ventricular myocytes in which PE increases ICa,L via PKC signalling. Moreover, in the present study PE elicited only stimulation of ICa,L, in contrast to rat ventricular myocytes where PE elicited a biphasic effect: an initial inhibition followed by a sustained stimulation of ICa,L (Zhang et al. 1998).
PE-induced stimulation of ICa,L and NOi release were each inhibited by LY294002 and therefore dependent on PI-3K signalling. PI-3K signalling leads to phosphorylation of Akt (Brazil & Hemmings, 2001) which in turn activates eNOS (Fulton et al. 1999) and NOi production. Indeed, the present findings show that PE increases Akt phosphorylation through a PI-3K-dependent mechanism. It is important to note that the Western blot experiments were performed on quiescent atrial myocytes treated with PE, i.e. in the absence of voltage-activated Ca2+ influx or release. This is consistent with reports that stimulation of Akt is Ca2+ independent (Conus et al. 1998). Therefore, the present results indicate that α1-AR stimulation of eNOS activity, i.e. NOi production, requires activation of both Ca2+-independent PI-3K/Akt signalling and Ca2+-dependent CaM signalling pathways. This dual signalling mechanism is similar to that responsible for muscarinic receptor stimulation of NOi release (Dedkova et al. 2003). In cat atrial myocytes both β2-ARs (Wang et al. 2002) and muscarinic receptors (Dedkova et al. 2003) act via PTX-sensitive Gi to release of NOi via PI-3K-dependent signalling. The present findings indicate that Gq also mediates activation of PI-3K signalling in atrial myocytes. This is consistent with reports in a variety of cell systems that receptor-coupled Gq activates PI-3K signalling (Murga et al. 1998; Graness et al. 2005; Xie et al. 2005).
The present study also shows that PE-induced stimulation of ICa,L was blocked by inhibition of guanylate cyclase or inhibition of cAMP-dependent PKA activity. This is consistent with NO acting via cGMP-inhibited PDE type III to raise endogenous cAMP-dependent PKA activity (Kirstein et al. 1995; Wang et al. 1998). The negative shift in maximum ICa,L activation voltage elicited by PE also is explained by cAMP/PKA signalling. In addition, PE-induced NOi release was decreased by approximately 50% by inhibition of cAMP-dependent PKA (H-89) while it was abolished by inhibition of IP3R signalling (2-APB). These findings suggest that PE acts initially via IP3-dependent Ca2+ signalling to indirectly stimulate NOi production, and NO in turn acts via cAMP/PKA signalling to stimulate ICa,L and CICR to further increase NOi production. Because NO signalling acts locally to regulate ion channel function (Dittrich et al. 2001; Wang et al. 2002; Dedkova et al. 2002) the effects of α1-AR stimulation may not correlate with changes in cellular cAMP levels (Schumann et al. 1975; Brodde et al. 1978; Bogoyevitch et al. 1993).
In rat atrial muscle, PE increases Ca2+ uptake possibly in part through secondary changes in Na+/Ca2+ exchange (Jahnel et al. 1991, 1992b, 1994). A proposed mechanism involves PE-induced depolarization and increases in TTX-sensitive Na+ influx (Jahnel et al. 1991, 1992b). It may therefore be argued that Ca+ influx via Na+/Ca2+ exchange contributes to PE-induced stimulation of Ca2+-dependent NOi production. However, any contribution by Na+/Ca2+ exchange is unlikely or at best very small. In the present study, the voltage clamp method used to analyse ICa,L precludes any increase in TTX-sensitive Na+ influx induced by PE. Moreover, the contribution of Ca+ influx via Na+/Ca2+ exchange is a small fraction of total Ca2+ influx (Weber et al. 2002). The fact that PE-induced NOi release is significantly stimulated by cAMP/PKA signalling also makes it unlikely that Ca2+ influx via Na+/Ca2+ exchange is a primary mechanism. Finally, PE-induced NOi production is strongly dependent on SR Ca2+ release. However, Ca2+ influx via the exchanger is a very inefficient trigger for stimulating SR Ca+ release (Sipido et al. 1997).
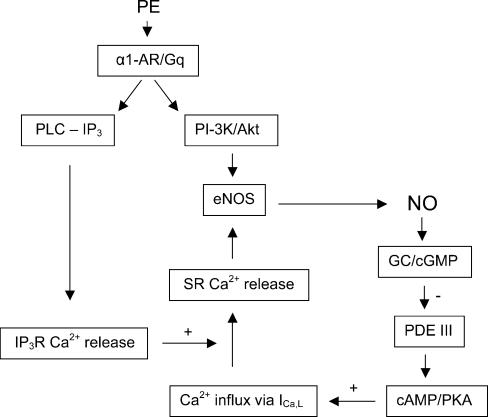
We therefore propose the scheme shown in Fig. 11 to explain the effects of PE to stimulate ICa,L in cat atrial myocytes. PE acts via α1-ARs coupled to Gq to stimulate two signalling pathways: PI-3K/Akt and PLC-induced IP3 signalling. In conjunction with stimulation of PI-3K/Akt signalling, IP3-mediated Ca2+ release via IP3Rs enhances CICR from a localized SR Ca2+ pool that targets stimulation of Ca2+/CaM-dependent eNOS contained within caveolae. The resulting increase in NOi release acts via GC/cGMP-induced inhibition of PDE III to enhance endogenous cAMP/PKA stimulation of ICa,L. Stimulation of Ca2+ influx via ICa,L stimulates CICR to further stimulate eNOS/NOi production. This positive feedback reaches steady-state presumably by the dose-dependent stimulation of PI-3K/Akt signalling. There also may be negative feedback mechanisms by various phosphatases not considered in the present experiments.
Figure 11. Proposed signalling mechanisms underlying PE-induced stimulation of ICa,L via NO signalling.
PE acts via α1-ARs coupled to Gq, presumably localized within caveolae, to activate both PLC – IP3 and PI-3K/Akt signalling. IP3 stimulates SR Ca2+ release via IP3Rs. IP3-mediated Ca2+ signalling enhances local SR Ca2+ release triggered by Ca2+ influx via ICa,L. In conjunction with receptor-mediated stimulation of PI-3K/Akt signalling, SR Ca2+ release activates CaM-dependent eNOS to stimulate NOi production. NO acts via cGMP-induced inhibition of PDE III to stimulate endogenous cAMP-dependent PKA activity. cAMP/PKA stimulates Ca2+ influx via ICa,L which in turn enhances Ca2+-mediated stimulation of eNOS and NOi production. Steady-state stimulation of NOi production may be determined by the dose-dependent receptor-mediated stimulation of PI-3K/Akt/eNOS signalling.
Finally, numerous reports which have studied parasympathetic nerve-induced stimulation of NOi release in vivo use PE as a pressor to elicit baroreflex responses. The assumption is that PE does not directly release NOi. The present study now indicates that these types of studies need to consider the possible direct effects of PE to induce NOi release from atrial muscle.
Acknowledgments
This work was supported by NIH grants HL63753 (to S.L.L), HL62231 (to L.A.B), American Heart Association fellowship grant AHA 0425761Z (to E.N.D) and a fellowship grant from Loyola University Medical Center, Cardiovascular Institute, Dr Ralph and Marian Falk Medical Research Trust Foundation (to X.J). We thank Linda Fox for her expert technical assistance in obtaining the electron micrographs and Dr J. Puglisi for generously providing the custom-made software (Spark Laboratory) used for measuring Ca2+ spark frequency.
References
- Bogoyevitch MA, Fuller SJ, Sugden PH. cAMP and protein synthesis in isolated adult rat heart preparations. Am J Physiol Cell Physiol. 1993;265:C1247–C1257. doi: 10.1152/ajpcell.1993.265.5.C1247. [DOI] [PubMed] [Google Scholar]
- Bootman MD, Collins TJ, Mackenzie L, Roderick EL, Berridge MJ, Peppiatt CM. 2-Aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J. 2002;16:1145–1150. doi: 10.1096/fj.02-0037rev. [DOI] [PubMed] [Google Scholar]
- Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem Sci. 2001;26:657–664. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- Brodde OE, Motomura S, Endoh M, Schumann HJ. Lack of correlation between the positive inotropic effect evoked by á-adrenoceptor stimulation and the levels of cyclic AMP and/or cyclic GMP in the isolated ventricle strip of the rabbit. J Mol Cellular Cardiol. 1978;10:207–219. doi: 10.1016/0022-2828(78)90344-9. [DOI] [PubMed] [Google Scholar]
- Brunner F, Schmidt K, Nielsen EB, Mayer B. Novel guanylyl cyclase inhibitor potently inhibits cyclic GMP accumulation in endothelial cells and relaxation of bovine pulmonary artery. J Pharmacol Exp Therapeutics. 1996;277:48–53. [PubMed] [Google Scholar]
- Chijiwa T, Mishima A, Hagiwara M, Sano M, Hayashi K, Inoue T, Naito K, Toshioka T, Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinoline sulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem. 1990;265:5267–5272. [PubMed] [Google Scholar]
- Conus NM, Hemmings BA, Pearson RB. Differential regulation by calcium reveals distinct signaling requirements for the activation of Akt and p70S6k. J Biol Chem. 1998;273:4776–4782. doi: 10.1074/jbc.273.8.4776. [DOI] [PubMed] [Google Scholar]
- Dedkova EN, Ji X, Wang YG, Blatter LA, Lipsius SL. Signaling mechanisms that mediate nitric oxide production induced by acetylcholine exposure and withdrawal in cat atrial myocytes. Circulation Res. 2003;93:1233–1240. doi: 10.1161/01.RES.0000106133.92737.27. [DOI] [PubMed] [Google Scholar]
- Dedkova EN, Wang YG, Blatter LA, Lipsius SL. Nitric oxide signalling by selective α2-adrenoceptor stimulation prevents ACh-induced inhibition of β2-stimulated Ca2+ current in cat atrial myocytes. J Physiol. 2002;542:711–723. doi: 10.1113/jphysiol.2002.023341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedkova EN, Wang YG, Blatter LA, Lipsius SL. Contractile activity stimulates nitric oxide production in cat ventricular myocytes via PI-(3)K-cytoskeletal signaling. Biophys J. 2004;86:399a. (Abstract) [Google Scholar]
- Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- Dittrich M, Jurevicius J, Georget M, Rochais F, Fleischmann BK, Hescheler J, Fischmeister R. Local response of L-type Ca2+ current to nitric oxide in frog ventricular myocytes. J Physiol. 2001;534:109–121. doi: 10.1111/j.1469-7793.2001.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertl R, Jahnel U, Nawrath H, Carmeliet E, Vereecke J. Differential electrophysiologic and inotropic effects of phenylephrine in atrial and ventricular heart muscle preparations from rats. Naunyn Schmeidebergs Arch Pharmacol. 1991;344:574–581. doi: 10.1007/BF00170655. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Inositol(1,4,5)-triphosphate-induced release of Ca2+ from the sarcoplasmic reticulum of skinned cardiac cells. Biophys J. 1986;49:190a. [Google Scholar]
- Feron O, Belhassen L, Kobzik L, Smith TW, Kelly RA, Michel T. Endothelial nitric oxide synthase targeting to caveolae. J Biol Chem. 1996;271:22810–22814. doi: 10.1074/jbc.271.37.22810. [DOI] [PubMed] [Google Scholar]
- Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiol Rev. 2002;82:893–922. doi: 10.1152/physrev.00013.2002. [DOI] [PubMed] [Google Scholar]
- Fujita T, Toya Y, Iwatsubo K, Onda T, Kimura K, Umemura S, Ishikawa Y. Accumulation of molecules involved in α1-adrenergic signal within caveolae: caveolin expression and the development of cardiac hypertrophy. Cardiovascular Res. 2001;51:709–716. doi: 10.1016/s0008-6363(01)00348-0. [DOI] [PubMed] [Google Scholar]
- Fulton D, Gratton J-P, McCabe TJ, Fontana J, Fuijo Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafni J, Munsch JA, Lam TH, Catlin MC, Costa LG, Molinski TF, Pessah IN. Xestospongins: potent memebrane permeable blockers of the inositol 1,4,5-trisphosphate receptor. Neuron. 1997;19:723–733. doi: 10.1016/s0896-6273(00)80384-0. [DOI] [PubMed] [Google Scholar]
- Gaughan JP, Hefner CA, Houser SR. Electrophysiological properties of neonatal rat ventricular myocytes with alpha1-adrenergic-induced hypertrophy. Am J Physiol Heart Circ Physiol. 1998;275:H577–H590. doi: 10.1152/ajpheart.1998.275.2.H577. [DOI] [PubMed] [Google Scholar]
- Graness A, Adomeit A, Heinze R, Wetzker R, Liebmann C. A novel mitogenic signaling pathway of bradykinin in the human colon carcinoma cell line SW-480 involves sequential activation of a Gq/11 protein, phosphatidylinositol-3-kinase beta and protein kinase C epsilon. J Biol Chem. 2005;273:32016–32022. doi: 10.1074/jbc.273.48.32016. [DOI] [PubMed] [Google Scholar]
- Han H-M, Robinson RB, Bilezikian JP, Steinberg SF. Developmental changes in guanine nucleotide regulatory proteins in the rat myocardial α1-adrenergic receptor complex. Circulation Res. 1989;65:1763–1773. doi: 10.1161/01.res.65.6.1763. [DOI] [PubMed] [Google Scholar]
- Hartmann HA, Mazzocca NJ, Kleiman RB, Houser SR. Effects of phenylephrine on calcium current and contractility of feline ventricular myocytes. Am J Physiol Heart Circ Physiol. 1988;255:H1173–H1180. doi: 10.1152/ajpheart.1988.255.5.H1173. [DOI] [PubMed] [Google Scholar]
- Herbert JM, Augereau JM, Gleye J, Maffrand JP. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1990;172:993–999. doi: 10.1016/0006-291x(90)91544-3. [DOI] [PubMed] [Google Scholar]
- Hescheler J, Nawrath H, Tang M, Trautwein W. Adrenoceptor-mediated changes of excitation and contraction in ventricular heart muscle from guinea-pigs and rabbits. J Physiol. 1988;397:657–670. doi: 10.1113/jphysiol.1988.sp017024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka H, Sasaki Y, Tanaka T, Endo T, Ohno S, Fujii Y, Nagata T. N-(6-Aminohexyl)-5-chloro-1-naphthalenesulfonamide, a calmodulin antagonist, inhibits cell proliferation. Proc Natl Acad Sci U S A. 1981;78:4354–4357. doi: 10.1073/pnas.78.7.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnel U, Duwe E, Pfennigsdorf S, Nawrath H. On the mechanism of action of phenylephrine in rat atrial heart muscle. Naunyn Schmeidebergs Arch Pharmacol. 1994;349:408–415. doi: 10.1007/BF00170888. [DOI] [PubMed] [Google Scholar]
- Jahnel U, Jakob H, Nawrath H. Electrophysiologic and inotropic effects of alpha-adrenoceptor stimulation in human isolated atrial heart muscle. Naunyn Schmeidebergs Arch Pharmacol. 1992a;346:82–87. doi: 10.1007/BF00167575. [DOI] [PubMed] [Google Scholar]
- Jahnel U, Nawrath H, Carmeliet E, Vereecke J. Depolarization-induced influx of sodium in response to phenylephrine in rat atrial heart muscle. J Physiol. 1991;432:621–637. doi: 10.1113/jphysiol.1991.sp018404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnel U, Nawrath H, Shieh RC, Sharma VK, Williford DJ, Sheu SS. Modulation of cytosolic free calcium concentration by alpha 1-adrenoceptors in rat atrial cells. Naunyn Schmeidebergs Arch Pharmacol. 1992b;346:88–93. doi: 10.1007/BF00167576. [DOI] [PubMed] [Google Scholar]
- Kanai AJ, Mesaros S, Finkel MS, Oddis CV, Birder LA, Malinski T. β-Adrenergic regulation of constitutive nitric oxide synthase in cardiac myocytes. Am J Physiol Cell Physiol. 1997;273:C1371–C1377. doi: 10.1152/ajpcell.1997.273.4.C1371. [DOI] [PubMed] [Google Scholar]
- Kaye DM, Wiviott SD, Balligand J-L, Simmons WW, Smith TW, Kelly RA. Frequency-dependent activation of a constitutive nitric oxide synthase and regulation of contractile function in adult rat ventricular myocytes. Circulation Res. 1996;78:217–224. doi: 10.1161/01.res.78.2.217. [DOI] [PubMed] [Google Scholar]
- Keung EC, Karliner JS. Complex regulation of calcium current in cardiac cells; dependence on a pertussis toxin-sensitive substrate, adenosine triphosphate, and an α1-adrenoceptor. J Clin Invest. 1990;85:950–954. doi: 10.1172/JCI114524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirstein M, Rivet-Bastide M, Hatem S, Benardeau A, Mercadier J-J, Fischmeister R. Nitric oxide regulates the calcium current in isolated human atrial myocytes. J Clin Invest. 1995;95:794–802. doi: 10.1172/JCI117729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima H, Nakatsubo N, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, Hirata Y, Nagano T. Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal Chem. 1998;70:2446–2453. doi: 10.1021/ac9801723. [DOI] [PubMed] [Google Scholar]
- Li K, He H, Li C, Sirois P, Rouleau JL. Myocardial α1-adrenoceptor: inotropic effect and physiologic and pathologic implications. Life Sci. 1997;60:1305–1318. doi: 10.1016/s0024-3205(96)00650-9. [DOI] [PubMed] [Google Scholar]
- Lipp P, Laine M, Tovey SC, Burrell KM, Berridge MJ, Li W. Functional InsP3 receptors that may modulate excitation-contraction coupling in the heart. Current Biol. 2000;10:939–942. doi: 10.1016/s0960-9822(00)00624-2. [DOI] [PubMed] [Google Scholar]
- Lipsius SL, Wang YG, Ji X, Blatter LA, Dedkova EN. Alpha-1 adrenoceptor stimulation by phenylephrine stimulates L-type calcium current via nitric oxide production in cat atrial myocytes. Circulation. 2003;108:IV–292. [Google Scholar]
- Liu QY, Karpinski E, Pang PK. The L-type calcium channel current is increased by alpha-1 adrenoceptor activation in neonatal rat ventricular cells. J Pharmacol Exp Therapeutics. 1994;271:935–943. [PubMed] [Google Scholar]
- Löhn M, Fürstenau M, Sagach V, Elger M, Schulze W, Luft FC, Haller H, Gollasch M. Ignition of calcium sparks in arterial and cardiac muscle through caveolae. Circulation Res. 2000;87:1034–1039. doi: 10.1161/01.res.87.11.1034. [DOI] [PubMed] [Google Scholar]
- Mackenzie L, Bootman MD, Laine M, Berridge MJ, Thuring J, Holmes A, Li W, Lipp P. The role of inositol 1,4,5-trisphosphate receptors in Ca2+ signalling and the generation of arrhythmias in rat atrial myocytes. J Physiol. 2002;541:395–409. doi: 10.1113/jphysiol.2001.013411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie L, Roderick HL, Berridge MJ, Conway SJ, Bootman MD. The spatial pattern of atrial cardiomyocyte calcium signaling modulates contraction. J Cell Sci. 2004;117:6327–6337. doi: 10.1242/jcs.01559. [DOI] [PubMed] [Google Scholar]
- Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J Biochem. 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- Murga C, Laguinge L, Wetzker R, Cuadrado A, Gutkind JS. Activation of Akt/protein kinase B by G protein-coupled receptors. A role for alpha and beta gamma subunits of heterotrimeric G proteins acting through phosphotidylinositol-3-OH kinasegamma. J Biol Chem. 1998;273:19080–19085. doi: 10.1074/jbc.273.30.19080. [DOI] [PubMed] [Google Scholar]
- Nakatsubo N, Kojima H, Kikuchi K, Nagoshi H, Hirata Y, Maeda D, Imai Y, Irimura T, Nagano T. Direct evidence of nitric oxide production from bovine aortic endothelial cells using new fluorescence indicators: diaminofluoresceins. FEBS Lett. 1998;427:263–266. doi: 10.1016/s0014-5793(98)00440-2. [DOI] [PubMed] [Google Scholar]
- Nosek TM, Williams M, Ziegler ST, Godt RE. Inositol trisphosphate enhances calcium release in skinned cardiac and skeletal muscle. Am J Physiol Cell Physiol. 1986;250:C807–C811. doi: 10.1152/ajpcell.1986.250.5.C807. [DOI] [PubMed] [Google Scholar]
- Perez DM, DeYoung MB, Graham RM. Coupling of expressed α1B- and α1D-adrenergic receptors to multiple signaling pathways is both G protein and cell type specific. Mol Pharmacol. 1993;44:784–795. [PubMed] [Google Scholar]
- Rees DD, Palmer RMJ, Schulz R, Hodson H, Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990;101:746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann HJ, Endoh M, Brodde O-E. The time course of the effects of β- and α-adrenoceptor stimulation by isoprenaline and methoxamine on the contractile force and cAMP levels of the isolated papillary muscle. Naunyn Schmiedebergs Arch Pharmacol. 1975;289:291–302. doi: 10.1007/BF00499982. [DOI] [PubMed] [Google Scholar]
- Schumann HJ, Wagner J, Knorr A, Reidemeister JC, Sadony V, Schramm G. Demonstration in human atrial preparations of α-adrenoceptors mediating positive inotropic effects. Naunyn Schmiedebergs Arch Pharmocol. 1978;302:333–336. doi: 10.1007/BF00508304. [DOI] [PubMed] [Google Scholar]
- Sipido KR, Maes MM, Van de Werf F. Low efficiency of Ca2+ entry through the Na/Ca exchanger as trigger for Ca2+ release from the sarcoplasmic reticulum. Circulation Res. 1997;81:1043–1044. doi: 10.1161/01.res.81.6.1034. [DOI] [PubMed] [Google Scholar]
- Skomedal T, Aass H, Osnes JB, Fjeld NB, Klingen G, Langslet A, Semb G. Demonstration of alpha adrenoceptor-mediated inotropic effect of norepinephrine in human atria. J Pharmacol Exp Therapeutics. 1985;233:441–446. [PubMed] [Google Scholar]
- Smart EJ, Anderson RGW. Alterations in membrane cholesterol that affect structure and function of caveolae. Meth Enzymol. 2002;353:131–139. doi: 10.1016/s0076-6879(02)53043-3. [DOI] [PubMed] [Google Scholar]
- Steinberg SF, Drugge ED, Bilezikian JP, Robinson RB. Acquisition by innervated cardiac myocytes of a pertussis toxin-specific regulatory protein linked to the alpha 1-receptor. Science. 1985;230:186–188. doi: 10.1126/science.2994230. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Kojima H, Urano Y, Kikuchi K, Hirata Y, Nagano T. Orthogonality of calcium concentration and ability of 4,5-diaminofluorescein to detect. J Biol Chem. 2002;277:47–49. doi: 10.1074/jbc.M108195200. [DOI] [PubMed] [Google Scholar]
- Terzic A, Puceat M, Clement O, Scamps F, Vassort G. Alpha 1-adrenergic effects on intracellular pH and calcium and on myofilaments in single rat cardiac cells. J Physiol. 1992;447:275–292. doi: 10.1113/jphysiol.1992.sp019002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vila Petroff MG, Kim SH, Pepe S, Dessy C, Marban E, Balligand J-L, Sollott SJ. Endogenous nitric oxide mechanisms mediate the stretch dependence of Ca2+ release in cardiomyocytes. Nature Cell Biol. 2001;3:867–873. doi: 10.1038/ncb1001-867. [DOI] [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- Wang YG, Dedkova EN, Steinberg SF, Blatter LA, Lipsius SL. β2-Adrenergic receptor signaling acts via NO release to mediate ACh-induced activation of ATP-sensitive K+ current in cat atrial myocytes. J General Physiol. 2002;119:69–82. doi: 10.1085/jgp.119.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YG, Rechenmacher CE, Lipsius SL. Nitric oxide signaling mediates stimulation of L-type Ca2+ current elicited by withdrawal of acetylcholine in cat atrial myocytes. J General Physiol. 1998;111:113–125. doi: 10.1085/jgp.111.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber CR, Piacentino V, Ginsburg KS, Houser SR, Bers DM. Na+-Ca2+ exchange current and submembrane [Ca2+] during the cardiac action potential. Circulation Res. 2002;90:182–189. doi: 10.1161/hh0202.103940. [DOI] [PubMed] [Google Scholar]
- Woo SH, Lee CO. Role of PKC in the effects of α1-adrenergic stimulation on Ca2+ transients, contraction, and Ca2+ current in guinea-pig ventricular myocytes. Pflügers Archive. 1999;437:335–344. doi: 10.1007/s004240050787. [DOI] [PubMed] [Google Scholar]
- Wu J, Vereecke J, Carmeliet E, Lipsius SL. Ionic currents activated during hyperpolarization of single right atrial myocytes from cat heart. Circulation Res. 1991;68:1059–1069. doi: 10.1161/01.res.68.4.1059. [DOI] [PubMed] [Google Scholar]
- Xie P, Browning DD, Hay N, Mackman N., RD Activation of NF-kappa B by bradykinin through a Galpha(q)- and Gbeta gamma-dependent pathway that involves phosphoinositide 3-kinase and Akt. J Biol Chem. 2005;275:24907–24914. doi: 10.1074/jbc.M001051200. [DOI] [PubMed] [Google Scholar]
- Zhang S, Hiraoka M, Hirano Y. Effects of alpha1-adrenergic stimulation on L-type Ca2+ current in rat ventricular myocytes. J Mol Cellular Cardiol. 1998;30:1955–1965. doi: 10.1006/jmcc.1998.0758. [DOI] [PubMed] [Google Scholar]
- Zima AV, Blatter LA. Inositol-1,4,5-trisphosphate-dependent Ca2+ signaling in cat atrial excitation–contraction coupling and arrhythmias. J Physiol. 2004;555:607–615. doi: 10.1113/jphysiol.2003.058529. [DOI] [PMC free article] [PubMed] [Google Scholar]